Abstract
Sarcoidosis is an inflammatory, granulomatous disease of unknown etiology that most commonly afflicts the lungs. Despite aggressive immunosuppressive therapies, many sarcoidosis patients still chronically present significant symptoms. Infliximab, a therapeutic tumor necrosis factor alpha (TNF-α) monoclonal antibody (MAb), produced a small but significant improvement in forced vital capacity (FVC) in sarcoidosis patients in a double-blind, placebo-controlled, phase II clinical trial. In the current study, serum samples from this clinical trial were assessed to evaluate the underlying hypothesis that treatment with infliximab would reduce systemic inflammation associated with sarcoidosis, correlating with the extent of clinical response. A 92-analyte multiplex panel was used to assess the expression of serum proteins in 134 sarcoidosis patients compared with sera from 50 healthy controls. A strong systemic inflammatory profile was associated with sarcoidosis, with 29 analytes significantly elevated in sarcoidosis (false-discovery rate, <0.05 and >50% higher than controls). The associated analytes included chemokines, neutrophil-associated proteins, acute-phase proteins, and metabolism-associated proteins. This profile was evident despite patients receiving corticosteroids and immunosuppressive therapies. Following infliximab treatment, sarcoidosis patients expressing the highest levels of TNF-α, who had more severe disease, had the greatest improvement in FVC and reduction in serum levels of the inflammatory proteins MIP-1β and TNF-RII. This study supports the need for further exploration of anti-TNF therapy for chronic sarcoidosis patients, particularly for those expressing the highest serum levels of TNF-α.
INTRODUCTION
Sarcoidosis is a chronic inflammatory disease characterized by noncaseating granulomas consisting primarily of T cells and macrophages, found most commonly in the lungs and lymphatic system (1, 15). Granulomas also form in other tissues, such as the skin (subcutaneous), eyes, liver, joints, and heart, in a subset of patients. The mechanisms initiating and promoting granuloma formation are not well established, and virus-, bacterium-, or inorganic-molecule-dependent mechanisms have been proposed (1). The inflammatory profile of sarcoidosis is generally characterized by Th1-associated cytokines (including interleukin 12 [IL-12], gamma interferon [IFN-γ], and IL-18) and molecules associated with chronic granulomatous inflammation (including angiotensin-converting enzyme and tumor necrosis factor alpha [TNF-α]). TNF-α is critical in the development of granulomas in various systems and is presumed to be important in the etiology of sarcoidosis.
For patients with persistent chronic sarcoidosis who would require continuous corticosteroid administration, alternative immunosuppressive (cytotoxic) and antimalarial agents are sometimes used. However, the unproven efficacy and considerable long-term toxicity of these agents underscore the need for safer alternatives with demonstrable efficacy (1).
In various case studies, the TNF-α-neutralizing monoclonal antibody (MAb) infliximab (Centocor Ortho Biotech, Inc., Malvern, PA) has been reported to be efficacious in the treatment of some patients with refractory sarcoidosis (3). To evaluate the safety and efficacy of infliximab in chronic pulmonary sarcoidosis, a multicenter, randomized, double-blind, placebo-controlled, phase II clinical study was undertaken (2). The study examined chronic pulmonary sarcoidosis patients who were symptomatic despite ongoing immunosuppressive therapy with corticosteroids and/or other agents. In this study, infliximab demonstrated a significant improvement in ventilatory restriction after 24 weeks of treatment (P = 0.038 compared with placebo). From post hoc analyses, improvement in extrapulmonary severity after 24 weeks was observed in the infliximab group, but not in the placebo group (12).
The underlying hypothesis for the current study is that treatment with infliximab will reduce systemic inflammation associated with sarcoidosis, correlating with the extent of clinical response. Patients expressing the highest levels of inflammatory burden before treatment are anticipated to respond best to infliximab. The aims of the current study to evaluate this hypothesis were to (i) evaluate the systemic inflammatory profile associated with sarcoidosis and the presentation of disease severity, (ii) establish the impact of TNF-α neutralization on systemic inflammation in sarcoidosis, and (iii) determine whether changes in inflammatory mediators correlate with clinical response to infliximab. To achieve these aims, we evaluated a broad panel of inflammation-associated proteins in serum samples obtained at baseline and week 24 from the sarcoidosis study population and compared them with serum samples from a healthy control population. Baseline concentrations of the serum analytes were tested for associations with disease status and correlations with clinical measurements of disease severity and clinical response to infliximab treatment. The impact of infliximab treatment on systemic inflammation associated with sarcoidosis was investigated by comparison to the placebo group for changes in serum levels from baseline to the week 24 endpoint.
MATERIALS AND METHODS
Inclusion and exclusion criteria, demographic and clinical characterization of the sarcoidosis patients, and clinical efficacy and safety results of the study (2), including percent predicted forced vital capacity (ppFVC), St. George's Respiratory Questionnaire (SGRQ) total score, 6-minute walk distance (6MWD), diffusing limit of carbon monoxide (DLCO), and extrapulmonary physician organ severity tool (ePOST) score (12), have been previously reported. Placebo or infliximab at 3 or 5 mg/kg of body weight was administered at weeks 0 (baseline), 2, 6, 12, 18, and 24. Peripheral venous blood samples were collected before study agent administration at baseline and week 24 (the primary endpoint time point).
Serum samples from 50 healthy control subjects were purchased from a commercial vendor (Bioreclamation, Hicksville, NY). Qualification for healthy status was evaluated from a self-reported questionnaire. Exclusion criteria for healthy controls included not feeling healthy at the time of donation; headache or fever in the past week; risky sexual activity in the past year; having ever tested positive for or been treated for sexually transmitted disease; a history of alcoholism or drug abuse; having or having had one or more of the diseases in a broad panel; having been under the care of a physician, having had surgery, or having taken any prescription medications in the past year; and, for females, being currently pregnant or having been pregnant in the past 6 weeks or being postmenopausal. Basic demographic information (age and gender) was available for the healthy control subjects.
The institutional review board for each institution approved the study. All subjects provided written informed consent.
Measurement of serum analyte concentrations.
Serum samples were analyzed for the concentrations of 92 inflammation-associated proteins by Rules Based Medicine, Inc., using their human MAP v1.6 panel (18) of Luminex-based multiplex assays, with measurements for IL-17, IL-18, and IL-23 added to the panel. The least detectable dose (LDD) was defined as the back-calculated concentration for the mean plus 3 standard deviations (SD) of the luminescence values of 20 blank readings (the LDD for each analyte is shown in Table S1 in the supplemental material). Concentrations below the lowest resolved standard and/or the LDD were transformed to a value of 0.5× LDD. Baseline serum samples from sarcoidosis patients and healthy controls were analyzed at the same time by Rules Based Medicine, and all serum samples underwent the same number of freeze-thaw cycles. The week 24 serum samples from sarcoidosis patients were analyzed independently of the baseline samples, with 10 baseline samples analyzed with week 24 samples to assess potential batch effects by Bland-Altman plot and correlation analyses.
Statistical analyses.
Nonparametric tests were generally used for analyses because the majority of analytes (original scale and log transformed) were not n-normally distributed (according to the D'Agostino and Pearson omnibus test for normality) for both sarcoidosis and healthy control populations. Mann-Whitney U tests and Kruskal-Wallis tests were used to compare continuous variables among 2 and 3 groups, respectively. Rank-based tests using Spearman's correlations were used to test for correlations among continuous variables. General linear model analyses, adjusted for multiple independent variables, were performed. For comparisons of treatment responses, the response for each individual patient was expressed as log2(week 24 value/baseline value), and these calculated relative values were tested for differences between infliximab and placebo treatment groups. To evaluate the validity of the analyses, model assumptions, such as normality and homogeneity of variance, were examined. The significance level was reported as a P value or a false-discovery rate (FDR) (20) to control for multiple-testing inflation of the false-positive rate based on the Benjamin-Hochberg procedure, calculated using the R program QVALUE v.1.0 (7). An FDR of <0.05 was considered significant, and a P value of <0.05 (with an FDR of >0.05) was considered a nominally significant trend. Unsupervised clustering was performed using hierarchical-standard clustering with complete linkage and a Euclidean similarity metric (OmniViz v6.0 software; BioWisdom, Inc.).
RESULTS
Associations of baseline serum analyte levels and sarcoidosis.
Serum samples were collected from 134 patients with chronic pulmonary sarcoidosis at baseline, before the first infusion of placebo (n = 44), infliximab at 3 mg/kg (n = 45), or infliximab at 5 mg/kg (n = 45). Serum samples were also obtained during the primary endpoint visit at week 24 (placebo, n = 42; infliximab at 3 mg/kg, n = 43; infliximab at 5 mg/kg, n = 45). Basic demographic and baseline clinical characterization of the sarcoidosis patients and their background therapy are provided in Table 1. In addition, serum samples were obtained from 50 healthy control subjects independently of the clinical trial. The serum concentrations of 92 inflammation-associated analytes were measured. For the baseline serum samples, the concentrations of 26 analytes were below the LDD of the assay for >75% of both sarcoidosis patients and healthy control subjects, and 2 additional analytes were below the LDD for >75% of sarcoidosis patients and >50% of healthy controls (see Table S1 in the supplemental material). These analytes were excluded from further statistical analyses of the baseline samples.
Table 1.
Demographics and baseline clinical phenotypes in sarcoidosis patients
| Characteristic | Total |
Female |
Male |
P value (female vs. male) | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Patients (n) | 134 | 77 | 57 | ||||
| Gender (% male) | 57.5 | 0.0 | 100.0 | ||||
| Age (yr) | 46.9 | 9.3 | 49.1 | 9.0 | 45.3 | 9.1 | 0.016 |
| ppFVC | 68.5 | 9.8 | 69.2 | 11.0 | 68.0 | 8.9 | 0.45 |
| SGRQ total score | 46.8 | 18.9 | 54.4 | 17.2 | 41.2 | 18.2 | 3.5 × 10−5 |
| 6MWD | 455 | 113 | 383 | 99 | 508 | 92 | <10−9 |
| DLCO | 17.6 | 6.6 | 12.7 | 4.1 | 21.2 | 5.8 | <10−9 |
| ePOST score | 2b | 0–6b | 4 | 0–8 | 1 | 0–4 | 0.018 |
| BMIa | 30.5 | 6.6 | 32.8 | 7.0 | 28.7 | 5.8 | 4.5 × 10−4 |
| Race | 0.0023 | ||||||
| Asian (%) | 1.5 | 1.8 | 1.3 | ||||
| Black (%) | 30.6 | 45.6 | 19.5 | ||||
| Caucasian (%) | 66.4 | 49.1 | 79.2 | ||||
| Other (%) | 1.5 | 3.5 | 0.0 | ||||
| Baseline therapy | 0.14 | ||||||
| Immunoc (%) | 10.4 | 15.8 | 6.5 | ||||
| OCS (%) | 51.5 | 43.9 | 57.1 | ||||
| OCS + Immuno (%) | 38.1 | 40.4 | 36.4 | ||||
| Organ involvement | |||||||
| Extrapulmonary (%) | 66.7 | 72.7 | 62.3 | 0.26 | |||
| Skin (%) | 12.7 | 17.5 | 9.1 | 0.15 | |||
| Eyes (%) | 4.5 | 5.3 | 3.9 | 0.71 | |||
BMI, body mass index.
Median and interquartile range are presented for ePOST scores.
Immuno, immunomodulators, including azathioprine, chloroquine, hydroxychloroquine, and methotrexate.
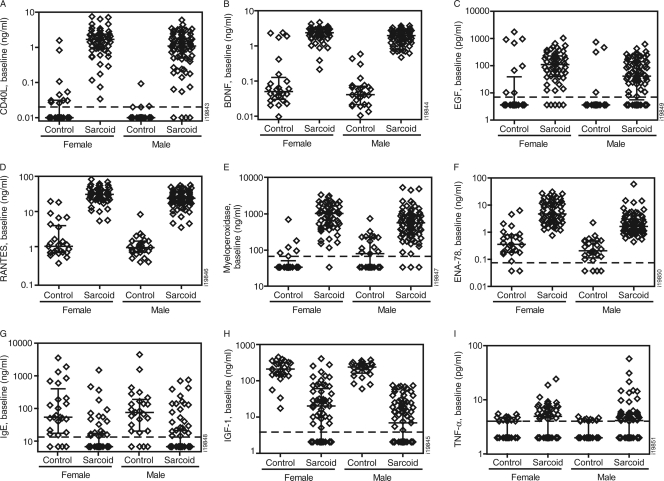
To establish the baseline systemic inflammatory profile associated with sarcoidosis, the serum analyte levels in the sarcoidosis and control populations were compared. In total, 35 analytes were associated with sarcoidosis, 29 were not associated with sarcoidosis, and 28 were below the LDD of the assay platform. Of the 35 analytes associated with sarcoidosis, the median serum levels of 29 analytes were >50% higher in sarcoidosis cases than in controls and those of 6 analytes were 50% lower in sarcoidosis cases than in controls, with an FDR of <0.05 (Table 2). The significantly associated analytes with the greatest differences between sarcoidosis cases and controls included CD40L, brain-derived neurotrophic factor (BDNF), epidermal growth factor (EGF), CC-chemokine ligand 5/RANTES (regulated upon activation, normally T-cell expressed, and presumably secreted), myeloperoxidase, and ENA-78 (epithelial-derived neutrophil-activating protein 78), each greater than 7-fold higher in the sarcoidosis than in the control population (Fig. 1 A to F); IGF-1 (insulin-like growth factor 1) and IgE were both decreased in sarcoidosis patients more than 8-fold relative to controls (Fig. 1G and H). TNF-α levels were significantly increased in sarcoidosis patients, but with nearly a majority (47%) of sarcoidosis patients having levels below the LDD (Fig. 1I). MIP-1β, an infliximab-sensitive chemokine (detailed in Changes in serum analyte concentrations after infliximab treatment below), was also significantly associated with sarcoidosis (3.5-fold; FDR < 10−8).
Table 2.
Analytes significantly associated with sarcoidosis
| Analytea | Females |
Males |
Males + females |
|||
|---|---|---|---|---|---|---|
| ± Foldb | FDR | ± Fold | FDR | ± Fold | FDR | |
| Sarcoidosis > control | ||||||
| CD40 ligand | 169 | <10−8 | 109 | <10−8 | 128 | <10−8 |
| Brain-derived neurotrophic factor | 47.5 | <10−8 | 48.0 | <10−8 | 48.7 | <10−8 |
| RANTES | 27.0 | <10−8 | 22.8 | <10−8 | 24.7 | <10−8 |
| Myeloperoxidase | 30.3 | <10−8 | 7.1 | 1.0 × 10−8 | 19.1 | <10−8 |
| EGF | 30.5 | 1.7 × 10−4 | 11.4 | 8.6 × 10−5 | 18.5 | 9.0 × 10−8 |
| ENA-78 | 13.16 | <10−8 | 7.88 | <10−8 | 7.59 | <10−8 |
| SGOT | 5.59 | 2.7 × 10−6 | 7.90 | <10−8 | 6.69 | <10−8 |
| Ferritin | 2.87 | 5.8 × 10−5 | 7.76 | <10−8 | 4.87 | <10−8 |
| Growth hormone | 5.88 | 1.9 × 10−4 | 3.94 | 3.8 × 10−3 | 4.81 | 6.4 × 10−6 |
| Eotaxin | 3.80 | 2.0 × 10−8 | 5.32 | <10−8 | 4.78 | <10−8 |
| Insulin | 2.11 | 0.026 | 6.06 | 8.9 × 10−4 | 3.56 | 8.4 × 10−5 |
| MIP-1β | 4.08 | 4.0 × 10−8 | 3.26 | <10−8 | 3.51 | <10−8 |
| MMP-3 | 3.04 | 6.6 × 10−5 | 3.25 | 5.0 × 10−8 | 3.24 | <10−8 |
| ENRAGE | 4.08 | 4.2 × 10−7 | 1.61 | 0.012 | 2.78 | 2.0 × 10−8 |
| IL-18 | 3.07 | <10−8 | 2.76 | <10−8 | 2.74 | <10−8 |
| PAI-1 | 2.47 | <10−8 | 2.62 | <10−8 | 2.69 | <10−8 |
| Leptin | 2.55 | 4.6 × 10−6 | 4.85 | 4.5 × 10−5 | 2.68 | 8.2 × 10−6 |
| TIMP-1 | 2.76 | <10−8 | 2.56 | <10−8 | 2.60 | <10−8 |
| IL-7 | 3.68 | 4.2 × 10−7 | 1.00 | 1.2 × 10−4 | 2.40 | <10−8 |
| CRP | 2.55 | 6.5 × 10−3 | 2.08 | 4.6 × 10−6 | 2.25 | 2.8 × 10−3 |
| IL-1RA | 2.65 | 9.5 × 10−5 | 1.46 | 0.022 | 2.18 | 1.3 × 10−3 |
| TNF-α | 1.23 | 0.061 | 2.08 | 0.030 | 2.16 | 5.3 × 10−3 |
| Glutathione S-transferase | 2.40 | 1.2 × 10−6 | 1.83 | 2.0 × 10−4 | 2.04 | <10−8 |
| Stem cell factor | 3.01 | 2.0 × 10−8 | 1.70 | 4.5 × 10−5 | 2.00 | <10−8 |
| Prostatic acid phosphatase | 2.26 | 1.0 × 10−8 | 1.70 | 1.2 × 10−4 | 1.94 | <10−8 |
| IL-16 | 2.13 | 5.0 × 10−8 | 1.80 | 8.0 × 10−8 | 1.84 | <10−8 |
| VEGF | 1.82 | 1.4 × 10−4 | 1.71 | 1.2 × 10−4 | 1.68 | 1.6 × 10−7 |
| MCP-1 | 1.47 | 7.4 × 10−5 | 1.70 | 1.9 × 10−6 | 1.63 | <10−8 |
| CD40 | 1.92 | 4.2 × 10−5 | 1.25 | 6.3 × 10−3 | 1.62 | 8.0 × 10−7 |
| Sarcoidosis < control | ||||||
| IGF-1 | −10.41 | <10−8 | −34.60 | <10−8 | −20.4 | <10−8 |
| IgE | −7.97 | 1.2 × 10−6 | −10.99 | 6.4 × 10−6 | −9.35 | <10−8 |
| α-2 Macroglobulin | −2.89 | 5.1 × 10−7 | −8.17 | <10−8 | −5.85 | <10−8 |
| MIP-1α | −1.90 | 2.7 × 10−4 | −3.90 | <10−8 | −2.99 | <10−8 |
| Tissue factor | −2.30 | 4.2 × 10−7 | −2.10 | 5.0 × 10−8 | −2.15 | <10−8 |
| Apolipoprotein CIII | −1.49 | 0.024 | −2.90 | 7.8 × 10−6 | −2.10 | 9.7 × 10−7 |
Analytes significantly associated with sarcoidosis (FDR < 0.05 and |signed-fold| > 1.5) in the total populations (males plus females) are reported. Analytes that were significantly associated with sarcoidosis in only females or only males are shown in boldface. SGOT, serum glutamic oxaloacetic transaminase; VEGF, vascular endothelial growth factor.
± Fold represents the signed fold for the median in sarcoidosis divided by the median in healthy control populations. When the median expression in the sarcoidosis population was lower than that in controls, the opposite of the reciprocal is reported.
Fig. 1.
Association between baseline analyte concentrations and sarcoidosis. The baseline concentrations for the indicated analytes (y axes) are shown for male and female subpopulations in the healthy control and sarcoidosis cohorts. Each symbol represents an individual patient. Median and interquartile range are shown for each population. The dashed lines indicate the LDDs for the analyte. (A to F) Top 6 of 29 analytes with increased concentrations associated with sarcoidosis. (G and H) Top 2 of 6 analytes with decreased concentrations associated with sarcoidosis. (I) TNF-α concentrations were increased in sarcoidosis but below the LDD in nearly half of patients.
The ratio of males to females was higher in the sarcoidosis patient population than in the healthy control population (57:77 versus 25:25, respectively), which had the potential to introduce gender bias in the results. Indeed, substantial differences were observed between males and females in the sarcoidosis population. Females had more severe disease than males, with a significantly higher SGRQ total score (P = 3.5 × 10−5) and lower 6MWD and DLCO (P < 10−9 for both) (Table 1). Fifteen of 64 (23%) analytes were significantly different (FDR < 0.05; >50% difference) between males and females in the sarcoidosis population. Because of the differences between males and females within sarcoidosis cases, statistical analyses for comparisons of cases and controls included stratification by gender. Thirty-one of the 35 analytes significantly associated with sarcoidosis remained significant when adjusted for gender, with TNF-α dropping out for females and IL-7, IL-1 receptor antagonist (IL-1RA), and CD40 dropping out for males. Of the 29 analytes not associated with sarcoidosis, 19 analytes had an FDR of >0.05 and/or an absolute value of signed fold of <1.5 for sarcoidosis cases versus controls for each of the gender-unrestricted or -restricted analyses, with the other 10 analytes showing an association only in gender-restricted analyses (see Table S2 in the supplemental material).
Correlation of baseline serum analyte levels with clinical measurements.
None of the analytes obtained at baseline were significantly associated with baseline clinical measurements (ppFVC, SGRQ total score, 6MWD, DLCO, or ePOST score) after FDR adjustment in both male and female sarcoidosis populations. Among analytes significantly associated with sarcoidosis, ENRAGE (extracellular newly identified receptor for advanced glycation end product)/S100A12 was correlated with the ppFVC with nominal significance for both males and females (r = −0.32; P = 0.014), and leptin was significantly correlated with SGRQ total scores in males only (r = 0.44; FDR = 0.0039) (see Fig. S1 in the supplemental material). A list of r, P, and FDR values for analytes that achieved significant correlations (FDR < 0.05) in either gender or nominal significant correlations in the total population is provided in Table S3 in the supplemental material.
Extrapulmonary organ involvement was significantly correlated (FDR < 0.05) with 7 analytes in the total sarcoidosis study population, including the acute-phase proteins C-reactive protein (CRP) and ferritin (see Table S4 in the supplemental material). For the severity of extrapulmonary organ involvement (i.e., the ePOST score), no significant correlations were seen in gender-stratified analyses (data not shown).
The correlation between baseline concentrations of the serum analytes and treatment-associated changes in clinical parameters from baseline to week 24 was evaluated. There were no significant correlations (FDR < 0.05) between baseline analyte concentrations and changes from baseline to week 24 in ppFVC, SGRQ, 6MWD, or ePOST in infliximab treatment groups.
Changes in serum analyte concentrations after infliximab treatment.
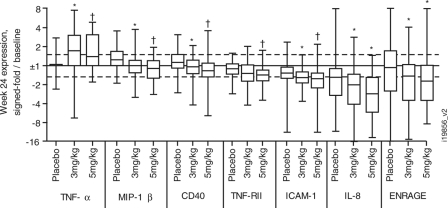
Having established the baseline systemic inflammatory profile associated with sarcoidosis, whether treatment with infliximab could significantly down-modulate this inflammatory profile was examined. After exclusion of 30 analytes for being below the detection level and 12 analytes for uncorrectable batch effects, the change in the serum concentrations of 50 analytes from baseline to week 24 was examined for the 3 treatment groups in the sarcoidosis patient population. The results for analytes with FDRs of <0.05 or P values of <0.05 for differences between the infliximab and placebo groups are shown in Table S5 in the supplemental material and are shown for select analytes in Fig. 2. The ratio of posttreatment concentrations to baseline concentrations for each analyte was calculated for each individual patient. The significance of the differences in the median concentrations between the 3-mg/kg and 5-mg/kg infliximab treatment groups and the placebo group was tested. TNF-α concentrations increased during treatment with infliximab, consistent with observations for other infliximab studies using this multiplex assay (unpublished data). Despite an FDR of <0.05 for the 5-mg/kg infliximab treatment compared with placebo, the difference in the median concentrations of MIP-1β, CD40, TNF-RII, and ICAM-1 were less than 40% from the placebo and not significant for the 3-mg/kg infliximab group versus placebo. Denser time courses of analyte levels in serum were not available for analysis using the Rules Based Medicine panel. However, clinical laboratory measurements for CRP and ICAM-1 were performed more frequently (see Fig. S2 in the supplemental material). There was a modest decrease in CRP levels at 2 weeks postdosing with 10 mg/kg infliximab relative to baseline (median, 25% decrease; P = 0.003 versus placebo), but it rebounded to baseline levels by week 6 (median, 0% change from baseline). ICAM-1 levels also were significantly decreased at week 2, but in both the 3- and 10-mg/kg infliximab groups (33% decrease; P < 10−6 versus placebo). Unlike CRP, the decrease in ICAM-1 levels was maintained throughout the study period, consistent with the results from the Rules Based Medicine panel.
Fig. 2.
Change in serum analyte concentrations after 24-week infliximab treatment. Results are shown for analytes demonstrating FDRs of <0.05 (†) for differences between the infliximab treatment group (3 or 5 mg/kg) and the placebo group or P < 0.05 (*) for both the 3- and 5-mg/kg treatment groups versus placebo. The ratio of the analyte concentration after 24-week treatment to the baseline concentration per patient is expressed as the signed fold (y axis) for each treatment condition. Box (median and interquartile range) and whiskers (range) plots are presented.
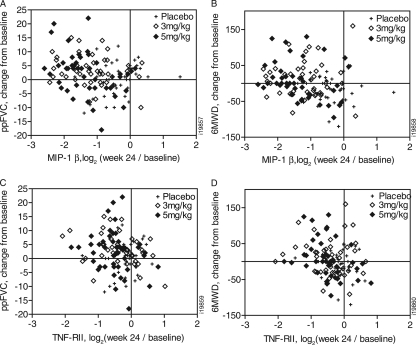
Changes from baseline in concentrations of MIP-1β and TNF-RII were inversely correlated (with nominal significance) with changes from baseline in both ppFVC (r = −0.39 and −0.30) and 6MWD (r = −0.38 and −0.48) for the 5-mg/kg infliximab treatment group (Fig. 3). Importantly, changes in these analytes had (nonsignificant) direct correlations with changes in ppFVC and 6MWD in the placebo group (Fig. 3). Multivariate modeling with adjustments for gender and the respective baseline clinical measurement did not significantly impact the observed correlations. Changes in TNF-RII and MIP-1β were strongly correlated (r = 0.70) and thus were not additive in their correlation with changes in ppFVC and 6MWD.
Fig. 3.
Correlation between changes in analyte concentrations and clinical measures after infliximab treatment. Changes in the concentrations of MIP-1β as correlated with ppFVC (A) and 6MWD (B) and changes in the concentrations of TNF-RII as correlated with ppFVC (C) and 6MWD (D), expressed as log2 of the week 24/baseline level (x axis), for 24-week treatment of sarcoidosis patients with placebo, 3 mg/kg infliximab, or 5 mg/kg infliximab are plotted for each patient analyzed.
Stratification based on TNF-α levels.
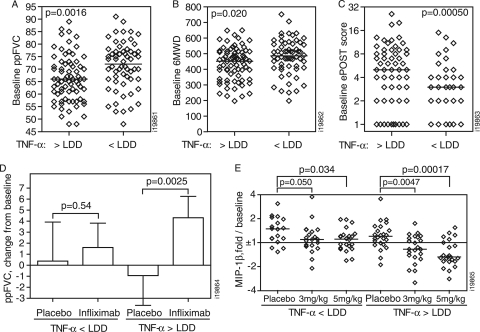
Infliximab targets TNF-α, and therefore, baseline serum concentrations of the molecule may be critical to consider when interpreting the impact of infliximab on clinical and biomarker endpoints. Because of the high proportion of samples with TNF-α levels below the LDD of 4 pg/ml, correlation analyses for TNF-α may be unreliable. As an alternative approach, sarcoidosis patients were classified as having TNF-α levels within (>LDD [TNF-α-high], 53%) or below (<LDD [TNF-α-low], 47%) the limits of quantification. The baseline ppFVC (P = 0.0016) (Fig. 4 A) and 6MWD (P = 0.020) (Fig. 4B) were both significantly lower in the “TNF-α-high” than in the “TNF-α-low” subset. Significance was retained (P = 0.0025 and 0.024, respectively) after multivariate adjustment for age, gender, and baseline therapy (oral corticosteroid [OCS], immunomodulator, or OCS plus immunomodulator). The frequency of extrapulmonary presentations in the sarcoidosis population was significantly higher in the TNF-α-high (78%) than in the TNF-α-low subset (53%) (P = 0.0051; Fisher's exact test). The cumulative severity of extrapulmonary involvement was also higher in the TNF-α-high subset than in the TNF-α-low subset (median [interquartile range] = 4 [1 to 8] and 1 [0 to 3.5], respectively; P = 0.00050) (Fig. 4C).
Fig. 4.
Stratification by TNF-α expression. Sarcoidosis patients were stratified into subsets based on having TNF-α levels below (<) or above (>) the LDD. (A to C) The baseline ppFVC (A) and 6MWD (B) in the total study population and the baseline ePOST score (C) in sarcoidosis patients presenting with extrapulmonary involvement are shown for each patient. Medians (bars) and P values for differences between the TNF-α-defined subsets are reported. (D) Adjusted least squares means (plus 95% confidence intervals) for the change in ppFVC from baseline to week 24 after infliximab (3- and 5-mg/kg) treatment or placebo. The means were adjusted for gender, age, baseline ppFVC, and baseline therapy (OCS versus OCS plus immunomodulator), and P values for infliximab versus placebo treatment were calculated in General Linear Model analyses. (E) Changes in MIP-1β serum levels after 24-week treatment with placebo or infliximab at the indicated dose are expressed as signed fold over baseline for the TNF-α-defined subsets for each patient. Medians (bars) and P values for infliximab versus placebo treatment are shown.
ppFVC significantly improved after infliximab treatment compared with placebo in the TNF-α-high subset (P = 0.022), but not in the TNF-α-low subset (P = 0.34). Importantly, this difference was maintained after adjustment for gender, age, baseline ppFVC, and baseline therapy (OCS versus OCS plus immunomodulator) in the multivariate general linear model analysis (P = 0.0025 versus 0.54 for the TNF-α-high and -low subsets, respectively). The adjusted least-squares means from the multivariate model were increases of 4.3 and 1.6 in ppFVC after infliximab treatment for the TNF-α-high and -low subsets, respectively (compared with a 0.9 decrease and a 0.4 increase, respectively, with placebo) (Fig. 4D). Changes in the SGRQ total score, 6MWD, and ePOST score were not significantly different for infliximab treatment compared with placebo in either subset.
Expression levels of 11 of the 34 proteins significantly over- or underexpressed in sarcoidosis patients (excluding TNF-α) were significantly higher in the TNF-α-high than in the TNF-α-low subset (Table 3). This includes increased expression of MIP-1α, which was decreased in the overall sarcoidosis study population versus healthy controls. In addition, 4 analytes not significantly elevated in the sarcoidosis population overall (ICAM-1, IL-8, monocyte-derived chemokine, and thyroid-stimulating hormone) were increased in the TNF-α-high subset.
Table 3.
Baseline analyte levels in TNF-α-defined subsets
| Analytea | Foldb | FDRc |
|---|---|---|
| MIP-1α | 4.62 | 2.6 × 10−7 |
| IL-1RA | 2.91 | 5.1 × 10−6 |
| EGF | 2.90 | 0.00039 |
| TNF-α | 2.82 | 3.2 × 10−8 |
| CRP | 2.52 | 0.031 |
| Myeloperoxidase | 2.43 | 5.4 × 10−7 |
| ENRAGE | 2.11 | 0.0016 |
| Growth hormone | 2.00 | 0.044 |
| CD40 Ligand | 1.84 | 0.00035 |
| IL-8 | 1.70 | 5.0 × 10−5 |
| ICAM-1 | 1.62 | 3.9 × 10−6 |
| CD40 | 1.61 | 3.2 × 10−8 |
| MDC | 1.60 | 3.4 × 10−5 |
| Thyroid-stimulating hormone | 1.53 | 0.0012 |
| TNF-RII | 1.51 | 1.8 × 10−6 |
| IL-18 | 1.51 | 6.2 × 10−5 |
Listed are analytes that are significantly different between the TNF-α > LDD subset and the TNF-α < LDD subset (FDR < 0.05 and fold > 1.5).
Fold expression of the analyte in the TNF-α > LDD subset over that in the TNF-α < LDD subset.
FDR for the difference in expression of the analyte in the TNF-α > LDD subset and in the TNF-α < LDD subset.
When the sarcoidosis population was stratified into subsets based on TNF-α expression, the effect of infliximab on MIP-1β was more pronounced than in the total population. The decrease in MIP-1β serum levels was greater in the TNF-α-high subset than the TNF-α-low subset after 5-mg/kg infliximab treatment (1.82- versus 1.35-fold relative to placebo, respectively; P = 0.0015) (Fig. 4E). Levels of TNF-RII also decreased in the TNF-α-high subset (21% and 29%, respectively, for the 3-mg/kg and 5-mg/kg infliximab groups) compared with placebo.
Overall, the results demonstrate that patients expressing higher levels of TNF-α presented with more severe pulmonary and extrapulmonary disease and demonstrated greater improvement in ppFVC and a greater decrease in MIP-1β after treatment with infliximab.
DISCUSSION
Using a 92-analyte panel of inflammation-associated markers, we have demonstrated a strong systemic inflammatory profile associated with chronic sarcoidosis. Among the associated inflammatory mediators, serum levels of MIP-1β and TNF-RII were significantly reduced after treatment with infliximab and were associated with greater improvements in FVC and 6MWD. Sarcoidosis patients expressing the highest levels of TNF-α, who had more severe pulmonary and extrapulmonary disease, had the greatest improvement in percent predicted FVC and reduction in MIP-1β and TNF-RII levels after infliximab treatment.
Twenty-nine of 64 analytes quantifiable in the sera of sarcoidosis patients and/or healthy controls were significantly elevated in sarcoidosis patients by at least 1.5-fold over controls. Importantly, these associations were independently observed in both the male and female populations in the gender-stratified analyses. Associated markers include chemokines, neutrophil-associated proteins, acute-phase proteins, coagulation factors, and regulators of metabolism. Many of these associations have not been previously reported for sarcoidosis or have been reported only for associations with expression in airways, but not systemic expression. This strong systemic inflammatory profile prior to infliximab therapy was evident despite the use of corticosteroids and immunosuppressive therapy, and many of these proteins remained elevated after anti-TNF therapy. This suggests that current therapies are still unable to completely suppress the inflammatory response in these patients with sarcoidosis. Why inflammation was substantially reduced in only a small fraction of the infliximab-treated population remains unclear. Potential explanations include the fact that longer active treatment periods are required; there is a limited subset of patients in which TNF-α is a central driver of systemic inflammation, for example, patients with severe extrapulmonary pathology as discussed above; and TNF-α contributes to only a limited component of the overall ongoing inflammation associated with sarcoidosis.
The serum disease profile illustrates an important role for chemokines in the ongoing inflammation associated with sarcoidosis. All chemokines in the panel were significantly associated with sarcoidosis (MCP-1, RANTES, ENA-78, IL-16, etc.). Our findings of these elevated chemokines in sarcoidosis support the following published findings. MCP-1 has been the chemokine most commonly reported to be overexpressed in sarcoidosis, with reports of increased expression in the airways (6, 10, 11, 16, 21) and systemically in serum (9, 11, 21). RANTES (10, 14, 17) and ENA-78 (22) were reported to have increased expression in the airways in sarcoidosis; we report here an elevation in serum. IL-8, a neutrophil chemoattractant, was reported to be elevated in the airways (6) and in plasma (4) and was found to be inversely correlated with FVC (23) in sarcoidosis patients. We report here that serum levels of IL-8 were modestly inversely correlated with FVC. Our finding of elevated eotaxin in the sera of patients with sarcoidosis is novel.
MIP-1β was reported to be elevated in the bronchoalveolar lavage (BAL) fluid of sarcoidosis patients at all stages of the disease, whereas MIP-1α was elevated only in chronic or progressive disease (5). Increased serum concentrations of MIP-1α have also been reported by Hashimoto et al. to be associated with sarcoidosis (9). Although serum levels of MIP-1α were decreased in the sarcoidosis population in the current study, MIP-1α was overexpressed in the TNF-α-high subset of sarcoidosis patients. This subset also was associated with lower percent predicted FVC, consistent with the previous observations of MIP-1α being upregulated only in progressive disease. Treatment with infliximab resulted in a significant decrease in MIP-1β, as well as a trend toward decreases in IL-8 and eotaxin, thus supporting a role for TNF-α in regulating chemokine levels in sarcoidosis.
Interestingly, the elevation of myeloperoxidase and ENRAGE and the inverse correlation of IL-8 with FVC suggests a role for neutrophil activation and phagocytic function in sarcoidosis. It has been previously demonstrated that increased neutrophils in the BAL fluid are associated with a worse prognosis (8) and that increased neutrophils in BAL fluid correlate with the BAL fluid levels of IL-8 (8). Based on these results, the utility of monitoring neutrophil-associated proteins in the serum during the course of disease and treatment warrants further investigation.
Sarcoidosis is a disease in which a Th1 phenotype is known to predominate (24), and IL-12, IL-18, and IFN-γ have been reported to be elevated in sarcoidosis (19). While IL-18 was elevated in the sarcoidosis patients in our study, IL-12 and IFN-γ were not, as these analytes were below the limit of detection of the assay platform. This platform is designed for large multianalyte profiling, and thus, the detection of the individual analytes is typically not as sensitive as a standard single-analyte assay would be. In addition, these proteins are labile and may be affected by long-term storage and freeze-thaw cycles.
The importance of TNF-α in the development of granulomatous inflammation has been demonstrated in various model systems (24). However, it is unclear how this translates to sarcoidosis pathology. TNF-α was found at low levels in sarcoidosis patients, but below the quantification limit in almost half of that population. When the sarcoidosis population was stratified into subsets based on relative levels of TNF-α (above versus below the LDD), the subset with higher TNF-α expression presented with more severe pulmonary and extrapulmonary disease and demonstrated a greater increase in percent predicted FVC than the subset with low TNF-α. Patients in the higher TNF-α-expressing group also had a more pronounced inflammatory profile and larger decreases in MIP-1β and TNF-RII serum levels following infliximab treatment. MIP-1β gene expression has previously been demonstrated to decrease in peripheral blood mononuclear cells after treatment with a TNF inhibitor, etanercept, in patients with rheumatoid arthritis (13). Decreases in TNF-RII following anti-TNF treatment have not previously been reported. These markers may represent a positive pharmacodynamic and response signature to anti-TNF treatment. Overall the results for the TNF-α-stratified subsets support the hypothesis that elevated systemic levels of TNF-α are associated with more severe disease and therefore represent a population potentially more responsive to anti-TNF-α therapy. Therefore, stratification of disease populations based on TNF-α levels may be warranted in future studies of anti-TNF-α therapeutics to assess whether greater clinical efficacy can indeed be attained.
Increased TNF-α levels were observed following infliximab treatment, an apparently paradoxical observation that was nevertheless anticipated. Infliximab stably binds circulating TNF-α, thus stabilizing generally short-lived circulating TNF. It is hypothesized that as new TNF-α is released into circulation, it is likewise bound by infliximab. This would result in an increased pool of nonactive, antibody-bound TNF-α. From previously reported pharmacokinetic analyses (2), there were substantial levels of free infliximab in circulation at the week 24 endpoint, suggesting that most TNF-α detected was drug bound. Although free versus bound TNF-α has not been formally tested, this is a common observation for anti-TNF therapies in diseases for which they are potently efficacious. Indeed, this was expected and served as a positive control.
In summary, we have demonstrated a 35-analyte disease profile of sarcoidosis that is reflective of a significant degree of systemic inflammation, with many of the inflammation-associated analytes being novel findings. The results reinforce a major role for chemokines in sarcoidosis and provide evidence for granulocyte-mediated inflammation. Overall, infliximab treatment did not result in a substantial reduction of the proteins in the 35-analyte panel; however, MIP-1β and TNF-RII did show decreases following treatment with infliximab.
The current study supports the need for further exploration of anti-TNF therapy for sarcoidosis patients who remain symptomatic despite the use of glucocorticosteroids and/or cytotoxic agents, particularly those expressing the highest serum levels of TNF-α. The impacts of novel therapies with different mechanisms of action on the disease profile will be important in understanding the heterogeneity of the disease. Identification of biomarkers of treatment response may lead to improved management of the unpredictable clinical course of sarcoidosis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jennifer Han and Robert Achenbach of Centocor Ortho Biotech Services, LLC, for editorial support in preparing the manuscript.
This study was funded by Centocor Ortho Biotech, Inc.
Footnotes
Supplemental material for this article may be found at http://cvi.asm.org/.
Published ahead of print on 20 April 2011.
REFERENCES
- 1. Anonymous. 1999. Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am. J. Respir. Crit. Care Med. 160:736–755 [DOI] [PubMed] [Google Scholar]
- 2. Baughman R. P., et al. 2006. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am. J. Respir. Crit. Care Med. 174:795–802 [DOI] [PubMed] [Google Scholar]
- 3. Baughman R. P., Lower E. E., Drent M. 2008. Inhibitors of tumor necrosis factor (TNF) in sarcoidosis: who, what, and how to use them. Sarcoidosis Vasc. Diffuse Lung Dis. 25:76–89 [PubMed] [Google Scholar]
- 4. Boots A. W., et al. 2009. Antioxidant status associated with inflammation in sarcoidosis: a potential role for antioxidants. Respir. Med. 103:364–372 [DOI] [PubMed] [Google Scholar]
- 5. Capelli A., Di Stefano A., Lusuardi M., Gnemmi I., Donner C. F. 2002. Increased macrophage inflammatory protein-1alpha and macrophage inflammatory protein-1beta levels in bronchoalveolar lavage fluid of patients affected by different stages of pulmonary sarcoidosis. Am. J. Respir. Crit. Care Med. 165:236–241 [DOI] [PubMed] [Google Scholar]
- 6. Car B. D., et al. 1994. Elevated IL-8 and MCP-1 in the bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am. J. Respir. Crit. Care Med. 149:655–659 [DOI] [PubMed] [Google Scholar]
- 7.Dabney A., Storey J.Q-value. 2008. http://genomics.princeton.edu/storeylab/qvalue/
- 8. Drent M., et al. 1999. Does the cellular bronchoalveolar lavage fluid profile reflect the severity of sarcoidosis? Eur. Respir. J. 13:1338–1344 [DOI] [PubMed] [Google Scholar]
- 9. Hashimoto S., et al. 1998. Correlation of plasma monocyte chemoattractant protein-1 (MCP-1) and monocyte inflammatory protein-1alpha (MIP- 1alpha) levels with disease activity and clinical course of sarcoidosis. Clin. Exp. Immunol. 111:604–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iida K., et al. 1997. Analysis of T cell subsets and beta chemokines in patients with pulmonary sarcoidosis. Thorax 52:431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iyonaga K., et al. 1998. Measurement of serum monocyte chemoattractant protein-1 and its clinical application for estimating the activity of granuloma formation in sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 15:165–172 [PubMed] [Google Scholar]
- 12. Judson M. A., et al. 2008. Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomised trial. Eur. Respir. J. 31:1189–1196 [DOI] [PubMed] [Google Scholar]
- 13. Koczan D., et al. 2008. Molecular discrimination of responders and nonresponders to anti-TNF alpha therapy in rheumatoid arthritis by etanercept. Arthritis Res. Ther. 10:R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kodama N., et al. 1998. Expression of RANTES by bronchoalveolar lavage cells in nonsmoking patients with interstitial lung diseases. Am. J. Respir. Cell Mol. Biol. 18:526–531 [DOI] [PubMed] [Google Scholar]
- 15. Newman L. S., et al. 2004. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am. J. Respir. Crit. Care Med. 170:1324–1330 [DOI] [PubMed] [Google Scholar]
- 16. Petrek M., Kolek V., Szotkowska J., du Bois R. M. 2002. CC and C chemokine expression in pulmonary sarcoidosis. Eur. Respir. J. 20:1206–1212 [DOI] [PubMed] [Google Scholar]
- 17. Petrek M., et al. 1997. The source and role of RANTES in interstitial lung disease. Eur. Respir. J. 10:1207–1216 [DOI] [PubMed] [Google Scholar]
- 18.Rules Based Medicine. Human MAP version 1.6. 2008. http://www.rulesbasedmedicine.com/products-services/humanmap-services/humanmap/
- 19. Shigehara K., et al. 2001. IL-12 and IL-18 are increased and stimulate IFN-gamma production in sarcoid lungs. J. Immunol. 166:642–649 [DOI] [PubMed] [Google Scholar]
- 20. Storey J. 2002. A direct approach to false discovery rates. J. R. Stat. Soc. B 64:479–498 [Google Scholar]
- 21. Suga M., et al. 1999. Clinical significance of MCP-1 levels in BALF and serum in patients with interstitial lung diseases. Eur. Respir. J. 14:376–382 [DOI] [PubMed] [Google Scholar]
- 22. Sugiyama K., et al. 2006. Elevated levels of interferon gamma-inducible protein-10 and epithelial neutrophil-activating peptide-78 in patients with pulmonary sarcoidosis. Respirology 11:708–714 [DOI] [PubMed] [Google Scholar]
- 23. Vasakova M., et al. 2009. Bronchoalveolar lavage fluid cellular characteristics, functional parameters and cytokine and chemokine levels in interstitial lung diseases. Scand. J. Immunol. 69:268–274 [DOI] [PubMed] [Google Scholar]
- 24. Zissel G., Prasse A., Muller-Quernheim J. 2007. Sarcoidosis—immunopathogenetic concepts. Semin. Respir. Crit. Care Med. 28:3–14 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.