Abstract
Western blot analysis of Orientia tsutsugamushi whole-cell lysates with scrub typhus patient sera has identified at least five protein antigens of O. tsutsugamushi with molecular sizes of 22 kDa, 47 kDa, 56 kDa, 58 kDa, and 110 kDa. In this study, sera from serial bleedings of 108 patients were used to study the kinetics and the magnitude of specific antibody responses against the 47-kDa and 56-kDa antigens. Recombinant protein of the conserved 47-kDa antigen (r47b) or a mixture of truncated 56-kDa antigen (r56s) from three prototype strains was used as the antigen in an enzyme-linked immunosorbent assay (ELISA). Our results showed that 76% and 93% of these patients had elevated IgM and IgG against r47b, respectively, and 98% and 100% had elevated IgM and IgG against r56s, respectively. The kinetics of antibody responses against r47b and r56s can be grouped into three patterns. In the first type of response, IgM and IgG against r47b and r56s appeared about the same time. The IgM and IgG titers against r56s were much higher than those against r47b. In the second type of response, induction of IgM appeared to be similar to that in the first type. The major difference to the first type is that the IgG titers against r47b were induced at least 1 week later than those against the r56s. The third type showed strong IgG responses against both r47b and r56s, and low or no IgM responses indicated a secondary infection. This is the first systematic investigation of antibody response kinetics against the conserved 47-kDa antigen versus the variable 56-kDa antigen in scrub typhus patients.
INTRODUCTION
Scrub typhus is an acute, febrile, and potentially fatal disease, caused by the infection of Orientia tsutsugamushi, an obligate, intracellular, Gram-negative bacterium. It accounts for up to 23% of all febrile episodes in areas of the Asia-Pacific region where scrub typhus is endemic (2, 3). Mortality rates for scrub typhus range from less than 1% to 50% depending upon proper antibiotic treatment, physical status of the individual patient, and the virulence of the particular strain of O. tsutsugamushi encountered (11, 12, 26). Symptoms may include fever, headache, rash, and other complications, including pneumonitis and meningitis. Differentiating scrub typhus from other acute tropical febrile illness, such as leptospirosis, murine typhus, malaria, and dengue fever, can be difficult because of similarities in signs and symptoms. At this time there is no vaccine for scrub typhus. The investigation of the immune response to a particular antigen should be beneficial for providing guidance to develop a potential vaccine candidate.
Western blot analysis of whole-cell antigen with naturally infected patient sera revealed several potential antigens, including 22-kDa, 47-kDa, 56-kDa, and 110-kDa proteins (9, 18, 19, 28). The recognition of these proteins by patient sera indicates the immunogenicity of these proteins and their potential for being used in diagnostic assays or vaccine candidates. Among these antigens, the 56-kDa protein which accounted for 10 to 15% of the total amount of cellular proteins appeared to be the most immunodominant protein (9). This protein, present in a large amount on the outer membrane, is strain specific and has been shown to induce neutralizing antibodies in an animal model (19, 24). Sequence analysis of the 56-kDa protein from more than 135 isolates confirmed that the gene has four regions of hypervariability (13, 20). The four regions roughly correspond to regions of hydrophilic residues in the protein (20). Many serotype-specific monoclonal antibodies to Orientia have been shown to bind to the 56-kDa protein (16, 21, 30). This 56-kDa protein is reactive with group-specific and strain-specific monoclonal antibodies, implying the existence of those specific epitopes in this protein (28). It is recognized by sera from almost all scrub typhus patients (5, 20), suggesting that it is a good candidate for use as a diagnostic antigen. The 47-kDa protein is another antigen recognized by patient sera (4, 9, 17). This protein belongs to the high-temperature requirement A (HtrA) family of serine proteases. Bacterial HtrAs are located in the periplasm and outer membrane of Gram-negative bacteria (7). They are widely conserved in single and multicellular organisms (22). The 47-kDa protein is also highly conserved (>97% identity) in 25 highly disparate strains of O. tsutsugamushi (4). The 47-kDa protein contains both scrub typhus group-reactive and strain-specific B-cell epitopes (10).
Bourgeois et al. (1) described two types of antibody responses in scrub typhus patient samples by IFA using whole-cell antigens. Type 1 responders exhibited an earlier and greater increase in IgM compared to IgG. In contrast, type 2 responders had suppressed and delayed IgM responses. Another report by Ching et al. showed that in an enzyme-linked immunosorbent assay (ELISA) using r56Kp as an antigen, both IgM and IgG are detectable as early as day four after onset of fever (5). No systematic investigation of the humoral responses against the 47-kDa antigen has been reported. In this study, we investigated the humoral responses against these two potential vaccine candidates in patients with natural infection. Recombinant 47-kDa antigen (r47b from strain Karp) or a mixture of truncated 56-kDa antigen (r56s) from three prototype strains (Karp, Kato, and Gilliam) was used as the antigen in an ELISA format. The serological reactivity of scrub typhus patient sera against r47b was measured and compared to the reactivity against r56s. From samples from 58 patients who had four or more serial bleedings, three kinetically distinct types of antibody response against the r47b and r56s were observed. In the first type, IgM and IgG against r47b and r56s appear at about the same time, with much higher titers in ELISA against r56s than r47b. In the second type, the IgM response is similar to that in the first type, but the IgG response against r47b occurs at least 1 week after that of the r56s, and in the third type, a burst of IgG response and almost no IgM response indicate a secondary infection. This is the first systematic investigation of antibody responses against the conserved 47-kDa antigen versus the variable 56-kDa antigen in scrub typhus patients.
MATERIALS AND METHODS
Bacterial strains and vectors.
Escherichia coli Top10 (Invitrogen, California) cells were used for cloning the desired gene fragments. The cloned genes were inserted into the pET24a or pET11a vector (Novagen, CA) for the expression of r47b or r56s, respectively, in E. coli BL21(DE3) (Invitrogen) under the control of phage T7 lac promoter (27).
Recombinant 47-kDa (r47b) and 56-kDa (r56) proteins.
The recombinant r47b protein and recombinant 56-kDa protein from Karp (r56Kp) were prepared as previously described (4, 5). The same procedures were used to clone and express the recombinant 56-kDa protein from strain Kato (r56Kt) and strain Gilliam (r56Gm). A novel high-performance liquid chromatography (HPLC) procedure was used to purify the r56Kt and r56Gm, and the procedures were modified by adding dithiothreitol to the buffer used for purification and refolding (31).
IgM and IgG ELISA.
Microtiter plates (96 well) were coated for 40 h at 4°C with antigens (0.3 μg per well) diluted in phosphate-buffered saline (PBS) and subsequently blocked with 10% skim milk in PBS for 1 h. Patient sera were diluted 1:100 in PBS with 5% skim milk for r47b (or 2% skim milk and 1% bovine serum albumin [BSA] for r56s), incubated for 1 h at room temperature, and washed three times with 0.1% Tween 20 in PBS and one time with PBS. Peroxidase-conjugated rabbit anti-human IgM (Dako, Denmark) at a 1,000-fold (for r47b) or 4,000-fold (for r56s) dilution was added. The same dilutions were used for rabbit anti-human IgG (Santa Cruz Biotechnology, CA). After 1 h of incubation at room temperature, the plates were washed as previously described and the 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) (ABTS) substrate (Kirkegaard & Perry, MD) was added. After 30 min of incubation at room temperature, optical densities at 405 nm (OD405) were measured with a plate reader (Molecular Devices, CA). Titers of antibody were expressed as the reciprocal of the highest dilution with a positive reaction.
Human sera.
Patient sera from the Pescadore Islands of Taiwan were obtained from a Chinese military garrison stationed there during 1976 and 1977 and were aliquoted and stored at −80°C (1). The clinical diagnosis of scrub typhus was confirmed by the demonstration of rising anti-Orientia indirect fluorescent antibody assay (IFA) titers and agent isolation in many cases. A negative-control panel consisted of sera from patients without a history of scrub typhus and who were diagnosed with the following diseases or conditions (number of serum samples in parentheses) (29): bartonellosis (5), cholera (1), leptospirosis (2), malaria (2), rheumatoid arthritis (2), tularemia (6), typhoid (6), anti-nuclear antibody (21), and others of unknown etiology from the Pescadore Islands (21).
RESULTS
Seroreactivity with r47b and r56s by ELISA.
The refolded purified r47b (from strain Karp) and r56s (from strains Karp, Kato, and Gilliam) were used as antigens to detect the presence of specific IgG and IgM in an ELISA. A panel of 437 serum samples from serial bleedings of 108 individuals with clinically diagnosed scrub typhus and a panel of 125 negative-control serum samples (59 normal serum samples from local control individuals and 66 samples from patients without a history of scrub typhus and who were diagnosed with other diseases [non-scrub typhus patients]) were used. Of the 108 patients, samples from 100 patients (92.6%) and 82 patients (75.9%) had specific IgG and IgM antibodies against r47b (Table 1), respectively. These numbers were lower than the percentages of patients whose samples had specific IgG (108 patients, 100%) and IgM (106 patients, 98.1%) antibodies against r56s. All r47b-positive patient samples were also r56s positive. The low percentages (≤3%) of local control individuals and non-scrub typhus patients whose sera had either IgG or IgM against r47b and/or r56s antigens suggest that the differences we observed between r47b and r56s were specific.
Table 1.
Comparison of the IgM and IgG responses against r47b and r56s in patient sera by ELISA
| Group | No. of individuals (no. of serum samples) | No. (%) positive againsta: |
|||
|---|---|---|---|---|---|
| IgM |
IgG |
||||
| r47b | r56s | r47b | r56s | ||
| Scrub typhus | 108 (437) | 82 (75.9) | 106 (98.1) | 100 (92.6) | 108 (100.0) |
| Local control | 34 (59) | 1 (2.9) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Non-scrub typhus | 66 (66) | 0 (0.00) | 2 (3.0) | 1 (1.5) | 2 (3.0) |
The same patient may have multiple bleedings. Individuals that have at least one positive sample are considered scrub typhus-positive patients. All r47b-positive patients were also r56s positive.
IgM and IgG ELISA results for all patient sera.
Since the individuals who have at least one positive sample are considered scrub typhus-positive patients, the percentages of patient sera with antibodies against r47b and r56s are lower than the numbers listed in Table 1. The IgM ELISA results for the 437 scrub typhus patient serum samples against r47b and r56s are shown in Fig. 1 A and B. In Fig. 1B, IgM antibody against r56s generally appeared after the first week of the onset of fever and could be detected even after 120 days in most cases. Although the percentage of patients whose sera had specific IgM against r47b is lower than the percentage of those whose sera had specific IgM against r56s, the IgM against r47b also appeared after the first week of fever and in some cases could be detected after 120 days.
Fig. 1.
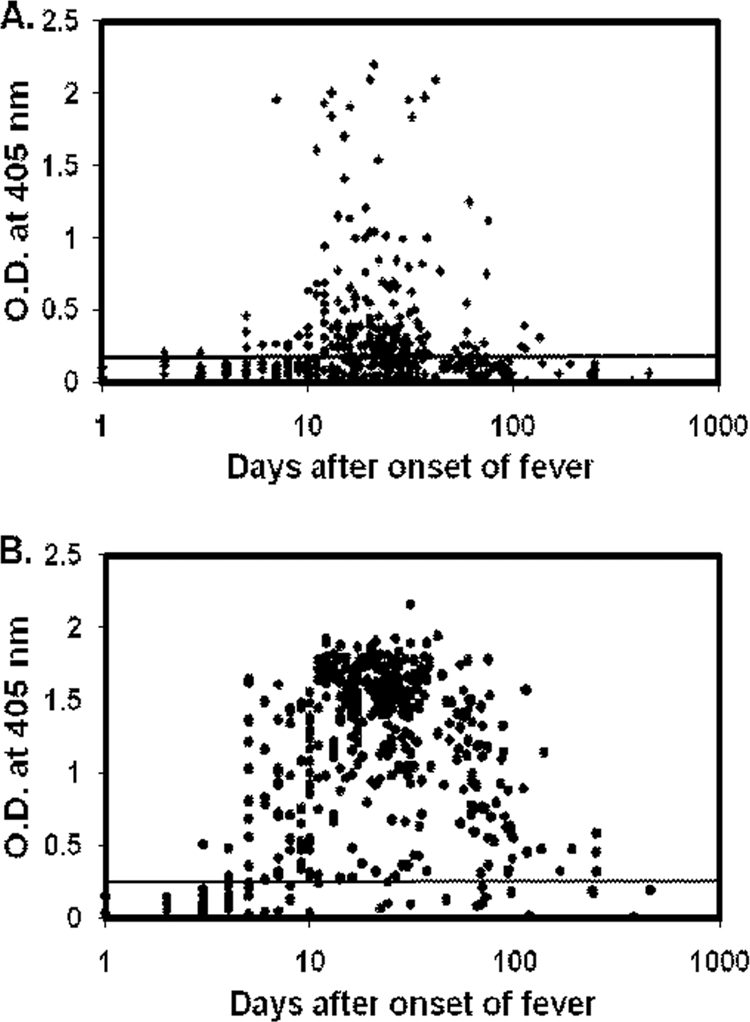
IgM ELISA results for 437 scrub typhus patient serum samples with recombinant antigens. Specific IgM against r47b (A) and r56s (B) were tested with 437 patient serum samples from serial bleeding of 108 scrub typhus patients. The x axis indicates the number of days after the onset of illness, and the y axis indicates the optical density (O.D.) at 405 nm. The cutoff values are 0.182 for r47b and 0.330 for r56s (mean of 125 negative controls plus 2 standard deviations).
The IgG ELISA results for the 437 scrub typhus patient serum samples against r47b and r56s are shown in Fig. 2 A and B. The IgG against both r47b and r56s generally appeared after the first week of the onset of fever and could be detected even after 120 days in most cases. The overall kinetic pattern is very similar to that of IgM.
Fig. 2.
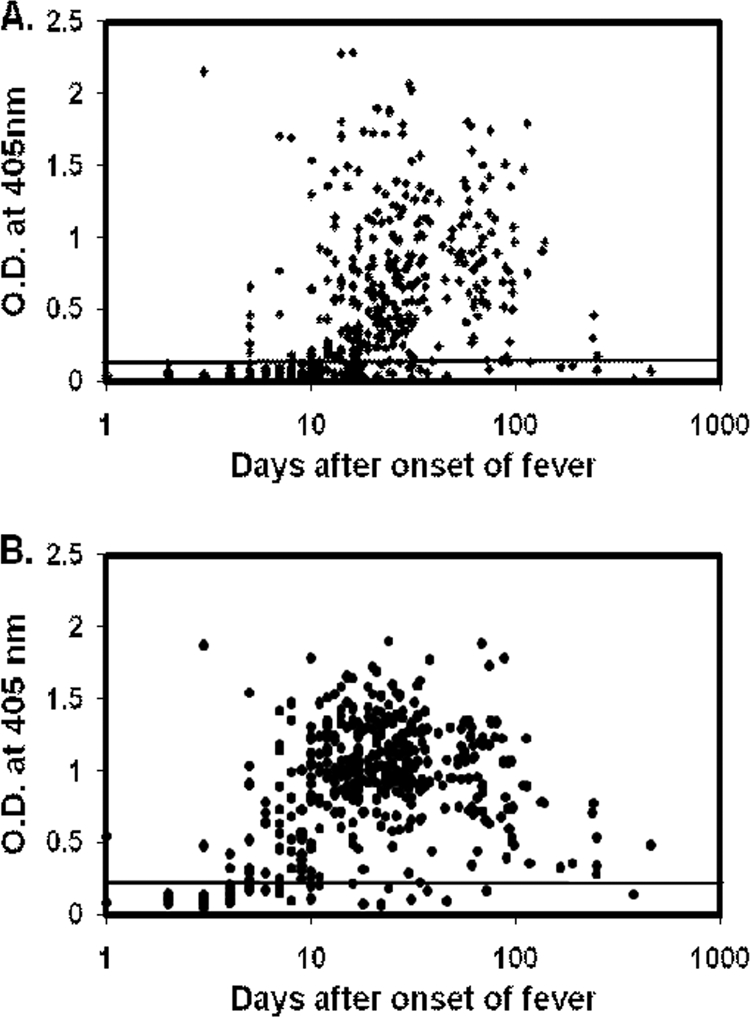
IgG ELISA results for 437 scrub typhus patient serum samples with recombinant antigens. Specific IgG against r47b (A) and r56s (B) were tested with 437 patient serum samples from serial bleeding of 108 scrub typhus patients. The x axis indicates the number of days after the onset of illness, and the y axis indicates the optical density (O.D.) at 405 nm. The cutoff values are 0.158 for r47b and 0.216 for r56s (mean of 125 negative controls plus 2 standard deviations).
IgG and IgM responses for sera from patients with serial bleedings.
The ELISA data for 69 patients who had four or more serial bleedings were further analyzed. Among these patients, only 58 patients had bleedings during the first week of illness. For those 58 patients, antibody responses against r47b and r56s can be grouped into three distinct types. Typical examples of type I are shown in Fig. 3 and Table 2, type II in Fig. 4 and 5 and Tables 3 and 4, and type III in Fig. 6 and Table 5.
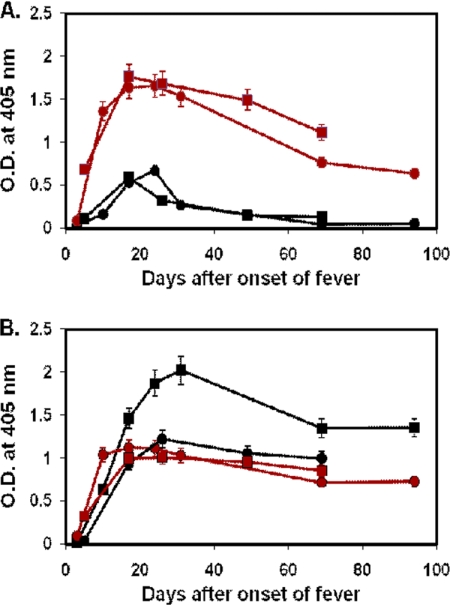
Fig. 3.
Time course of antibody responses of two typical type I patients to r47b and r56s antigens. The serum samples of patient MAK135 (circle) were collected at days 3, 10, 17, 24, 31, 69, and 94, and the serum samples of patient MAK229 (square) were collected at days 5, 17, 26, 49, and 69. IgM (A) and IgG (B) specific for r47b (black) or r56s (red) in patient sera were measured by ELISA. The cutoff values are the same as in Fig. 1 and 2. The symbols represent the averages of two experiments, and the error bars represent ranges. O.D., optical density.
Table 2.
ELISA titers of samples from two typical patients with type I responsea
| Patient | No. of days after onset of fever | Titer |
|||
|---|---|---|---|---|---|
| r47b |
r56s |
||||
| IgM | IgG | IgM | IgG | ||
| MAK 135 | 3 | − | − | 100 | |
| 10 | − | 100 | 1,600 | 3,200 | |
| 17 | 100 | 400 | 3,200 | 6,400 | |
| 24 | 200 | 1,600 | 6,400 | 12,800 | |
| 31 | − | 1,600 | 1,600 | 6,400 | |
| 69 | − | 800 | 400 | 3,200 | |
| 94 | − | 800 | 100 | 3,200 | |
| MAK 229 | 5 | 100 | − | 400 | 100 |
| 17 | 200 | 400 | 6,400 | 3,200 | |
| 26 | 100 | 800 | 3,200 | 1,600 | |
| 49 | 100 | 400 | 1,600 | 1,600 | |
| 69 | 100 | 400 | 800 | 1,600 | |
−, ELISA negative (titer less than 100).
Fig. 4.
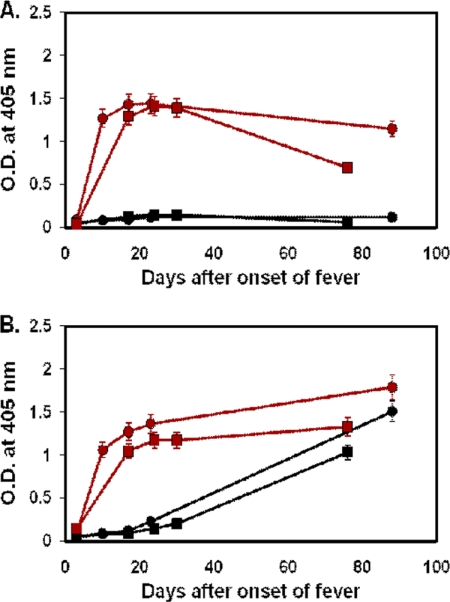
Time course of antibody responses of two typical type IIa patients to r47b and r56s antigens. The serum samples of patient MAK217 (circle) were collected at days 3, 12, 17, 26, and 52, and the serum samples of patient MAK240 (square) were collected at days 3, 10, 16, 25, and 63. IgM (A) and IgG (B) specific for r47b (black) or r56s (red) in patient sera were measured by ELISA. The cutoff values are the same as in Fig. 1 and 2. The symbols represent the averages of two experiments, and the error bars represent ranges. O.D., optical density.
Fig. 5.
Time course of antibody responses of two typical type IIb patients to r47b and r56s antigens. The serum samples of patient MAK142 (circle) were collected at days 3, 10, 17, 23, and 88, and the serum samples of patient MAK153 (square) were collected at days 3, 17, 24, 30, and 76. IgM (A) and IgG (B) specific for r47b (black) or r56s (red) in patient sera were measured by ELISA. The cutoff values are the same as in Fig. 1 and 2. The symbols represent the averages of two experiments, and the error bars represent ranges. O.D., optical density.
Table 3.
ELISA titers of samples from two typical patients with type IIa responsea
| Patient | No. of days after onset of fever | Titer |
|||
|---|---|---|---|---|---|
| r47b |
r56s |
||||
| IgM | IgG | IgM | IgG | ||
| MAK 217 | 3 | − | − | − | − |
| 12 | − | 100 | 800 | 800 | |
| 17 | − | 100 | 800 | 800 | |
| 26 | 100 | 200 | 3,200 | 6,400 | |
| 52 | − | 400 | 800 | 1,600 | |
| MAK 240 | 3 | − | − | − | − |
| 10 | − | − | 800 | 3,200 | |
| 16 | − | 100 | 1,600 | 3,200 | |
| 25 | − | 400 | 800 | 3,200 | |
| 63 | − | 400 | 100 | 1,600 | |
−, ELISA negative (titer less than 100).
Table 4.
ELISA titers of samples from two typical patients with type IIb responsea
| Patient | No. of days after onset of fever | Titer |
|||
|---|---|---|---|---|---|
| r47b |
r56s |
||||
| IgM | IgG | IgM | IgG | ||
| MAK 142 | 3 | − | − | − | − |
| 10 | − | − | 1,600 | 800 | |
| 17 | − | 100 | 1,600 | 800 | |
| 23 | − | 100 | 800 | 1,600 | |
| 88 | 100 | 800 | 800 | 1,600 | |
| MAK 153 | 3 | − | − | − | − |
| 17 | − | 100 | 400 | 200 | |
| 24 | 100 | 100 | 800 | 800 | |
| 30 | − | 100 | 800 | 800 | |
| 76 | − | 800 | 400 | 1,600 | |
−, ELISA negative (titer less than 100).
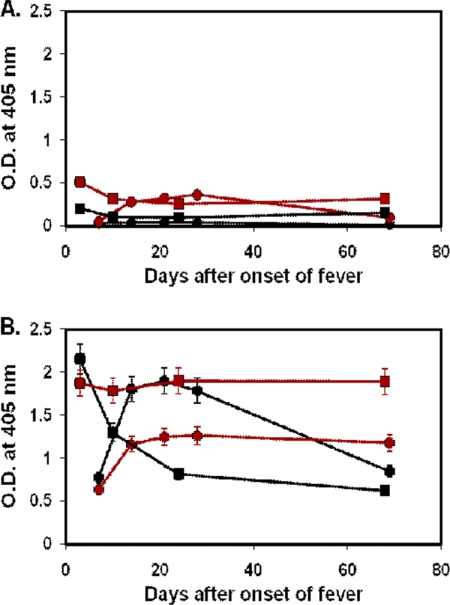
Fig. 6.
Time course of antibody responses of two typical type III (the secondary infection) patients to r47b and r56s antigens. The serum samples of patient MAK132 (circle) were collected at days 7, 14, 21, 28, and 69, and the serum samples of MAK168 (square) were collected at days 3, 10, 24, and 68. IgM (A) and IgG (B) specific for r47b (black) or r56s (red) in patient sera were measured by ELISA. The cutoff values are the same as in Fig. 1 and 2. The symbols represent the averages of two experiments, and the error bars represent ranges. O.D., optical density.
Table 5.
ELISA titers of samples from two typical patients with type III responsea
| Patient | No. of days after onset of fever | Titer |
|||
|---|---|---|---|---|---|
| r47b |
r56s |
||||
| IgM | IgG | IgM | IgG | ||
| MAK 132 | 7 | − | 400 | − | 800 |
| 14 | − | 6,400 | 100 | 51,200 | |
| 21 | − | 6,400 | 100 | 51,200 | |
| 28 | − | 6,400 | 200 | 12,800 | |
| 69 | − | 400 | 100 | 12,800 | |
| MAK 168 | 3 | 100 | 6,400 | 200 | 12,800 |
| 10 | − | 800 | 100 | 102,400 | |
| 24 | − | 400 | 100 | 25,600 | |
| 68 | − | 400 | 100 | 25,600 | |
−, ELISA negative (titer less than 100).
There were 20 patients whose samples exhibited type I responses (Fig. 3A and B; Table 2). Characteristic of the type I response is that both IgM and IgG appeared after the first week of the onset of fever as the signal rose above the cutoff. The antibodies against r47b appeared at the same time as antibodies against r56s. The IgM against r47b and r56s reached a peak level by 15 to 25 days after the onset of fever (Fig. 3A). For IgM against r47b, the signal declined to below the cutoff by 60 days. For IgM against r56s, the signal then gradually declined but remained above the cutoff even at 90 days. The IgG against r47b and r56s reached a peak by 20 to 35 days after the onset of fever and stayed above the cutoff at 90 days (Fig. 3B). At the peak of the antibody response, the titers of IgG against r47b (1:1,600 or 1:800) were much higher than the titers of IgM against r47b (1:200) (Table 2). The titers of IgG and IgM against r56s at the peak of the antibody response were very similar (Table 2). Although there was detectable IgM against r47b, most of the IgM was against r56s, as the ELISA titers of IgM against r56s (1:6,400) were much higher than those against r47b (1:200) at the peak of the response.
There were 34 type II patients. The characteristics of type II response are described as follows: the IgG and IgM responses against r56s were the same as type I; they appeared at about 1 week after the onset of fever, reached peak levels by about 20 days, and remained above the cutoff at 90 days (Fig. 4 and 5). There are two major differences between the type I and type II responses. The first one is the levels of IgM antibodies against r47b: unlike the findings for type I response, there is almost no IgM against r47b in type II response (Fig. 4A and 5A). The second difference is the response time of IgG against r47b: IgG against r47b appeared later than the IgG against r56s. Based on the time of delayed response, type II can be further subgrouped into type IIa and type IIb. Among the 34 type II patients, 23 of them belong to type IIa. The rise of IgG against r47b in samples from type IIa patients was delayed about 7 to 10 days (Fig. 4B). The IgG against r47b appeared at 15 to 20 days, reached a peak by 25 to 30 days, and remained detectable at 60 days. Samples from 11 patients belong to type IIb. The rise of IgG against r47b in samples from type IIb patients was more than 20 days delayed compared to that of IgG against r56s (Fig. 5B). The IgG level against r47b rose more slowly than the type IIa level and was still rising at 90 days. Two examples of each subgroup were illustrated in Tables 3 and 4 by the titers. The titers of the IgM against r47b were lower than 1:100 for most of the time during the time course for both type IIa and IIb patients (Tables 3 and 4).
Four patients had sera that demonstrated the type III response, showing strong IgG responses against both r47b and r56s and low or no IgM responses against both r47b and r56s, indicating a secondary infection (Fig. 6A and B; Table 5).
DISCUSSION
We investigated the kinetics of IgM and IgG responses induced by 56-kDa and 47-kDa antigens in O. tsutsugamushi-infected patients. Almost all patient sera (>98%) showed reactivity against the mixture of three r56 antigens, consistent with data previously reported showing that samples from more than 95% of patients with scrub typhus recognized the 56-kDa protein of O. tsutsugamushi (6, 8, 14, 15, 19). Seong et al. (25) also observed that fewer than 70% of patients had samples with antibody responses to the recombinant 47-kDa protein. In Table 1, our data showed that 93% of patients who had at least one sample among the serial bleedings had samples with detectable IgG against r47b. As many of our samples that had no detectable IgG against r47b were obtained during the first 3 weeks after the onset of fever (especially for type II patients), the percentage of samples with detectable IgG against r47b dropped. Overall, 65% of our serum samples had detectable IgG against r47, which is very similar to the observation of Seong et al.
The protein sequences derived from the 56-kDa antigen genes of multiple Orientia strains have showed great diversity. Although the strains that exist in Taiwan are not identical to the three prototypes (23), most of them are similar to the Karp or the Karp-like strain and many are similar to strain Gilliam (13, 23). However, all patients had samples with antibodies that recognized these r56s antigens, suggesting cross-reactivity due to the conserved regions in 56-kDa antigen. The 47-kDa is a relatively conserved protein which belongs to the HtrA family. The protein sequence alignment between O. tsutsugamushi strains Karp, Kato, and Gilliam showed that the amino acid sequences of the 47-kDa protein are different at only 7 positions (amino acids 24, 71, 101, 106, 140, 275, and 309) out of 466 amino acids, which is less than a 2% difference. Samples from about 93% patients were IgG positive against Karp r47b compared to 100% against r56s. The 7% difference in antibody responses against r47b and r56s was most likely due to the collection date of the sera and not to the differences in the sequences. In fact, all of the eight r47b IgG-negative patient serum samples were collected in the early phases of the illness (fewer than 25 days after the onset of fever), suggesting the slow antibody response to 47-kDa protein. The signal of IgG against r47b in those patients might increase above the negative cutoff during the later days.
Previously, Bourgeois et al. (1) found that two types of IgM responses against whole-cell antigens occurred in scrub typhus patient samples by IFA. Type 1 responses exhibited an early and greater increase in IgM compared to responses in IgG. In contrast, type 2 responders had suppressed and delayed IgM responses. Their results suggested that the type I response reflects primary infection, while type 2 indicates secondary infection. Our current study dissected the total antibody responses against the whole cell into responses against individual antigens. The r56s and r47b ELISAs may be more sensitive to differentiate antigen-specific immune responses because, unlike whole-cell antigens, no other cross-reactive antigens in the whole-cell preparation are present. We found very few cases in which IgM antibodies were detected earlier than IgG antibodies. For all 58 patients with multiple serum samples, the kinetics of their IgG and IgM responses against r56s were similar. However, two distinct types of antibody responses against r47b were observed. In the type I response, 20 patients showed similar IgG responses against r47b and r56s. In type II responses, 34 patients showed delayed IgG and weak IgM responses against r47b compared to those against r56s. The titers of IgG against 47-kDa and 56-kDa proteins in scrub typhus patients were very close, especially in type IIb response.
O. tsutsugamushi exhibits great antigenic diversity, and the variation of human responses to different serotypes is not well understood. The variable 56-kDa protein is the major surface protein of O. tsutsugamushi. Sera from most patients and animals with a history of Orientia infection react strongly with this protein. This reflects both the abundance of the 56-kDa antigen on the cell surface and its high immunogenicity. The 47-kDa protein is another antigen recognized by antibodies against O. tsutsugamushi (4, 9, 17). As Ohashi et al. (19) reported, most scrub typhus sera reacted with the 56-kDa protein but only some sera reacted with the 47-kDa protein. We did observe delayed immune response against the 47-kDa protein as described for the type II response. The apparent weak IgM response to r47b in the patient probably reflects its lower quantity on the cell surface and its low immunogenicity.
In general, Orientia infection led to early, pronounced, and long-lasting antibody responses against the 56-kDa protein, while the responses against the 47-kDa protein were delayed and less pronounced.
ACKNOWLEDGMENTS
We thank Daryl Kelly for his critical reading of the manuscript and helpful comments.
This research was supported by the Naval Medical Research Center, research work unit 6000.RAD1.J.A0310. The study protocol was approved by the Naval Medical Research Center Institutional Review Board (case number PJT44) in compliance with all applicable Federal regulations governing the protection of human subjects.
The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government. Chien-Chung Chao and Wei-Mei Ching are employees of the U.S. Government. This work was prepared as part of their official duties.
Footnotes
Published ahead of print on 20 April 2011.
REFERENCES
- 1. Bourgeois A. L., et al. 1982. Humoral and cellular responses in scrub typhus patients reflecting primary infection and reinfection with Rickettsia tsutsugamushi. Am. J. Trop. Med. Hyg. 31:532–540 [DOI] [PubMed] [Google Scholar]
- 2. Brown G. W., Robinson D. M., Huxsoll D. L., Ng T. S., Lim K. J. 1976. Scrub typhus: a common cause of illness in indigenous populations. Trans. R. Soc. Trop. Med. Hyg. 70:444–448 [DOI] [PubMed] [Google Scholar]
- 3. Brown G. W., Robinson D. M., Huxsoll D. L. 1978. Serological evidence for a high incidence of transmission of Rickettsia tsutsugamushi in two Orang Asli settlements in Peninsular Malaysia. Am. J. Trop. Med. Hyg. 27:121–123 [DOI] [PubMed] [Google Scholar]
- 4. Chen H. W., et al. 2009. Identification of cross-reactive epitopes on the conserved 47-kilodalton antigen of Orientia tsutsugamushi and human serine protease. Infect. Immun. 77:2311–2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ching W. M., Wang H., Eamsila C., Kelly D. J., Dasch G. A. 1998. Expression and refolding of truncated recombinant major outer membrane protein antigen (r56) of Orientia tsutsugamushi and its use in enzyme-linked immunosorbent assays. Clin. Diagn. Lab Immunol. 5:519–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ching W. M., et al. 2001. Early diagnosis of scrub typhus with a rapid flow assay using recombinant major outer membrane protein antigen (r56) of Orientia tsutsugamushi. Clin. Diagn. Lab Immunol. 8:409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clausen T., Kaiser M., Huber R., Ehrmann M. 2011. HTRA proteases: regulated proteolysis in protein quality control. Nat. Rev. Mol. Cell Biol. 12:152–156 [DOI] [PubMed] [Google Scholar]
- 8. Eisemann C. S., Osterman J. V. 1981. Antigens of scrub typhus rickettsiae: separation by polyacrylamide gel electrophoresis and identification by enzyme-linked immunosorbent assay. Infect. Immun. 32:525–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanson B. 1985. Identification and partial characterization of Rickettsia tsutsugamushi major protein immunogens. Infect. Immun. 50:603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hickman C. J., Stover C. K., Joseph S. W., Oaks E. V. 1993. Murine T-cell response to native and recombinant protein antigens of Rickettsia tsutsugamushi. Infect. Immun. 61:1674–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawamura A., Tanaka H. 1988. Rickettsiosis in Japan. Jpn. J. Exp. Med. 58:169–184 [PubMed] [Google Scholar]
- 12. Kelly D. J., Richards A. L., Temenak J., Strickman D., Dasch G. A. 2002. The past and present threat of rickettsial diseases to military medicine and international public health. Clin. Infect. Dis. 34:S145–S169 [DOI] [PubMed] [Google Scholar]
- 13. Kelly D. J., Fuerst P. A., Ching W. M., Richards A. L. 2009. Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin. Infect. Dis. 48(Suppl. 3):S203–S230 [DOI] [PubMed] [Google Scholar]
- 14. Kim I. S., Seong S. Y., Woo S. G., Choi M. S., Chang W. H. 1993. High-level expression of a 56-kilodalton protein gene (bor56) of Rickettsia tsutsugamushi Boryong and its application to enzyme-linked immunosorbent assays. J. Clin. Microbiol. 31:598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim I. S., et al. 1993. Rapid diagnosis of scrub typhus by a passive hemagglutination assay using recombinant 56-kilodalton polypeptides. J. Clin. Microbiol. 31:2057–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murata M., et al. 1986. Production and characterization of monoclonal strain-specific antibodies against prototype strains of Rickettsia tsutsugamushi. Microbiol. Immunol. 30:599–610 [DOI] [PubMed] [Google Scholar]
- 17. Niu D., et al. 2003. Immunogenicity of a 40kDa fragment of the 47kDa recombinant protein and DNA vaccine from Karp strain of Orientia tsutsugamushi. Ann. N. Y. Acad. Sci. 990:527–534 [DOI] [PubMed] [Google Scholar]
- 18. Oaks E. V., Rice R. M., Kelly D. J., Stover C. K. 1989. Antigenic and genetic relatedness of eight Rickettsia tsutsugamushi antigens. Infect. Immun. 57:3116–3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ohashi N., Tamura A., Suto T. 1988. Immunoblotting analysis of anti-rickettsial antibodies produced in patients of Tsutsugamushi disease. Microbiol. Immunol. 32:1085–1092 [DOI] [PubMed] [Google Scholar]
- 20. Ohashi N., Nashimoto H., Ikeda H., Tamura A. 1992. Diversity of immunodominant 56-kDa type-specific antigen (TSA) of Rickettsia tsutsugamushi. Sequence and comparative analyses of the genes encoding TSA homologues from four antigenic variants. J. Biol. Chem. 267:12728–12735 [PubMed] [Google Scholar]
- 21. Ohashi N., et al. 1996. Demonstration of antigenic and genotypic variation in Orientia tsutsugamushi which were isolated in Japan, and their classification into type and subtype. Microbiol. Immunol. 40:627–638 [DOI] [PubMed] [Google Scholar]
- 22. Page M. J., Di Cera E. 2008. Evolution of peptidase diversity. J. Biol. Chem. 283:30010–30014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qiang Y., et al. 2003. Phylogenetic characterization of Orientia tsutsugamushi isolated in Taiwan according to the sequence homologies of 56-kDa type-specific antigen genes. Microbiol. Immunol. 47:577–583 [DOI] [PubMed] [Google Scholar]
- 24. Seong S. Y., et al. 1997. Induction of neutralizing antibody in mice by immunization with recombinant 56 kDa protein of Orientia tsutsugamushi. Vaccine 15:1741–1747 [DOI] [PubMed] [Google Scholar]
- 25. Seong S. Y., Choi M. S., Kim I. S. 2001. Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect. 3:11–21 [DOI] [PubMed] [Google Scholar]
- 26. Smadel J. E., Elisberg B. L. 1965. Scrub typhus rickettsia, p. 1130–1143 In Horsfall F. L., Tamm I. (ed.), Viral and rickettsial infections of man, 4th ed. JB Lippincott, Philadelphia, PA [Google Scholar]
- 27. Studier F. W., Moffatt B. A. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113–130 [DOI] [PubMed] [Google Scholar]
- 28. Tamura A., Ohashi N., Urakami H., Takahashi K., Oyanagi M. 1985. Analysis of polypeptide composition and antigenic components of Rickettsia tsutsugamuushi by polyacrylamide gel electrophoresis and immunoblotting. Infect. Immun. 48:671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weddle J. R., et al. 1995. Effectiveness of a dot-blot immunoassay of anti-Rickettsia tsutsugamushi antibodies for serologic analysis of scrub typhus. Am. J. Trop. Med. Hyg. 53:43–46 [PubMed] [Google Scholar]
- 30. Yamamoto S., et al. 1986. Immunological properties of Rickettsia tsutsugamushi, kawasaki strain, isolated from a patient in Kyushu. Microbiol. Immunol. 30:611–620 [DOI] [PubMed] [Google Scholar]
- 31. Yang Q., Ching W. M., Jiang J., Lousteau L., Richards A. L. 2003. An improved method for the purification and refolding of r56-kDa proteins from Gilliam and Kato strains of Orientia tsutsugamushi. Ann. N. Y. Acad. Sci. 990:375–385 [DOI] [PubMed] [Google Scholar]