Abstract
All-trans-retinoic acid (RA) promotes the maturation and differentiation of B cells, which are known as a type of professional antigen-presenting cells. We show here that CD1d, a major histocompatibility complex class I-like molecule that presents lipid antigens, is expressed in the mouse spleen B cells and is increased by RA. Thus, we hypothesized that RA and the CD1d ligand, α-galactosylceramide (αGalCer), could interact to promote the differentiation, maturation, and antibody response of antigen-activated B cells. In isolated B cells, αGalCer alone markedly stimulated, and RA further increased B cell proliferation, synergizing with the B cell antigen receptor ligation via anti-μ antibody (P < 0.05). The significantly increased cell proliferation stimulated by αGalCer was abrogated in the B cells of CD1d-null mice. RA alone and combined with αGalCer also promoted B cell differentiation by the enrichment of sIgG1-, CD138-, and PNA/Fas-positive B cells (P < 0.05), suggesting a plasmacytic cell differentiation. In vivo, wild-type mice treated with RA and/or αGalCer during primary immunization with tetanus toxoid produced a higher serum anti-tetanus IgG response and had more bone marrow anti-tetanus antibody-secreting cells as determined by enzyme-linked immunospot assay (P < 0.05) in the secondary response, a finding indicative of heightened long-term memory; however, the increased antibody secretion after αGalCer treatment was abolished in CD1d-null mice. We provide evidence here that RA, together with αGalCer, can effectively regulate B cell proliferation and differentiation, ultimately promoting a more efficient antibody response to protein antigen. The results suggest that the combination of RA and αGalCer could be a useful adjuvant combination in vaccine strategies.
INTRODUCTION
An adequate supply of vitamin A has been shown to be life-saving in young children (35), and maintenance of appropriate immune functions is widely believed to underlie its beneficial effects. Vitamin A and its active metabolite all-trans-retinoic acid (RA) are capable of rescuing poor immune responses in vitamin A-deficient animals and of augmenting anti-infectious responses in the vitamin A-adequate state (28). Various processes leading to improved immune response have been shown to be regulated by RA, including lymphopoiesis, cell differentiation, T cell proliferation, cytokine production, and tissue trafficking of B and T cells (7, 24, 25, 30).
Previously, we reported that RA significantly upregulates the gene expression and activity of CD1d, a major histocompatibility complex class I-like molecule that functions by presenting glycolipid antigens to invariant NKT cells (iNKT) (6, 23) and then rapidly secretes both T-helper (Th) type 1 and type 2 cytokines, such as gamma interferon and interleukin-4, bridging the innate and adaptive immune systems (9, 14, 16, 31, 32, 34). α-Galactosylceramide (αGalCer) is a model glycolipid antigen that binds specifically to CD1d (13). The activation of iNKT cells by αGalCer and endogenous glycolipids presented by CD1d has been shown to be beneficial during several bacterial and viral infections, in certain antitumor responses, and in the regulation of certain autoimmune diseases such as diabetes (3, 10, 12, 19, 29, 33).
Our present studies were based on previous findings that: (i) B cells express CD1d and process CD1d-associated antigen (1, 9, 16); (ii) RA is a strong regulator of CD1d expression, at least in monocytic cells (6); and (iii) RA serves to promote the maturation of B cells (4, 26). We hypothesized that RA and glycolipid (αGalCer) could work together to promote B cell activation and differentiation, leading to increased antibody production. In the present study, we have first tested the regulatory effects of RA on CD1d-mediated splenic B-cell activation and differentiation in vitro, and then determined whether RA and αGalCer combined are able to enhance the antibody response to tetanus toxoid (TT) in terms of the antigen-induced primary humoral response and long-term memory response in vivo.
MATERIALS AND METHODS
Antibody and reagents.
CD19-PEcy7, Fas-PE, and CD138-PE antibodies were purchased from BD Biosciences. IgG1-Alexa 488 was obtained from Invitrogen. Peanut agglutinin (PNA) conjugated with fluorescein was from Vector Laboratories, Inc. (Burlingame, CA). αGalCer was from Alexis Biochemicals (San Diego, CA), and βGalCer was from Sigma. Anti-μ antibody used in the B cell proliferation analyses was from Jackson Laboratory (Bar Harbor, ME). Reagents and cell culture medium were determined to be endotoxin-free by using a Limulus amebocyte lysate endotoxin assay kit from GenScrip (Piscataway, NJ).
Animals, splenocytes, and B cell isolation and culture.
Animal protocols were approved by the Institutional Animal Use and Care Committee of Pennsylvania State University. Adult female BALB/c (∼8 weeks old [Charles River Laboratories]) were used to obtain spleen B cells for in vitro study as described previously (5). Female CD1d-null mice (CD1tm1Gru/J) and age-matched control BALB/cJ mice, 8 weeks old, were from Jackson Laboratory.
Spleen B cells were isolated by using a negative B cell enrichment kit according to the manufacturer's instructions (StemCell Technology, Vancouver, British Columbia, Canada). The purity of isolated B cells was ∼94% based on CD19 staining. Cells were cultured in RPMI 1640 medium, which was supplemented with 10% fetal bovine serum and 5 × 10−5 M β-mercaptoethanol, all from Invitrogen.
In vivo animal experimental design.
BALB/c female mice, or CD1d-null and BALB/cJ control mice, 8 weeks old, were injected subcutaneously with TT (10 μg/mouse [22]). One dose of αGalCer (5 μg/mouse) was injected simultaneously subcutaneously. βGalCer was given similarly as αGalCer to control animals. RA was given orally (Sigma; 37.5 μg/mouse/day) in canola oil, with oil only as the vehicle control, daily for 7 consecutive days (22). Blood was collected from the retro-orbital sinus prior to and after TT immunization. The treatment and sampling times in the present study are further described and illustrated with the results from the study.
Cell proliferation assay.
[3H]thymidine incorporation assay was performed to determine B cell proliferation as described previously (4).
Flow cytometry analysis and sorting.
For each experimental condition, 105 isolated B cells were incubated with 0.1 μg of fluorescence-labeled antibody. After a washing step, unstained and isotype-control antibody stained cells were used to set up gates as described previously (4).
Enzyme-linked immunospot (ELISPOT) assay.
The procedure was performed based on a previous report (22). The antigen-specific spots were counted and calculated as number of spots per 106 bone marrow cells.
Enzyme-linked immunosorbent assay (ELISA) for plasma anti-TT antibody.
A plasma anti-tetanus assay was performed as previously described (22). A standard plasma sample was serially diluted on each assay plate to assure that the measurements were in a linear dose-response range and that there was comparability across the assays. Titers of antibody (i.e., the fold dilution) were calculated based on the standard curve developed for each plate.
Statistical methods.
Means, standard errors, and P values were determined by using Prism 5 software (GraphPad Software, Inc). P values were calculated by t test or analysis of variance, followed by Tukey's post hoc test. A P value of <0.05 was considered significant.
RESULTS
RA increases CD1d expression in B cells.
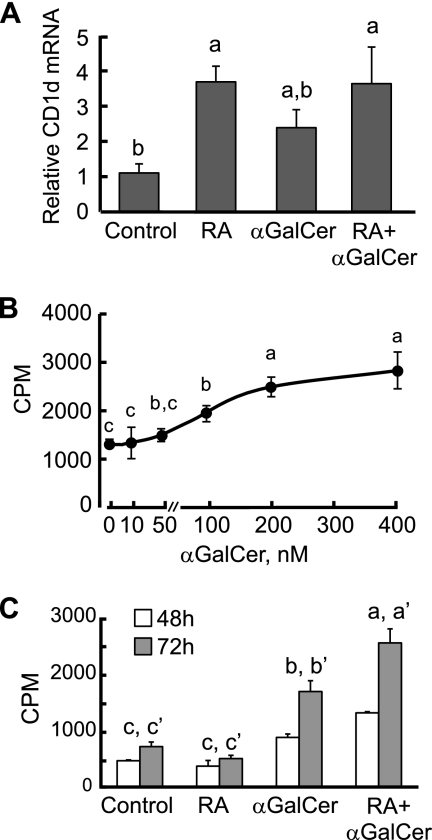
Spleen B cells were isolated that had a purity ca. 94% according to CD19 staining. CD1d mRNA expression level was determined by quantitative PCR both after and in the absence of treatment with RA (20 nM, 24 h). We also stimulated B cells with αGalCer (100 nM), which is known as a ligand for CD1d (2). Treatment with RA increased CD1d mRNA during the 24-h experiment (P < 0.05), which was consistent with the results we observed in monocytic cells, whereas αGalCer failed to regulate the CD1d mRNA level (Fig. 1 A).
Fig. 1.
Regulation of CD1d expression and cell proliferation by RA and αGalCer in mouse splenic B cells. (A) RA increased CD1d expression in spleen B cells. B cells were cultured in the presence or absence of RA (20 nM) or αGalCer (100 nM) for 24 h. Total RNA was extracted and subjected to quantitative PCR analysis. The data are presented as the ratio of CD1d to tubulin mRNAs, representing three independent experiments with treatments in triplicate. a > b, P < 0.05. (B) αGalCer increases B cell proliferation dose dependently. B cells were isolated and cultured in the presence of different concentrations of αGalCer for 72 h. [3H]thymidine was added for the last 6 h of culture to monitor cell proliferation activity. a > b > c, P < 0.05. (C) B cells were isolated and cultured in the presence or absence of αGalCer and/or RA or anti-μ antibody (0.1 μg/ml). [3H]thymidine was added on day 2 or 3 for the last 6 h of culture to monitor cell proliferation activity. αGalCer strongly increased B cell proliferation, which was further enhanced by RA. a > b > c (48 h) and a′ > b′ > c′ (72 h), P < 0.05.
RA and αGalCer differentially regulate B cell proliferation and differentiation.
The CD1d molecule, as a lipid antigen receptor, could potentially act as an alternative or additional type of B cell receptor (BCR). Thus, we measured B cell proliferation by thymidine incorporation after the treatment of isolated B cells with αGalCer and RA for 48 and 72 h; moreover, to test for cooperation or cross talk between αGalCer stimulation and the BCR, we incubated cells with anti-μ antibody in the presence or absence of αGalCer. We first tested the doses of αGalCer used to stimulate B cell and selected 100 nM as an optimal subthreshold dose for the following experiments (Fig. 1B). As shown in Fig. 1C, αGalCer exerted a strong mitogenic effect, evident at both 48 and 72 h, whereas βGalCer, an isomer that binds to CD1d but fail to activate iNKT cells (27) and was used in the control group, did not show any effect. Although RA alone had no stimulatory effect on B cell proliferation, it potentiated the mitogenic effect of αGalCer (Fig. 1C).
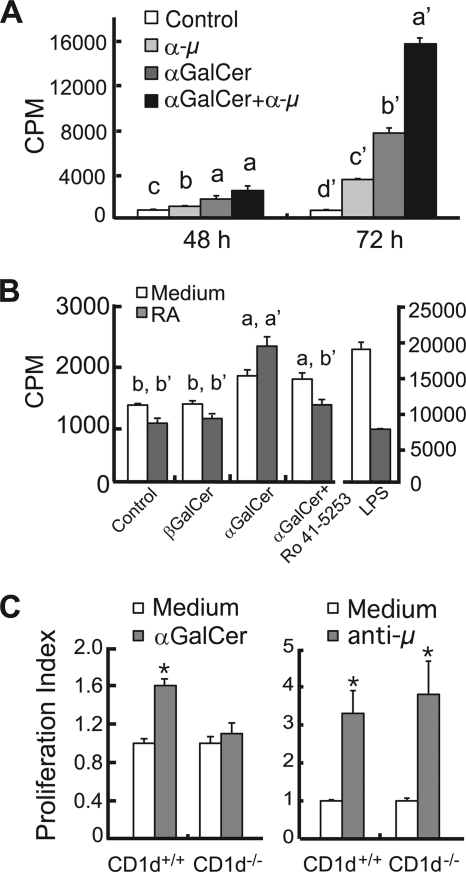
Moreover, both anti-μ and αGalCer were mitogenic to B cells and, in combination, produced a more than additive increase in B cell proliferation, evident on day 3 (Fig. 2A). In these experiments, the retinoid antagonist inhibited the effect of RA (compare results for αGalCer with RA to αGalCer with RA plus antagonist, Fig. 2B). Lipopolysaccharide (LPS), used as a positive control, strongly stimulated B cell proliferation as expected, but RA inhibited the stimulatory effect of LPS, as we have reported previously (4). Thus, the promotion by RA of αGalCer-induced B cell proliferation is specific for this compound compared to that for LPS.
Fig. 2.
αGalCer synergized with B cell receptor to stimulate B cell proliferation. (A) B cells were isolated and cultured in the presence or absence of αGalCer and/or anti-μ antibody (0.1 μg/ml). “a > b > c” (48 h) and “a′ > b′ > c′ > d′” (72 h) denote differences of P < 0.05. (B) RA increases αGalCer stimulated B cell proliferation. B cells were cultured with stimuli in the presence or absence of RA (20 nM) for 72 h. An RAR antagonist (Ro 41-5253, 100 nM) was added to the culture as indicated in the chart. LPS (100 ng/ml) was used in the experiment as a positive control. a > b and a′ > b′, P < 0.05. (C) αGalCer stimulated B cell proliferation via CD1d expression, whereas anti-μ does not. *, P < 0.05.
To test the specificity of the CD1d-mediated B cell activation, we also conducted the assay in CD1d-null mice. The deletion of CD1d abrogated the stimulatory effect of αGalCer while these cells retained the responsiveness to anti-μ stimulation, similar to wild-type cells, in the B cell proliferation assay (Fig. 2C). These results imply that RA can enhance the signaling of αGalCer through CD1d, while CD1d- and BCR-mediated signaling interact in a manner that suggests complementary actions.
RA and αGalCer help to regulate B cell differentiation into plasmacytic cells.
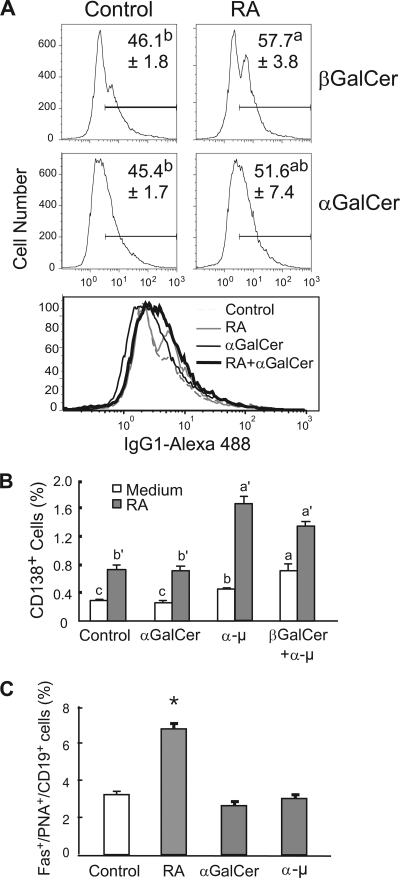
Next, we tested whether RA and αGalCer interact with each other to regulate B cell differentiation into plasma cells. Surface IgG1 (sIgG1) and CD138 (also known as syndecan 1, a plasmacytic B cell marker [36]), as well as Fas and PNA staining, which serve as biomarkers of germinal center B cells (15, 21), were used to determine the B cell phenotype after treatment with RA and αGalCer. B cells were treated with RA and/or αGalCer, and anti-μ was used as a positive stimulus to induce B cell activation and differentiation. After 4 days of culture, cells were subjected to flow cytometry analysis. As shown in Fig. 3A, RA significantly increased the percentage of sIgG1+ cells (P < 0.05), while αGalCer failed to increase sIgG1 expression. These results are consistent with the known cell differentiation-inducing effects of RA (4, 5), suggesting that αGalCer, while effective in regulating cell proliferation, may be insufficient in promoting B-cell differentiation. It was also observed that RA, or anti-μ treatment, increased the proportion of CD138+ B cells (Fig. 3B, all P < 0.05). Although αGalCer alone had no observable effect, RA and αGalCer in combination augmented the increase in CD138 (syndecan 1)-expressing cells after BCR ligation with anti-μ antibody.
Fig. 3.
RA and αGalCer differentially regulate plasma cell differentiation. B cells were cultured in the presence or absence of RA, αGalCer, or anti-μ for 4 days and then stained with antibodies that recognize CD19, sIgG1, CD138, and Fas/PNA. (A) RA increased sIgG1 expression, while αGalCer did not. Representative flow histograms of B cell sIgG on day 4 of culture are illustrated; the percentage of sIgG1-positive cells is shown as the mean ± the standard error (n = 4). a > b, P < 0.05. An overlay of the histograms for each condition is presented below to compare the change in peak positions. (B) RA and anti-μ increased CD138+ B cells. a > b > c and a′ > b′, P < 0.05. (C) RA increased the germinal center B cell population via costaining of Fas, PNA, and CD19. RA increased Fas+ and PNA+ B cell populations. a > b > c > d, P < 0.05.
The splenic microenvironment is crucial for B cell plasmacytic development. The germinal center B cell markers Fas and PNA were detected to test whether RA and/or αGalCer promote the development of germinal centers. Fas- and PNA-positive cells were consistently increased by RA (Fig. 3C), while the number was not affected by either αGalCer or anti-μ. These data further imply a differential role of RA and αGalCer on B cell differentiation. Whereas RA promoted B cell differentiation, as shown by several criteria, αGalCer may need an extra signal, such as BCR ligation, and signals initiated by RA to regulate B cell differentiation.
RA and αGalCer increase the primary antibody response and memory B cell formation in vivo after tetanus immunization.
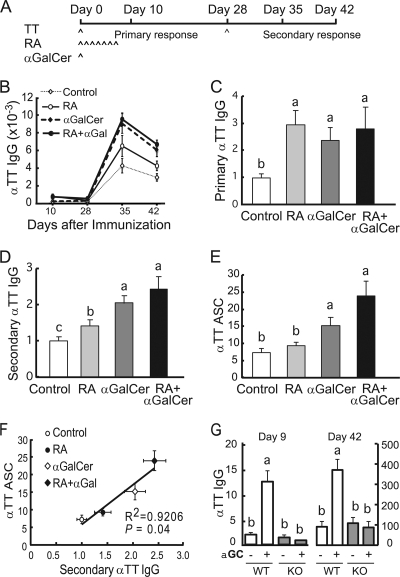
To explore the role of RA and αGalCer in the regulation of immune response, we used a mouse model of TT immunization, since TT is a clinically relevant antigen that is commonly used experimentally as a recall antigen. A single dose of αGalCer was given simultaneously with the primary TT immunization, followed by treatment with RA orally during the development of the primary antibody response. A boost injection was given 4 weeks after the primary immunization (Fig. 4A). Plasma was collected for anti-TT IgG measurement at both primary and secondary responses. At the end of the study, bone marrow mononuclear cells were collected to detect memory B cells by ELISPOT assay. As expected, immunization elicited a TT-specific IgG response, which was relatively low during the primary phase (days 10 and 28) but increased prominently after boosting (days 35 and 42) (Fig. 4B). RA and αGalCer significantly increased the plasma IgG level during both primary and secondary responses (Fig. 4C and D). Consistent with the ELISA titers measured 14 days after boosting, a TT-specific ELISPOT assay also showed that αGalCer significantly increased the number of TT-specific antibody-secreting cells (ASC), suggesting the stimulation of memory B cell formation by αGalCer given at the time of priming (Fig. 4E). Although the effect of RA was not statistically significant in the ELISPOT assay, the trend was consistent with ELISA results (Fig. 4F) with a strong overall correlation (R = 0.9263, P < 0.05). We further tested the antibody response in CD1d-null mice. Treatment with αGalCer increased the production of anti-TT specific IgG in control mice but failed to affect the antibody response in CD1d-null mice (Fig. 4G), indicating the importance of CD1d in this immune enhancement. Together, these data demonstrated that RA and αGalCer administered, along with TT antigen could enhance the antibody response during both the primary and the secondary (memory) B cell response.
Fig. 4.
RA and αGalCer promote B cell differentiation and anti-tetanus IgG secretion in vivo. (A) BALB/c mice were immunized with TT antigen, along with αGalCer, and treated with RA orally (for details, see Materials and Methods). Blood was collected at the times indicated for plasma anti-TT IgG ELISA. Bone marrow cells were collected at the end of experiment for ELISPOT assay. (B) TT-specific primary and secondary antibody responses. Day 10, primary response; day 28, immediately before boosting; days 35 and 42, recall response to TT at days 7 and 14, respectively, after the second immunization. (C and D) Plasma TT-specific IgG after primary immunization (day 10) and secondary immunization (day 42). The data are presented as the fold increase versus the control group (a > b or a > b > c, P < 0.05). (E) Number of antibody-secreting cells in bone marrow. The data from two independent experiments were combined (n = 8/group; a > b > c, P < 0.05). (F) Correlation of plasma anti-TT IgG with bone marrow anti-TT ASC (memory B cells). (G) Serum IgG primary and secondary responses in wild-type and CD1d-null mice (n = 4/group).
DISCUSSION
The present studies have shown that RA interacts with αGalCer to regulate B cell proliferation and differentiation and that CD1d may be one of the mediators for their effect. B cells are an important type of antigen-presenting cells that recognize protein antigen through BCR, as well as lipid antigen via CD1d molecules on its surface. Lang et al. also reported that B cell CD1d expression is required for the NKT cell activity in the regulation of the antibody response (16). Moreover, Leadbetter et al. (17) showed that the CD1d-iNKT arm promotes B cell function in anti-lipid antibody production. According to several lines of evidence, RA and αGalCer acted in a complementary manner, and the combination augmented B cell differentiation in vitro and the plasma antibody response in vivo.
First, αGalCer alone significantly stimulated B cell proliferation, which was additive with BCR ligation by anti-μ (Fig. 1 and 2). The additivity of anti-μ with αGalCer is consistent with a previous report that the presentation of a CD1d-restricted antigen such as αGalCer by the BCR can activate B cells (1). Whereas we (4) and others (11) have shown that RA typically inhibits B cell proliferation and is a strong inhibitor of anti-μ or LPS stimulated B cell proliferation (4) (Fig. 2B), even while inducing B cell differentiation, it nonetheless enhanced B cell proliferation stimulated by αGalCer. Since B cell expansion is the critical initial response that increases the pool of antigen-activated B cells, the interaction of RA and αGalCer to increase B cell proliferation may contribute to the amplification of the overall immune response. In spite of the BCR-dependent pathway (1), CD1d may also induce B cell proliferation through a BCR-independent way.
Since RA works together with αGalCer to enhance B cell proliferation, RA and αGalCer diverge in the regulation of the B cell differentiation process, a multistep and complex journey. The processes in which RA and αGalCer may exert their differential effects on B cell activation and differentiation are summarized in Fig. 5. Mature naive B cells go through expansion and differentiation upon encountering stimulation. The differential effect of RA and αGalCer on the expression of several essential molecules such as sIgG, CD138, and Fas/PNA, which represent the B cell differentiation and plasmacyte formation, may suggest an adequate differentiation program controlling the stages of B cell activation. The differential effects of RA and αGalCer are also interesting from the viewpoint that they may complement one another, leading to a heightened response when applied in combination. The demonstration that treatment in vivo with RA and/or αGalCer at the time of primary immunization with TT can significantly increase the specific anti-TT plasma antibody level during both the primary and the secondary responses indicates the potential of glycolipid antigen stimulation to affect the response to a protein antigen, while the similar elevation in the bone marrow ASC response suggests that the production of a larger pool of long-lived anti-TT memory cells was initiated when mice were treated with RA and/or αGalCer at the time of initial exposure to antigen (Fig. 4). The absence of the stimulatory affect in the CD1d-null mice suggests the importance of iNKT cells in the adaptive immune response.
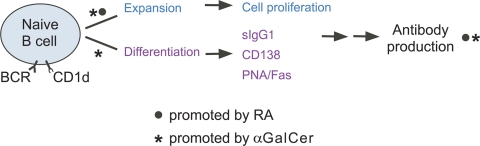
Fig. 5.
Schematic model. The diagram illustrates the positive regulatory effects of RA (•) and αGalCer (*) on different phases of B cell proliferation and differentiation, leading to the formation of the plasma cell phenotype, increased B memory cell population, and a heightened recall response to reimmunization, as shown experimentally in Fig. 4.
An interesting question raised here is whether concurrent stimulation with a glycolipid and a protein antigen activates the same B cell or whether “bystander” effects on nearby B cells are in play. Compared to protein and polysaccharide antigens, glycolipids have received relatively little attention, and it is only recently, with the understanding that αGalCer and endogenous glycolipids such as isoglobosides (37) can activate iNKT cells, that attention has begun to turn to these molecules as important antigens. Their natural antigenicity in vivo, however, is not well understood. The concept that B cells, as carriers of BCR and CD1d, could be importantly involved in the response to a protein antigen, while being stimulated by a glycolipid antigen, seems worthy of further investigation. RA acted in a complementary manner to αGalCer to enhance the B cell differentiation program, leading to increased B cell maturation and increased plasma antibody titers and memory response, also as evidenced by increased formation of bone marrow ASC indicative of a long-term memory pool.
To conclude, our data provide novel evidence that RA, which induced CD1d, is involved in αGalCer-stimulated B cell activation and differentiation and that both together are effective in stimulating the humoral B cell antibody response. Several recent reports provide evidence that αGalCer and its analog could be an effective adjuvant in the immunization protocol (8, 18, 20). With the understanding of the interaction of RA and αGalCer, the combination may be promising for improving vaccine effectiveness in the future.
ACKNOWLEDGMENTS
We thank the Center for Quantitative Cell Analysis, Penn State, for assistance with flow cytometry.
This study was supported by NIH grant DK-41479 and ARRA supplement R03 DK41479-20S.
We have no financial conflicts or other interests to disclose.
Footnotes
Published ahead of print on 6 April 2011.
REFERENCES
- 1. Barral P., et al. 2008. B-cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc. Natl. Acad. Sci. U. S. A. 105:8345–8350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borg N. A., et al. 2007. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature 448:44–49 [DOI] [PubMed] [Google Scholar]
- 3. Brigl M., Brenner M. B. 2010. How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin. Immunol. 22:79–86 [DOI] [PubMed] [Google Scholar]
- 4. Chen Q., Ross A. C. 2005. Vitamin A and immune function: retinoic acid modulates population dynamics in antigen receptor and CD38-stimulated splenic B cells. Proc. Natl. Acad. Sci. U. S. A. 102:14142–14149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen Q., Ross A. C. 2007. Retinoic acid promotes mouse splenic B cell surface IgG expression and maturation stimulated by CD40 and IL-4. Cell. Immunol. 249:37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen Q., Ross A. C. 2007. Retinoic acid regulates CD1d gene expression at the transcriptional level in human and rodent monocytic cells. Exp. Biol. Med. (Maywood) 232:488–494 [PMC free article] [PubMed] [Google Scholar]
- 7. Chen X., et al. 2008. Retinoids accelerate B lineage lymphoid differentiation. J. Immunol. 180:138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Devera T. S., et al. 2010. CD1d-dependent B-cell help by NK-like T cells leads to enhanced and sustained production of Bacillus anthracis lethal toxin-neutralizing antibodies. Infect. Immun. 78:1610–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Devera T. S., Shah H. B., Lang G. A., Lang M. L. 2008. Glycolipid-activated NKT cells support the induction of persistent plasma cell responses and antibody titers. Eur. J. Immunol. 38:1001–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dufour F. D., Baxter A. G., Silveira P. A. 2008. Interactions between B-lymphocytes and type 1 NKT cells in autoimmune diabetes. J. Immunotoxicol. 5:249–257 [DOI] [PubMed] [Google Scholar]
- 11. Ertesvag A., Naderi S., Blomhoff H. K. 2009. Regulation of B cell proliferation and differentiation by retinoic acid. Semin. Immunol. 21:36–41 [DOI] [PubMed] [Google Scholar]
- 12. Florence W. C., Bhat R. K., Joyce S. 2008. CD1d-restricted glycolipid antigens: presentation principles, recognition logic, and functional consequences. Expert Rev. Mol. Med. 10:e20. [DOI] [PubMed] [Google Scholar]
- 13. Godfrey D. I., Kronenberg M. 2004. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 114:1379–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Godfrey D. I., Stankovic S., Baxter A. G. 2010. Raising the NKT cell family. Nat. Immunol. 11:197–206 [DOI] [PubMed] [Google Scholar]
- 15. Hao Z., et al. 2008. Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity 29:615–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lang G. A., Devera T. S., Lang M. L. 2008. Requirement for CD1d expression by B cells to stimulate NKT cell-enhanced antibody production. Blood 111:2158–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leadbetter E. A., et al. 2008. NK T cells provide lipid antigen-specific cognate help for B cells. Proc. Natl. Acad. Sci. U. S. A. 105:8339–8344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee Y. S., et al. 2010. An alpha-GalCer analogue with branched acyl chain enhances protective immune responses in a nasal influenza vaccine. Vaccine 29:417–425 [DOI] [PubMed] [Google Scholar]
- 19. Li D., Xu X. N. 2008. NKT cells in HIV-1 infection. Cell Res. 18:817–822 [DOI] [PubMed] [Google Scholar]
- 20. Li X., et al. 2010. Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc. Natl. Acad. Sci. U. S. A. 107:13010–13015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luzina I. G., et al. 2001. Spontaneous formation of germinal centers in autoimmune mice. J. Leukoc. Biol. 70:578–584 [PubMed] [Google Scholar]
- 22. Ma Y., Chen Q., Ross A. C. 2005. Retinoic acid and polyriboinosinic:polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J. Immunol. 174:7961–7969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsuda J. L., Mallevaey T., Scott-Browne J., Gapin L. 2008. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr. Opin. Immunol. 20:358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maynard C. L., et al. 2009. Contrasting roles for all-trans retinoic acid in TGF-β-mediated induction of Foxp3 and Il10 genes in developing regulatory T cells. J. Exp. Med. 206:343–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mora J. R., et al. 2006. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314:1157–1160 [DOI] [PubMed] [Google Scholar]
- 26. Morikawa K., Nonaka M. 2005. All-trans-retinoic acid accelerates the differentiation of human B lymphocytes maturing into plasma cells. Int. Immunopharmacol. 5:1830–1838 [DOI] [PubMed] [Google Scholar]
- 27. Ortaldo J. R., et al. 2004. Dissociation of NKT stimulation, cytokine induction, and NK activation in vivo by the use of distinct TCR-binding ceramides. J. Immunol. 172:943–953 [DOI] [PubMed] [Google Scholar]
- 28. Ross A. C., Chen Q., Ma Y. 2009. Augmentation of antibody responses by retinoic acid and costimulatory molecules. Semin. Immunol. 21:42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shimizu K., Goto A., Fukui M., Taniguchi M., Fujii S. 2007. Tumor cells loaded with alpha-galactosylceramide induce innate NKT and NK cell-dependent resistance to tumor implantation in mice. J. Immunol. 178:2853–2861 [DOI] [PubMed] [Google Scholar]
- 30. Sigmundsdottir H., Butcher E. C. 2008. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat. Immunol. 9:981–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sullivan B. A., et al. 2010. Mechanisms for glycolipid antigen-driven cytokine polarization by Vα14i NKT cells. J. Immunol. 184:141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taniguchi M., Seino K., Nakayama T. 2003. The NKT cell system: bridging innate and acquired immunity. Nat. Immunol. 4:1164–1165 [DOI] [PubMed] [Google Scholar]
- 33. Venkataswamy M. M., et al. 2009. Incorporation of NKT cell-activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis bacillus Calmette-Guerin. J. Immunol. 183:1644–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Venkataswamy M. M., Porcelli S. A. 2010. Lipid and glycolipid antigens of CD1d-restricted natural killer T cells. Semin. Immunol. 22:68–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Villamor E., Fawzi W. W. 2005. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin. Microbiol. Rev. 18:446–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang Y., Tung J. W., Ghosn E. E., Herzenberg L. A., Herzenberg L. A. 2007. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc. Natl. Acad. Sci. U. S. A. 104:4542–4546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou D., et al. 2004. Lysosomal glycosphingolipid recognition by NKT cells. Science 306:1786–1789 [DOI] [PubMed] [Google Scholar]