Abstract
Aptima Combo 2 (AC2) Neisseria gonorrhoeae testing of 81,405 patients who were tested by culture and 14,666 who were AC2 tested for Chlamydia trachomatis detected 142 extra infections and confirmed 106 culture-positive samples (the positivity rate increased from 0.13 in testing by culture to 0.26 in testing by AC2). Retrievable AC2 positive samples were confirmed (98.5%) by an alternate AGC test.
TEXT
Lower genital tract infections with Neisseria gonorrhoeae may be asymptomatic and accompanied by Chlamydia trachomatis infection(13). Efforts are needed to identify and treat lower tract infections to prevent upper tract complications, such as pelvic inflammatory disease, ectopic pregnancy, or tubal infertility in women and less commonly epididymitis or prostatitis in men, as well as transmission between asymptomatically infected patients and their uninfected partners. Attempts to culture N. gonorrhoeae from clinical specimens can be unsuccessful. Testing for N. gonorrhoeae with nucleic acid amplification tests (NAATs) has increased diagnostic sensitivity (3, 4, 9, 11, 18, 19) using traditional and less-invasive sampling. Although not all are FDA cleared, first-void urine (FVU), self-collected vaginal swabs (VS), and anal and oral swabs have been shown to yield positive results (1, 7, 15). The commercially available transcription-mediated amplification (TMA) test Aptima Combo 2 (AC2) is able to detect N. gonorrhoeae and C. trachomatis RNA in clinical specimens collected in specimen transportation media (STM), with no cross-reactions with non-N. gonorrhoeae strains. Positives can be confirmed in alternate individual TMA tests, Aptima GC (AGC) (for N. gonorrhoeae) and APTIMA CT (ACT) (for C. trachomatis) (2, 12).
Community physicians practicing in Southern Ontario who suspect that their patient may have an N. gonorrhoeae infection traditionally submit cervical swabs (CS) from women and urethral swabs (US) from men for culture. If a C. trachomatis infection is suspected, an NAAT will be ordered for a CS or FVU. If both infections are suspected, specimens are collected for N. gonorrhoeae culture and C. trachomatis nucleic acid amplification testing. We evaluated the utility of performing additional AC2 testing for N. gonorrhoeae on specimens submitted for N. gonorrhoeae culture and C. trachomatis TMA. The objectives were as follows: (i) to perform N. gonorrhoeae testing by AC2 on STM samples from patients receiving N. gonorrhoeae culture, (ii) to test STM samples submitted for C. trachomatis testing for N. gonorrhoeae, and (iii) to confirm AC2-GC positives using the AGC assay.
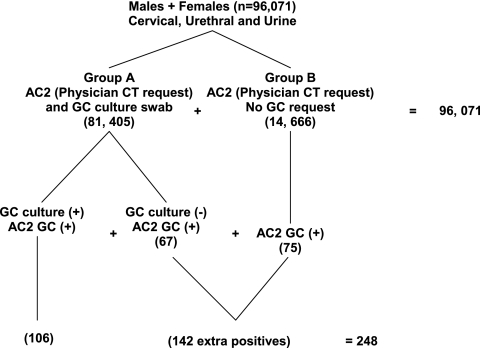
From March to August 2008, the microbiology laboratory at Gamma Dynacare Medical Laboratories in Brampton, Canada, received 96,071 urogenital samples from 961 men (FVU or US) and 95,110 women (FVU or CS) for C. trachomatis and N. gonorrhoeae testing or C. trachomatis testing only (Fig. 1). There were 81,405 patients in group A, whose physicians submitted a swab collected in an M40 Transystem specimen transport system (Copan Diagnostics Inc.) for N. gonorrhoeae culture and an additional sample collected in STM (GenProbe) for AC2 testing for C. trachomatis. Group B consisted of 14,666 men and women whose physicians collected FVU samples or swabs into STM for AC2 testing for C. trachomatis only. Specimens tested for C. trachomatis or N. gonorrhoeae RNA were processed on the semiautomated GenProbe DTS 1600 system or on an automated Tigris instrument. N. gonorrhoeae positives were confirmed using the AGC assay. Samples for N. gonorrhoeae culture were inoculated onto modified Martin-Lewis chocolate agar biplates (catalogue no. P4100; PML Microbiologicals). Cultures were confirmed with Vitek NH1 test cards and Gonogen serological tests.
Fig. 1.
Algorithm of testing shows 142 extra cases of N. gonorrhoeae.
Figure 1 shows that from group A, 106 of the patients with a positive N. gonorrhoeae culture (0.13% prevalence) were also positive by AC2 testing and an additional 67 were negative by culture but positive by AC2, an increase of 63%. There were no culture-positive, NAAT-negative findings. These findings are similar to those of a previous study (10) comparing Cobas Amplicor (AMP) PCR to culture of 3,023 clinical specimens from woman and men, which demonstrated an increase from 58 to 85 positives, an increase of 46% due to PCR testing. From group B, in the present study, the 14,666 samples yielded 75 N. gonorrhoeae positives. The total number of extra N. gonorrhoeae positives from AC2 testing in both groups was 142, more than doubling the number of N. gonorrhoeae positives by culture, for a total of 248 (0.26% prevalence). An examination of the gender and specimen types revealed the 248 N. gonorrhoeae positives to be distributed in 115 males (55 FVU and 60 US) and 133 females (28 FVU and 105 CS). For all patients, the prevalence of N. gonorrhoeae infection by culture was 0.13% (106/81,405): in women it was 0.09% (73/80,590) by culture and 0.14% (133/95,110) by AC2 (P < 0.001). The prevalence in men by culture was 6% (49/815) and increased to 11.9% (115/961) (P < 0.001) when all were tested by AC2.
Of 142 extra positive samples, 65 were available for confirmatory testing by AGC and 64 (98.5%) were confirmed to be positive. Confirmatory testing of GC Amplicor (AMP)-positive results in a previous study (10) showed a 56.1% rate of confirmation (87/155) using a 16S rRNA N. gonorrhoeae PCR (Roche), and only 57 were culture positive. This lower rate of confirmation may have been due to the AMP PCR cross-reacting with nongonorrhoea Neisseria (NGN), a phenomenon which has been widely reported (6, 7, 17). In contrast, there have been no reports of AC2 or AGC assays falsely reacting with NGN (2, 12), but because the AC2 assays have such high analytical sensitivity (4), original low-level positives may not always repeat positive and confirm (12).
Several studies have examined populations for dual N. gonorrhoeae/C. trachomatis infections (5, 8, 14, 16), showing a wide range depending on the population examined. Although we were unable to examine our database for dual infections, a current 2-month determination showed similar prevalence rates due to dual combo testing (C. trachomatis, 2.9%; N. gonorrhoeae, 0.29%) with 48 patients infected with both. Thus, combination testing for both organisms provides data on dual and single infections, providing information preventing unnecessary antibiotic treatment for both infections in patients infected with only one pathogen, since current clinical guidelines are to treat both. Dual infections and culture failures for patients investigated in community referral settings suggest that Aptima Combo 2 testing of samples submitted for C. trachomatis testing can identify extra N. gonorrhoeae-positive patients who would benefit from treatment of the appropriate pathogen. This may also be a cost-beneficial strategy if testing for both C. trachomatis and N. gonorrhoeae costs the same as testing for C. trachomatis alone. However, until molecular methods are available for detection of antibiotic-resistant N. gonorrhoeae from samples positive by NAATs, some form of sentinel culturing will be required (19).
Footnotes
Published ahead of print on 16 March 2011.
REFERENCES
- 1. Bachmann L. H., et al. 2009. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae oropharyngeal infections. J. Clin. Microbiol. 47:902–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boyadzhyan B., Yashina T., Yatabe J. H., Patnaik M., Hill C. S. 2004. Comparison of the APTIMA CT and GC assays with the AMPTIMA Combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J. Clin. Microbiol. 42:3089–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan E. L., Brandt K., Olienus K., Antonishyn N., Horsman G. B. 2000. Performance characteristics of the Becton Dickinson ProbeTec System for the direct detection of Chlamydia trachomatis and Neisseria gonorrhoeae in male and female urine specimens in comparison with the Roche Cobas System. Arch. Pathol. Lab. Med. 124:1649–1652 [DOI] [PubMed] [Google Scholar]
- 4. Chernesky M., Castriciano S., Jang D., Smieja M. 2006. Use of flocked swabs and a universal transport medium to enhance molecular detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J. Clin. Microbiol. 44:1084–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Creighton S., Tenant-Flowers M., Taylor C. B., Miller R., Low N. 2003. Co-infection with gonorrhoea and chlamydia: how much is there and what does it mean. Int. J. STD AIDS 142:109–113 [DOI] [PubMed] [Google Scholar]
- 6. Diemert D. J., Libman M. D., Lebel P. 2002. Confirmation by 16S rRNA PCR of the COBAS AMPLICOR CT/NG test for diagnosis of Neisseria gonorrhoeae infection in a low-prevalence population. J. Clin. Microbiol. 40:4056–4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farrell D. J., Sheedy T. J. 2001. Urinary screening for Neisseria gonorrhoeae in asymptomatic individuals from Queensland, Australia: an evaluation using three nucleic acid amplification methods. Pathology 33:204–205 [DOI] [PubMed] [Google Scholar]
- 8. Forward K. R. 2010. Risk of coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in Nova Scotia. Can. J. Infect. Med. Microbiol. 21:e84–e86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gaydos C. A., et al. 2003. Performance of the APTIMA Combo 2 assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in female urine and endocervical swab specimens. J. Clin. Microbiol. 41:304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luijt D. S., Bos P. A. J., van Zwet A. A., van Voorst Vader P. C., Schirm J. 2005. Comparison of COBAS AMPLICOR Neisseria gonorrhoeae PCR, including confirmation with N. gonorrhoeae-speific 16S rRNA PCR, with traditional culture. J. Clin. Microbiol. 43:1445–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martin D. H. C., et al. 2000. Multicenter evaluation of AMPLICOR and automated COBAS AMPLICOR CT/NG tests for Neisseria gonorrhoeae. J. Clin. Microbiol. 38:3544–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Munson E., et al. 2007. Evaluation of Gen-Probe APTIMA-based Neisseria gonorrhoeae and Chlamydia trachomatis confirmatory testing in a metropolitan setting of high disease prevalence. J. Clin. Microbiol. 45:2793–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Newman L. M., Moran J. S., Workowski K. A. 2007. Update on the management of gonorrhea in adults in the United States. Clin. Infect. Dis. 44(Suppl. 3):S84–S101 [DOI] [PubMed] [Google Scholar]
- 14. Nsuami M., Cammarata C. L., Brooks B. N., Taylor S. N., Martin D. H. 2004. Chlamydia and gonorrhea co-occurrence in a high school population. Sex. Transm. Dis. 31:424–427 [DOI] [PubMed] [Google Scholar]
- 15. Schachter J., Moncada J., Liska S., Shayevich C., Klausner J. D. 2008. Nucleic acid amplification tests in the diagnosis of Chlamydia and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex. Transm. Dis. 35:637–642 [DOI] [PubMed] [Google Scholar]
- 16. van Bergen J. E., et al. 2006. Population prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae in the Netherlands. Should asymptomatic persons be tested during population-based Chlamydia screening also for gonorrhoea or only if chlamydial infection is found? BMC Infect. Dis. 6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Der Pol B., et al. 2001. Enhancing the specificity of the COBAS AMPLICOR CT/NG tests for Neisseria gonorrhoeae by retesting specimens with equivocal results. J. Clin. Microbiol. 39:3092–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Der Pol B. D., et al. 2001. Multicenter evaluation of the BDProbeTec ET system for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine specimens, female endocervical swabs, and male urethral swabs. J. Clin. Microbiol. 39:1008–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Whiley D. M., Tapsall J. W., Sloots T. P. 2006. Nucleic acid amplication testing for Neisseria gonorrhoeae. J. Mol. Diagn. 8:3–15 [DOI] [PMC free article] [PubMed] [Google Scholar]