Abstract
Light-emitting diode fluorescence microscopy is being scaled up for tuberculosis control, but fading of auramine-stained slides could compromise external quality assurance. We stored auramine-stained slides and reexamined them over time. Slides stored in all environments faded quickly, with significant changes in the proportion of positive slides in as little as 1 week.
TEXT
Fluorescence microscopy (FM) can increase sensitivity and decrease the time required for sputum smear examination compared to Ziehl-Neelsen (ZN) staining and light microscopy (6). Low-cost, robust light-emitting diode (LED) microscopes have facilitated the expansion of FM into low-resource settings (2, 3), and in 2010, the World Health Organization (WHO) recommended that LED FM be phased in to replace ZN microscopy for tuberculosis (TB) diagnosis (9). As part of FM expansion, a system for external quality assurance (EQA) is needed and is currently being developed (9). While many EQA programs for ZN microscopy involve saving a sample of ZN-stained smears for blinded rereading by a centralized reference laboratory (1, 8), fluorochrome-based stains fade over time (4). However, there is a wide spectrum of opinion regarding the rate of fading and its effect on the sensitivity of fluorochrome-stained smears. To generate evidence for the development of EQA strategies for LED FM, we examined the effect of storing auramine-stained slides at different temperatures and in different environments on the concordance of smear readings over time.
All of the specimens submitted for mycobacterial diagnostics at Hinduja Hospital and Medical Research Centre, Mumbai, India, had an extra slide prepared and stained with auramine O-KMnO4 (HiMedia, Mumbai, India). These smears were examined by two independent microscopists using a Lumin LED objective lens attachment (LW Scientific) in conjunction with an LED FM evaluation (5). A subset of slides was selected for storage and inclusion in this study. The sample was enriched with low-positive smears as follows: approximately 25% scanty, 25% 1+, 25% 2+, 15% 3+, and 10% negative. All included smears had been identically scored by the two microscopists on initial reading. A total of 330 slides were allocated to one of four storage environments, darkness at room temperature (22°C), darkness in a humidified incubator (30°C), darkness in a refrigerator (4°C), and light at room temperature (22°C). Sixty slides in each of the 3 dark environments (closed slide boxes) were read monthly, 30 additional slides in all 4 environments were read weekly, and 30 slides were stored in the dark at room temperature and read once after 3 months. All slides were reread by both original microscopists during routine daily work and recorded as negative, scant, 1+, 2+, or 3+. Blinding, reassortment, and restorage were performed by an unblinded researcher not involved in smear reading. Smear examination results were dichotomized as positive/negative, with smears read as scanty, 1+, 2+, or 3+ considered positive.
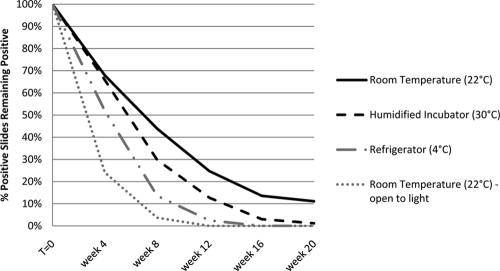
In all of the storage environments, there was a rapid increase in the proportion of slides that were read as negative over time (Fig. 1). When considering slides originally read as positive, the proportion continuing to be read as positive was significantly lower for all of the storage environments by the first rereading, regardless of the reading schedule (P < 0.05, paired two-tailed Fisher exact tests). Thus, even slides read 1 week after staining had faded significantly. The proportion of originally positive slides remaining positive dropped to <50% by 8 weeks for all of the storage environments. Slides that were exposed to light faded the quickest, with only 24% of the originally positive slides remaining positive at 4 weeks and none positive at 10 weeks.
Fig. 1.
Fading of auramine-stained smears. Shown is the proportion of initially positive slides that continued to be read as positive over time after storage in different environments. Slides undergoing weekly and monthly readings were combined.
Slides stored in the dark at 22°C remained positive the longest. Contrary to expectations, storage at 4°C did not prevent or delay fading. In fact, slides stored at 4°C faded sooner than those stored at 30°C in a humidity-enriched environment. The explanation for this is not clear. Perhaps condensation accumulated on the cold smears after removal from the refrigerator, and this direct contact with moisture accelerated fading more than constant increased ambient humidity did. It is also possible that the fluorochrome stain is less efficient at light production at a lower temperature, although the smears were allowed to warm to room temperature before being examined.
There were no statistically significant differences in the rate of fading between slides read at weekly, monthly, or quarterly intervals, suggesting that the process of briefly exposing slides to light for intermittent examinations did not have a large effect on fading compared to the duration of storage itself.
Slides which were strongly positive (2+ or 3+) tended to remain positive for a longer time than weakly positive (scant or 1+) slides (Table 1). Nevertheless, even when only 3+ slides were considered, only 36% remained positive at 3 months.
Table 1.
Proportions of slides remaining positive by original slide readings
| Time (mo) or parameter | % of slides remaining positive after original readinga (no. of slides) of: |
|||
|---|---|---|---|---|
| 3+ (53) | 2+ (71) | 1+ (78) | Scant (69) | |
| 0 | 100 | 100 | 100 | 100 |
| 1 | 83 | 64 | 45 | 42 |
| 2 | 61 | 27 | 14 | 14 |
| 3 | 36 | 11 | 6 | 2 |
| 4 | 17 | 3 | 1 | 3 |
| 5 | 14 | 2 | 1 | 0 |
| Linear regression slopeb | −0.19 (−0.21, −0.17) | −0.24 (−0.30, −0.18) | −0.25 (−0.34, −0.17) | −0.26 (−0.35, −0.17) |
All storage environments combined; monthly readings combined with respective weekly readings at 4 weeks, 8 weeks, etc.
Intercept forced through 1.0. Slopes indicate the change in the proportion of positive slides per month; negative values denote decreasing proportions remaining positive. The values in parentheses represent 95% confidence intervals.
Using linear least-squares regression, the proportion of positive smears that faded to negative was 21%/month (95% confidence interval [CI], 21 to 17%) for slides stored at room temperature, 23%/month (95% CI, 28 to 19%) for slides stored in the humidified incubator, and 26%/month (95% CI, 33-18%) for slides stored in the refrigerator. All slopes were significantly greater than null but not significantly different from one another. Performing similar regression analysis, we found that slides with high bacillary burdens faded the most slowly and paucibacillary slides faded more quickly (Table 1).
We enriched our sample with low positives in an effort to target those most at risk of being missed during rereading and to detect small amounts of fading. However, given the large effect we observed, this likely exaggerated our results compared to what would be seen in a normal sample. Not only would these paucibacillary slides fade faster and lose their positive signal more quickly, but their readings are inherently less reproducible, which would lead to some regression to the mean. While blinded rechecking reflects the practice of many ZN EQA programs, there is inherent subjectivity involved since the readings will vary depending on the fields examined and the care taken to quantify acid-fast bacilli, even without a fading effect. However, while these factors are likely to add variability to the readings over time, they should not affect overall trends.
Due to resource constraints, we unfortunately were not able to compare the concordance of reading stored slides after restaining. It has been advocated that routine restaining be performed before EQA controllers' examinations, even for ZN smears due to the (less significant) fading of ZN-stained slides, especially in warm, humid environments (7, 8). Many programs have not adopted this practice, however, citing the increased costs and resources required for already strained centralized laboratories. Appropriate and feasible EQA procedures are needed for TB laboratories planning to implement LED FM, and this evidence should inform new EQA guidelines that are being developed.
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research (CIHR MOP-88918) and the European Commission (TBSusgent, EU-FP7). Madhukar Pai was supported by a CIHR New Investigator Award, Dick Menzies was supported by a Chercheur-national award of the Fonds de Recherche en Santé de Quebec, and Jessica Minion was supported by a Quebec Respiratory Health Training Fellowship.
We acknowledge the National Health and Education Centre, Hinduja Hospital.
None of the supporting agencies were involved in the conduct or review of this study or the decision to publish its results.
Footnotes
Published ahead of print on 23 March 2011.
REFERENCES
- 1. Gilpin C., Kim S. J., Lumb R., Rieder H. L., Van Deun A. 2007. Critical appraisal of current recommendations and practices for tuberculosis sputum smear microscopy. Int. J. Tuberc. Lung Dis. 11:946–952 [PubMed] [Google Scholar]
- 2. Hänscheid T. 2008. The future looks bright: low-cost fluorescent microscopes for detection of Mycobacterium tuberculosis and Coccidiae. Trans. R. Soc. Trop. Med. Hyg. 102:520–521 [DOI] [PubMed] [Google Scholar]
- 3. Minion J., Sohn H., Pai M. 2009. Light-emitting diode technologies for TB diagnosis: what is on the market? Expert Rev. Med. Devices 6:341–345 [DOI] [PubMed] [Google Scholar]
- 4. Sanborn W. R., Heuck C. C., El Aouad R., Storch W. B. 2005. Fluorescence microscopy for disease diagnosis and environmental monitoring. World Health Organization Regional Office for the Eastern Mediterranean, Cairo, Egypt [Google Scholar]
- 5. Shenai S., et al. 2011. Evaluation of light emitting diode-based fluorescence microscopy for the detection of mycobacteria in a tuberculosis-endemic region. Int. J. Tuberc. Lung Dis. 15:483–488 [DOI] [PubMed] [Google Scholar]
- 6. Steingart K. R., et al. 2006. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect. Dis. 6:570–581 [DOI] [PubMed] [Google Scholar]
- 7. Van Deun A., Chambugonj N., Hye A., Hossain A. 1997. Rapid fading of carbolfuchsin stained AFB smears under extreme conditions of temperature and humidity. Int. J. Tuberc. Lung Dis. 1:384–385 [PubMed] [Google Scholar]
- 8. Van Deun A., Portaels F. 1998. Limitations and requirements for quality control of sputum smear microscopy for acid-fast bacilli. Int. J. Tuberc. Lung Dis. 2:756–765 [PubMed] [Google Scholar]
- 9. World Health Organization 2010. Fluorescent light emitting diode (LED) microscopy for diagnosis of tuberculosis: policy statement. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/dots/laboratory/who_policy_led_microscopy_july10.pdf [PubMed] [Google Scholar]