Abstract
Molecular DNA-based diagnostics are increasingly being used for diagnosis of viral infections. For enteric viruses, PCR assays have also been developed. The aims of this study were to compile and evaluate a comprehensive panel of PCR assays for diagnosis of viruses causing diarrheal disease and to evaluate its use in a largely pediatric population in a 750-bed university medical center. The PCR panel was designed to include assays for detection of adenovirus, astrovirus, enterovirus, norovirus, parechovirus, rotavirus, and sapovirus. The results of the PCR panel were evaluated in relation to conventional viral diagnostics consisting of viral culture and/or rotavirus and adenovirus rapid antigen tests on samples that were taken for routine diagnostics. Comparing conventional with PCR-based testing, the number of viruses detected increased dramatically from 25 to 106 when PCR assays were used. This increase was due mainly to detection of previously undetected viruses, i.e., astrovirus, norovirus, and sapovirus. In 24% of the samples, norovirus was detected. Also, the lower detection limit of PCR-based adenovirus, enterovirus, parechovirus, and rotavirus diagnostics further increased the detection rate. By focusing on samples from patients with complaints of gastroenteritis, detection of a causative agent was increased from 49% by conventional tests to 97% by molecular diagnostics. However, many samples containing low viral loads were found in patients with complaints other than intestinal complaints. In conclusion, the proposed comprehensive PCR panel with appropriate cutoff values can be used for sensitive, rapid, and clinically relevant diagnosis of gastrointestinal viruses.
INTRODUCTION
Diarrheal disease is one of the main worldwide causes of morbidity and mortality. Globally, an estimated 2.5 million children die from these illnesses each year, despite increasing knowledge of pathogenesis and advances in treatment strategies. Viral infections are the most common causative agents of diarrheal disease, with rotavirus alone being responsible for 60% of all diarrheal episodes in developing countries and 40% in developed countries (11, 17, 19). Traditionally, viral culture, electron microscopy, and rapid latex agglutination tests have been used in diarrheal disease to detect viral pathogens, mainly rotavirus, adenovirus, and enteroviruses. Still, these detection methods remain to be the gold standard today, although earlier studies have indicated an explosive increase in detection rates by using molecular PCR-based assays (9, 15). Additionally, in recent years, due largely to the use of new molecular detection technologies, the role of other (mostly difficult-to-culture) viruses, such as astrovirus, norovirus, and sapovirus, is becoming more apparent (10). Norovirus is now widely established as a leading cause of gastroenteritis, especially among adults (3). Also, astrovirus and sapovirus are being found mainly in children with gastroenteritis (2, 13), although questions remain on the exact role these viruses play in causing (outbreaks of) gastroenteritis. There are also conflicting data on the role of enterovirus and parechovirus in gastroenteritis. For example, Tapia et al. found that in a 24-month follow-up study of 102 children from birth, 86% of those children excreted parechovirus in at least 1 of 24 monthly fecal samples (18).
The goal of our study was to design and evaluate a broad panel of real-time PCR tests for viruses involved in diarrheal disease. For this purpose, we have compiled a comprehensive panel of real-time PCR-based tests, including adenovirus, astrovirus, enteroviruses, noroviruses (genogroups I [GI] and II [GII]), parechovirus, rotavirus, and sapovirus. The tests were then optimized to fit a generic PCR protocol, and finally the new panel was evaluated on 100 clinical fecal samples and compared to routine clinical diagnostics (fecal viral culture and/or rapid antigen testing [RAT]).
MATERIALS AND METHODS
Clinical samples.
In February and March 2009, a total of 100 fecal samples, all submitted for conventional viral diagnosis, were included in the study. These samples were from 82 patients admitted to Maastricht University Medical Center, a tertiary 750-bed hospital. The majority of the samples (n = 79) were from patients (n = 61) under 5 years of age, 5 patients (n = 5 samples) were between 5 and 10 years old, 4 patients (n = 4 samples) were between 10 and 20 years old, and 12 patients (n = 12 samples) were older than 20 years old.
By retrospective chart review, we collected where possible clinical data from the patients and determined the clinical diagnosis. Data were than categorized in the following groups: (i) gastroenteritis; (ii) intestinal complaints other than gastroenteritis, such as lactose intolerance; (iii) other clinical syndromes, e.g., respiratory tract infection or meningitis; and (iv) no data available.
Routine clinical viral diagnostics.
Care as usual generally included rotavirus and adenovirus rapid antigen tests (RATs) (Rota/Adeno Combistick, Novamed, Israel) and viral culture. Fifty micrograms or 50 μl of fecal sample was suspended in dilution buffer. Subsequently, RAT was performed according to the manufacturer's instructions.
Approximately 0.5 g of fecal sample was suspended in 4 ml virus transport medium (Eagle's minimal essential medium [EMEM]; Gibco/Invitrogen, Breda, Netherlands) supplied with 0.1% gelatin, 250 μg/ml vancomycin, 75 μg/ml gentamicin, 5 μg/ml amphotericin B, 2 mM l-glutamine, and 0.1 mM nonessential amino acids (NEAA; Gibco). The fecal sample was divided into three tubes and centrifuged for 1 min at 9,000 × g, followed by filtration (0.2-μm pores) to eliminate fecal bacteria and other large particles. The remaining suspension was used for viral culture on HEp2, MRC5, Vero, and LLc-MK2 cells. Microscopic evaluation of the viral shell culture was performed 1 day after inoculation, after 3, 7, 10, and 14 days. Staining of the suspected viral culture with monoclonal fluorescent antibodies was performed when specific cytopathogenic effects were seen (6).
Molecular diagnostics.
For DNA and RNA isolation, 1 g of fecal sample was homogenized using the MagNA Lyser (Roche Diagnostics), MagNA Lyser green beads, and MagNA Pure LC RNA isolation tissue lysis buffer. In brief, 1 g of fecal sample was added to 400 μl lysis buffer in tubes containing green beads. Consequently, the sample was lysed for 90 s at 6,000 rpm. Afterward, the tubes were centrifuged for 2 min at 16,000 × g, and 100 μl of the supernatant was mixed with 1 ml of MagNA Pure LC RNA isolation tissue lysis buffer. After 10 min of incubation at room temperature, 200 μl was used for consequent nucleic acid extraction on the MagNA Pure LC. For this, the MagNA Pure total NA extraction kit (Roche Diagnostics) was used according to the manufacturer's instructions. Final elution of the nucleic acids occurred in 60 μl. Real-time PCR was performed using primers and probes described in Table 1. For sapovirus, the PCR assay included the use of two forward primers and four probes to cover the sapoviral heterogeneity (10). For parechovirus, mutations, compared to the published probe sequence, were found in several patient samples. For this reason, an additional degenerative probe was designed as part of this study. Before extraction, all samples were spiked with murine cytomegalovirus (CMV) RNA, which was used as an extraction and amplification control. A separate reverse transcription (RT) step was performed using TaqMan reverse transcriptase reagents by using random hexamers (Applied Biosystems, Foster City, CA) and consisted of 10 min at 25°C, 30 min at 48°C, and finally 5 min at 95°C. Consequently, the PCR mix consisted of 20 μl isolated DNA, primers and probes, and 1× Absolute quantitative PCR mix (Abgene, Epsom, United Kingdom). The PCR protocol consisted of 15 min at 95°C, followed by 42 cycles of 15 s at 95°C and 1 min at 60°C. All qualitative real-time PCRs were performed using an ABI prism 7900HT system (Applied Biosystems), and threshold cycle (CT) values were determined using a threshold value of 0.05 and automatic baselining. The quality of the assays was ensured by positive and negative controls as well as a test on amplification inhibition in each sample by an external amplification control. As positive controls, positive cultures (for enterovirus and parechovirus) or RNA controls were used. Artificial RNA controls were constructed using pGEM-3Z vectors containing T7 RNA polymerase promoters flanking the multiple cloning region (Promega, Leiden, Netherlands), into which the respective amplicons were cloned. Using T7 RNA polymerase, RNA constructs containing the amplicons were generated and used as artificial RNA controls. Detection limits of the different assays were determined by serial dilution of positive controls. At least 10 independent dilution series were used to determine the detection probability (8). Detection limits were defined at a detection probability of at least 80%.
Table 1.
Primers and probes used in the comprehensive panel of real-time PCR tests
| Target | Primer/probe sequence (5′→3′)a | Reference |
|---|---|---|
| Adenovirus | GCCCCAGTGGTCTTACATGCACATC | 5 |
| GCCACGGTGGGGTTTCTAAACTT | 5 | |
| FAM-TGCACCAGACCCGGGCTCAGGTACTCCGA-TAMRA | 5 | |
| Astrovirus | TCTYATAGACCGYATTATTGG | 21 |
| TCAAATTCTACATCATCACCAA | 21 | |
| FAM-CCCCADCCATCATCATCTTCATCA-BQ1 | 21 | |
| Enterovirus | CCCTGAATGCGGCTAATCC | 12 |
| ATTGTCACCATAAGCAGCCA | 12 | |
| FAM-AACCGACTACTTTGGGTGTCCGTGTTTC-BQ1 | 12 | |
| Norovirus GI | CGYTGGATGCGNTTYCATGA | 21 |
| CCTTAGACGCCATCATCATTTAC | 21 | |
| FAM-TYGCGRTCTCCTGTCCA-MGBNFQ | 21 | |
| Norovirus GII | CARGARBCNATGTTYAGRTGGATGAG | 21 |
| TCGACGCCATCTTCATTCACA | 21 | |
| VIC-AGATYGCGATCSCCCTC-MGBNFQ | 21 | |
| Parechovirus | CACTAGTTGTAAGGCCCACGAA | 20 |
| GGCCCCAGATCAGATCCA | 20 | |
| JOE-CAGTGTCTCTTGTTACCTGCGGGTACCTTCT-BQ1 | 20 | |
| NED-YAGTGTCTCTTGTTACCTRCRGGTACCTYCT-BQ1 | This study | |
| Rotavirus | ACCATCTTCACGTAACCCTC | 15 |
| ACCATCTACACATGACCCTC (2nd forward primer) | 15 | |
| CACATAACGCCCCTATAGCC | 15 | |
| FAM-ATGAGCACAATAGTTAAAAGCTAACACTGTTCAA-BQ1 | 15 | |
| Sapovirus | ACCAGGCTCTCGCCACCTA | 10 |
| ATTTGGCCCTCGCCACCTA (2nd forward primer) | 10 | |
| GCCCTCCATYTCAAACACTAWTTT | 10 | |
| FAM-CTGTACCACCTATGAACCA-TAMRA | 10 | |
| FAM-TTGTACCACCTATGAACCA-TAMRA | 10 | |
| FAM-TGTACCACCTATAAACCA-TAMRA | 10 | |
| FAM-TGCACCACCTATGAAC-TAMRA | 10 |
FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine; BQ1, black hole quencher 1; MGBNFQ, minor groove binding nonfluorescent quencher; VIC, fluorescent label (Applied Biosystems); JOE, 6-carboxy-4,5-dichloro-2,7-dimethyoxyfluorescein; NED, fluorescent-label (Applied Biosystems).
Samples with a positive adenovirus PCR with a CT value of <28 were submitted to genotyping. First of all, a real-time PCR using SYBR green as described by Echavarria et al. was performed (1). After PCR, amplicons were purified using PCRapace spin columns (Invitek GmbH, Berlin, Germany). Subsequently, sequence reactions were performed using 0.2 μM each primer, 1 μl of 1.1 BigDye Terminator reaction mix (Applied Biosystems, Carlsbad, CA), 1× accompanying sequencing buffer, and 1 μl of purified amplicon. Readout of the sequences was performed on a model 3130 DNA sequencer (Applied Biosystems). Finally, sequences were analyzed using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Statistics.
For comparison of the proportions in different patient groups, the chi-square test was used. For comparison of CT values, the Mann-Whitney test was used.
RESULTS
Test panel characteristics.
Several previously described real-time RT-PCRs were combined to form a comprehensive panel of tests for detection of viruses involved in diarrheal disease. The different assays were adjusted and optimized to fit one generic PCR protocol. Imposing a detection probability of at least 80%, the detection limits were 100 copies/ml for adenovirus, 9.3 × 103 copies/ml for astrovirus, 625 copies/ml for enterovirus, 750 copies/ml for norovirus GI, 1.5 × 103 copies/ml for norovirus GII, 500 copies/ml for parechovirus, 750 copies/ml for rotavirus, and 4.7 × 103 copies/ml for sapovirus. The specificities of the different PCRs were tested within the current panel of targets, where no cross-reactivity was seen between any of the species (data not shown). However, in 5 samples, we found an unspecific signal (CT values of 37 to 39) in the norovirus GI PCR when a high viral load was found in the norovirus GII PCR (CT value 16 to 30).
Overview of conventional testing versus molecular testing.
Conventional diagnostics of viruses causing gastrointestinal complaints, consisting of viral culture (n = 16 samples), rapid antigen testing (RAT) for the presence of rotavirus and adenovirus (n = 2 samples), or viral culture and RAT (n = 82 samples), were compared to molecular diagnostics (n = 100 samples) (Fig. 1). Results showed that in 100 patient fecal samples, 25 viruses were detected in 25 samples with conventional testing, while with molecular testing 106 viruses were detected in 70 samples. Of the 81 extra viruses found with molecular diagnostics, 27 were either rotavirus, adenovirus, or enterovirus, i.e., viruses that can be detected by conventional testing. In two cases, rotavirus was missed by conventional testing, as no RAT was performed. Of the 70 samples in which viruses were detected with molecular diagnostics, 42 samples contained a single virus, whereas 28 samples contained multiple viruses, of which 21 contained 2 viruses, 6 contained 3 viruses, and 1 contained 4 viruses.
Fig. 1.
Overview of results from conventional testing compared to those of the panel of real-time PCRs. Conventional testing consisted of a rapid antigen test for adenovirus and rotavirus and/or viral culture.
Including clinical data in the analysis of the PCR result, we found that 39 samples were from gastroenteritis patients (n = 33), of which 38 samples (97.4%) contained a virus. Conventional testing on those 39 samples (including RAT on all 39 samples and culture on 38 samples) found a virus in 19 samples (49%). In 50 samples from patients that suffered from other complaints, 27 (54%) contained at least one virus (chi-square value of <0.0001). There was no significant correlation between any of the viruses with patients that suffered from other complaints.
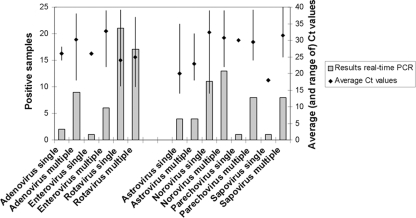
The range of the CT values found for the different viruses varied greatly between 16 and 40. Average CT values and the range of CT values per virus are shown in Fig. 2. This figure also compares the range of CT values found in samples that contain one virus to samples that contained multiple viruses. No large differences were found, but trends were seen toward lower CT values in the samples that contained only one virus.
Fig. 2.
Comparison of the number of viruses found by real-time PCR in samples where only one virus was detected (single) and in those samples where multiple viruses were found (multiple). On the second y axis, the average CT values found are represented by diamonds, and the lines represent the range of CT values found within each group.
Rotavirus diagnosis.
Rotavirus is the most frequently found virus in our collection of fecal samples, with 38 samples containing this pathogen, as determined by real-time PCR. Rotavirus was positively identified in only 19 samples by RAT (in 2 samples where PCR was positive, RAT was not performed). The results showed clearly that in a large part of the samples (10 out of 17) that were PCR positive and RAT negative, the CT values were high (≥30), indicating a low viral load. However, results also indicated that 7 out of 17 of these samples had CT values that fell within the range (19 to 29) of the samples that are commonly detected by RAT. Importantly, results showed that within this group, the four patients where rotavirus was the only agent found all had clinical symptoms of acute gastroenteritis (Fig. 3). Viral loads in gastroenteritis patients were significantly higher than in patients suffering from other clinical conditions (P = 0.007).
Fig. 3.
Comparison of clinical symptoms of patients who were PCR positive for rotavirus and those who had either rotavirus RAT-positive detection or rotavirus RAT-negative detection. The latter group is furthermore divided into those with a low CT value (higher rotavirus load) and high CT values (lower rotavirus loads). In this figure, only data of patients in which rotavirus was the only virus detected by any method have been included.
Comparison of viral culture and PCR testing.
In only six of the 98 cultured samples, a virus was cultured: one sample showed enterovirus, and five samples showed adenovirus. The enterovirus culture-positive sample was also found positive for enterovirus by PCR (CT value of 26). In this sample, no other viruses were detected, while in the additional six samples PCR positive for enterovirus (CT value range of 22 to 39), more viruses were detected. Unfortunately, clinical data from the culture-positive patient were not available. The culture-negative, PCR-positive samples were from six patients. Two of them experienced gastroenteritis and were coinfected with rotavirus. Three patients had neurological symptoms, either febrile convulsions or meningitis, and one patient was admitted to the hospital with respiratory complaints. The other four patients were coinfected with either rotavirus, parechovirus, adenovirus, sapovirus, or astrovirus.
In five samples, adenovirus was cultured, four of which were found positive by PCR (CT value range of 18 to 29); the PCR-negative sample was positive in the adenovirus RAT but remained negative with a second adenovirus PCR (1). The five adenovirus culture-positive samples were from three gastroenteritis patients, one patient with respiratory problems, and one patient suffering from lactose intolerance. In addition, seven culture-negative samples (originating from seven patients) were PCR positive for adenovirus, with CT values ranging from 24 to 38. The two samples with the lowest CT values (24 and 26) were samples in which adenovirus was the only virus detected; in the five other samples, multiple viruses were detected by PCR (one sample, in addition to adenovirus, contained rotavirus, one norovirus, one sapovirus, and one enterovirus, and one sample contained, in addition to adenovirus, sapovirus, rotavirus, and enterovirus). In the other adenovirus PCR-positive samples (CT values of 33 to 38), one to three other viruses were detected. One sample was from a patient with gastroenteritis, coinfected with sapovirus. From the other five PCR-positive samples, three were from patients with convulsions and respiratory problems, one was from a patient whose brother had an adenovirus respiratory infection, and one was from a patient who suffered from hyper-IgM syndrome and was evaluated for fever with increased inflammatory parameters. Adenovirus typing of the six samples with the lowest CT values showed that one sample from a gastroenteritis patient belonged to genogroup F (to which the gastroenteritis-causing adenovirus 40/41 belongs). The other adenoviruses belong to other genogroups, i.e., B and C.
Diagnosis of nonculturable viruses.
A remarkable finding was that norovirus was found in 24 samples (24%). In 11 samples, norovirus was the only virus detected, and in these samples CT values range from 16 to 27 for the genogroup II (n = 4 samples) PCR and from 37 to 39 for genogroup I (n = 7 samples). We found one sample with a high viral load for genogroup I (CT value of 22) (this sample also contained rotavirus and parechovirus). All nine other norovirus genogroup I-positive samples contained low viral loads, with CT values between 38 and 39. The 15 samples containing norovirus genogroup II (from 12 patients) were from 11 gastroenteritis patients and from one patient with neutropenia and diarrhea for which no cause was found in conventional diagnostics. In eight samples (originating from eight patients), low viral loads for norovirus genogroup I (CT values of 38 to 39) were not found in patients with clinical signs of gastroenteritis. When comparing the patients' clinical symptoms with the CT values of the PCRs for norovirus (either genogroup I or II), a statistically significant difference (P = 0.004) was found between gastroenteritis patients and patients without gastroenteritis.
Astrovirus and sapovirus were found in 8 and 9 samples, respectively, with CT values ranging from 14 to 35 and from 18 to 40. Astrovirus was found in 4 samples in combination with other viruses (CT values ranging from 14 to 32). In 3 samples from one patient with gastroenteritis, astrovirus was the only virus detected, with viral loads (CT values) between 14 and 16.
The majority of the samples that were PCR positive for sapovirus contained other viruses, except for one sample, the sample with the highest viral load (CT value of 18), which originated from a patient with diarrhea and underlying vasculitis syndrome. Finally, for parechovirus, nine samples were found positive, but only in one sample was parechovirus found to be the only virus detected. However, from this patient, three samples were analyzed in a 5-week period and all contained parechovirus. When comparing the patients' clinical symptoms with the CT values of the PCRs for parechovirus, astrovirus, and sapovirus, no significant differences were seen (P values were, respectively, 1.00, 0.43, and 0.38).
DISCUSSION
In many different areas of clinical microbiology, molecular diagnostics have been introduced in recent years. The main reasons for introduction of molecular diagnostics are the short time to results and the possibility to detect unculturable or difficult-to-culture viruses. Also in the field of viral gastroenteritis, more molecular diagnostic assays have been published for detection of causative viruses (10, 15, 20). Nonetheless, few reports have focused on the impact of replacing viral culture and rapid antigen testing by a panel of PCR tests.
In this study, we optimized a comprehensive panel of PCR assays to fit one generic PCR protocol. Due to the generic protocol, the assays can be easily run simultaneously in one PCR run, and the generic protocol facilitates multiplexing of targets as well. After optimization of the assay, the panel was evaluated and compared to our in-house routine of fecal diagnostics, consisting of viral culture and rapid antigen testing.
The main trend seen in this study is that by replacing conventional diagnostics by real-time PCRs, a dramatic increase in detection of viruses was found in the same set of samples. Previous studies comparing PCRs for smaller panels of enteric viruses or panels of respiratory viruses to conventional diagnostics had also shown a large increase in the number of detected viruses by PCR (9, 10, 14). The main reasons more viruses are detected by molecular diagnostics are the detection of nonculturable viruses (which could not be detected by the conventional methods we used), responsible for nearly two-thirds of the extra viruses found, and the lower detection limit. This was demonstrated for rotavirus, for example, where all samples with a CT value higher than 28 were negative by RAT (Fig. 3). During this study, the CT value measured during real-time PCR analysis was used as an indication of the viral load found in the sample. Although real-time PCR allows for the determination of a more exact viral load (in copies or genome equivalents), this was not determined in this study, as factors such as the consistency and water content of the fecal sample influence the viral load, for which no correction was included in this trial.
Current infection control measures, such as isolation measures or cohorting of patients in pediatric wards, regarding gastrointestinal viruses are based on clinical symptoms but have been influenced by results from conventional testing. With a dramatic increase noted in the number of positive samples and viruses found, the question arises what the clinical impact of these new findings is. Transmission of viruses in asymptomatic or subclinical patients may play a role on pediatric wards, where compliance with hand hygiene protocols is of great importance. Except for rotavirus and norovirus, viral loads did not differ in gastroenteritis patients compared to those of the other groups. For rotavirus, 20 to 40% of the infections are subclinical and therefore account for substantial nosocomial rotavirus infections (reviewed in reference 4). Nosocomial transmission of rotavirus occurs via infected hands of health care workers that take care of gastroenteritis patients (∼70% infection rate) or via infected surfaces (4). Better compliance with hand hygiene protocols, also in asymptomatic patients, is important to decrease transmission.
Looking at rotavirus (Fig. 3), it is clear that high CT values are less associated with gastroenteritis. These data are in agreement with previous studies, such as that by Kang et al., who reported that CT values for rotavirus were associated with the severity of disease as assessed by the Vesikari severity score (7). Philips et al. proposed a CT cutoff value for rotavirus real-time PCR results which is between 25 and 28 (16). Although CT values will change depending on the PCR assay used, a similar cutoff CT value of 28 based on our data of samples in which rotavirus was found (by any method) would result in a clinical sensitivity and specificity of 80% and 80%, respectively, in comparison to a specificity and sensitivity of RAT of 60% and 78%, respectively. When calculated for samples in which only rotavirus was found (by any method), the clinical sensitivity and specificity for PCR with a CT cutoff of 28 were 92% and 100%, respectively, in comparison to 62% and 100% by RAT. Although, based on our results as well as previously published studies, it seems appropriate to install a CT cutoff value for PCR results, it is clear that with its improved analytical sensitivity, real-time PCR can also improve the clinical sensitivity/specificity of rotavirus diagnostics. In future studies, when more data are available, the installation of cutoff values for PCRs other than that of rotavirus should be further investigated.
Regarding enterovirus, only one sample was detected positive for enterovirus by conventional culture, and this is most likely due to analytical sensitivity. All culture-negative samples, with one exception at a CT value of 22, had a CT value of >28. For adenovirus, the results were more complex. By RAT, two samples were found both rotavirus and adenovirus positive. In both cases, culture and repeated rotavirus and adenovirus PCRs remained negative. Although it is possible that culture and PCR gave false-negative results, it is likely that the RAT results in these cases were due to unspecific hybridization and were in this study considered false-positive results. One RAT- and culture-positive sample for adenovirus was not detected by PCR or a confirmatory PCR. Further results showed that 11 samples were found to be PCR positive and RAT negative. Of these samples, six had a CT value of <28. These samples were subjected to genotyping. The results showed that three adenoviruses belonged to species C, two to species B, and one to species F. These results mostly explain why these samples were missed by RAT, as RAT detects only species F (containing serotypes 40 and 41).
Switching to detection by real-time PCRs offers the possibility to detect four previously, by conventional testing, undetected viruses, i.e., norovirus, astrovirus, sapovirus, and parechovirus. Looking at the group of 39 samples (from 33 patients) submitted with a clinical diagnosis of gastroenteritis, by conventional testing in 49% of the cases a suspected causative viral agent was detected. By performing detection by real-time PCR, this improved to 97%, with only one sample remaining where no virus could be identified. In 19 of 39 samples, only one virus was detected in each sample, and these were either norovirus, astrovirus, or rotavirus. When results from samples submitted with a clinical diagnosis not involving gastrointestinal complaints were compared, 6% of the samples were positive by conventional testing, in comparison to 53% by real-time PCR. This can partly be explained by the fact that when no virus was found with conventional diagnostics, an incorrect diagnosis was made. Further, several viruses in our panel, such as enterovirus, adenovirus, and parechovirus, are known to also cause infections with diverse other complaints, such as respiratory complaints, sepsis-like illness, or meningitis. Another explanation for these findings can be asymptomatic or long-lasting carriage of these viruses, as has been described in other publications (7). Carriage or shedding of these viruses during a period of diarrhea may give the clinicians a false clue of a correct diagnosis. If there is another cause for the gastrointestinal complaints, the patient may be withdrawn from accurate care. However, clinical decisions are based on a combination of test results and clinical aspects and not on test results alone. For example, the study by Oosterheert et al. showed that although PCR results were available before culture results in respiratory samples, the decision to discontinue antibiotic use was not significantly changed by knowing these results (14).
Future prospective studies investigating carriage of these viruses in healthy volunteers and patients with specific complaints will shed more light on the clinical significance of our findings regarding shedding of gastrointestinal viruses and clinical relevance of astrovirus, sapovirus, and parechovirus. The current study is limited by the time frame (2 months) and sample number (n = 100) that is included in the study. Furthermore, all clinical diagnoses were derived from retrospective chart review, which limited the clinical data that were obtained. Finally, the analytical detection limit of certain PCRs was relatively high. It can be expected that with a lower detection limit, even more viruses would have been detected.
In conclusion, this study has designed a comprehensive panel of standardized real-time PCRs for detection of gastrointestinal viruses. Evaluation of the performance of the panel in comparison with conventional diagnostics, i.e., rotavirus and adenovirus RAT and viral culture, showed that for all samples, the detection of viruses by real-time PCR increased dramatically from 25 viruses by conventional methods to 106 viruses by the PCR panel. When focusing on patients with complaints of gastroenteritis, detection of a causative agent was increased from 49% by conventional diagnostics to 97% by molecular diagnostics. However, many samples containing especially low viral loads were found in patients with complaints other than intestinal complaints. Installing CT cutoff values, such as the one for rotavirus as proposed in this study, may improve the clinical sensitivity and specificity of real-time PCR detection and clear the road for sensitive, rapid, and clinically relevant diagnosis of gastrointestinal viruses.
Footnotes
Published ahead of print on 23 March 2011.
REFERENCES
- 1. Echavarria M., Forman M., Ticehurst J., Dumler J. S., Charache P. 1998. PCR method for detection of adenovirus in urine of healthy and human immunodeficiency virus-infected individuals. J. Clin. Microbiol. 36:3323–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gallimore C. I., Cubitt D. W., Richards A. F., Gray J. J. 2004. Diversity of enteric viruses detected in patients with gastroenteritis in a tertiary referral paediatric hospital. J. Med. Virol. 73:443–449 [DOI] [PubMed] [Google Scholar]
- 3. Glass R. I., Parashar U. D., Estes M. K. 2009. Norovirus gastroenteritis. N. Engl. J. Med. 361:1776–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gleizes O., et al. 2006. Nosocomial rotavirus infection in European countries: a review of the epidemiology, severity and economic burden of hospital-acquired rotavirus disease. Pediatr. Infect. Dis. J. 25:S12–S21 [DOI] [PubMed] [Google Scholar]
- 5. Heim A., Ebnet C., Harste G., Pring-Akerblom P. 2003. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J. Med. Virol. 70:228–239 [DOI] [PubMed] [Google Scholar]
- 6. Isenberg H. (ed.). 2004. Clinical microbiology procedures handbook, 2nd ed. ASM Press, Washington, DC [Google Scholar]
- 7. Kang G., et al. 2004. Quantitation of group A rotavirus by real-time reverse-transcription-polymerase chain reaction: correlation with clinical severity in children in South India. J. Med. Virol. 73:118–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knutsson R., Lofstrom C., Grage H., Hoorfar J., Radstrom P. 2002. Modeling of 5′ nuclease real-time responses for optimization of a high-throughput enrichment PCR procedure for Salmonella enterica. J. Clin. Microbiol. 40:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Logan C., O'Leary J. J., O'Sullivan N. 2006. Real-time reverse transcription-PCR for detection of rotavirus and adenovirus as causative agents of acute viral gastroenteritis in children. J. Clin. Microbiol. 44:3189–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Logan C., O'Leary J. J., O'Sullivan N. 2007. Real-time reverse transcription PCR detection of norovirus, sapovirus and astrovirus as causative agents of acute viral gastroenteritis. J. Virol. Methods 146:36–44 [DOI] [PubMed] [Google Scholar]
- 11. Lopman B. A., et al. 2003. Viral gastroenteritis outbreaks in Europe, 1995-2000. Emerg. Infect. Dis. 9:90–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Monpoeho S., et al. 2001. Best viral elution method available for quantification of enteroviruses in sludge by both cell culture and reverse transcription-PCR. Appl. Environ. Microbiol. 67:2484–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olesen B., et al. 2005. Etiology of diarrhea in young children in Denmark: a case-control study. J. Clin. Microbiol. 43:3636–3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oosterheert J. J., et al. 2005. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clin. Infect. Dis. 41:1438–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pang X. L., et al. 2004. Increased detection of rotavirus using a real time reverse transcription-polymerase chain reaction (RT-PCR) assay in stool specimens from children with diarrhea. J. Med. Virol. 72:496–501 [DOI] [PubMed] [Google Scholar]
- 16. Phillips G., et al. 2009. Diagnosing rotavirus A associated IID: using ELISA to identify a cutoff for real time RT-PCR. J. Clin. Virol. 44:242–245 [DOI] [PubMed] [Google Scholar]
- 17. Simpson R., Aliyu S., Iturriza-Gomara M., Desselberger U., Gray J. 2003. Infantile viral gastroenteritis: on the way to closing the diagnostic gap. J. Med. Virol. 70:258–262 [DOI] [PubMed] [Google Scholar]
- 18. Tapia G., et al. 2008. Longitudinal observation of parechovirus in stool samples from Norwegian infants. J. Med. Virol. 80:1835–1842 [DOI] [PubMed] [Google Scholar]
- 19. Thapar N., Sanderson I. R. 2004. Diarrhoea in children: an interface between developing and developed countries. Lancet 363:641–653 [DOI] [PubMed] [Google Scholar]
- 20. van Doornum G. J., Schutten M., Voermans J., Guldemeester G. J., Niesters H. G. 2007. Development and implementation of real-time nucleic acid amplification for the detection of enterovirus infections in comparison to rapid culture of various clinical specimens. J. Med. Virol. 79:1868–1876 [DOI] [PubMed] [Google Scholar]
- 21. van Maarseveen N. M., Wessels E., de Brouwer C. S., Vossen A. C., Claas E. C. 2010. Diagnosis of viral gastroenteritis by simultaneous detection of adenovirus group F, astrovirus, rotavirus group A, norovirus genogroups I and II, and sapovirus in two internally controlled multiplex real-time PCR assays. J. Clin. Virol. 49:205–210 [DOI] [PubMed] [Google Scholar]