Abstract
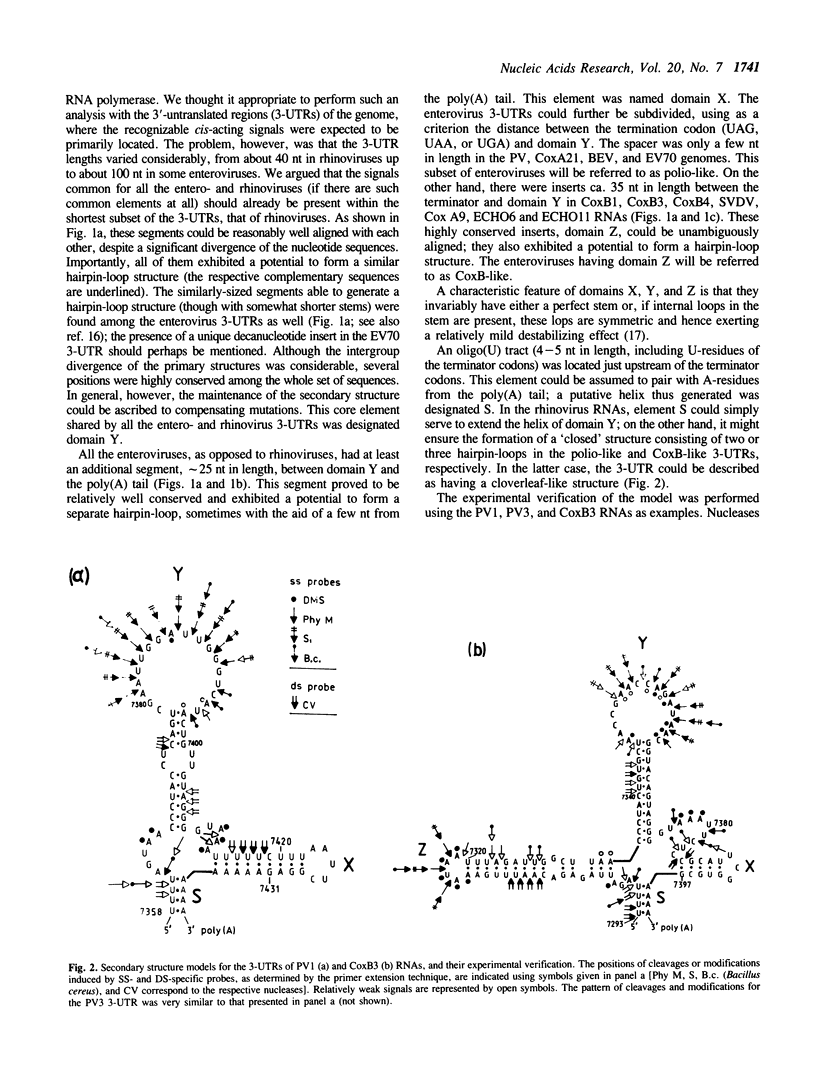
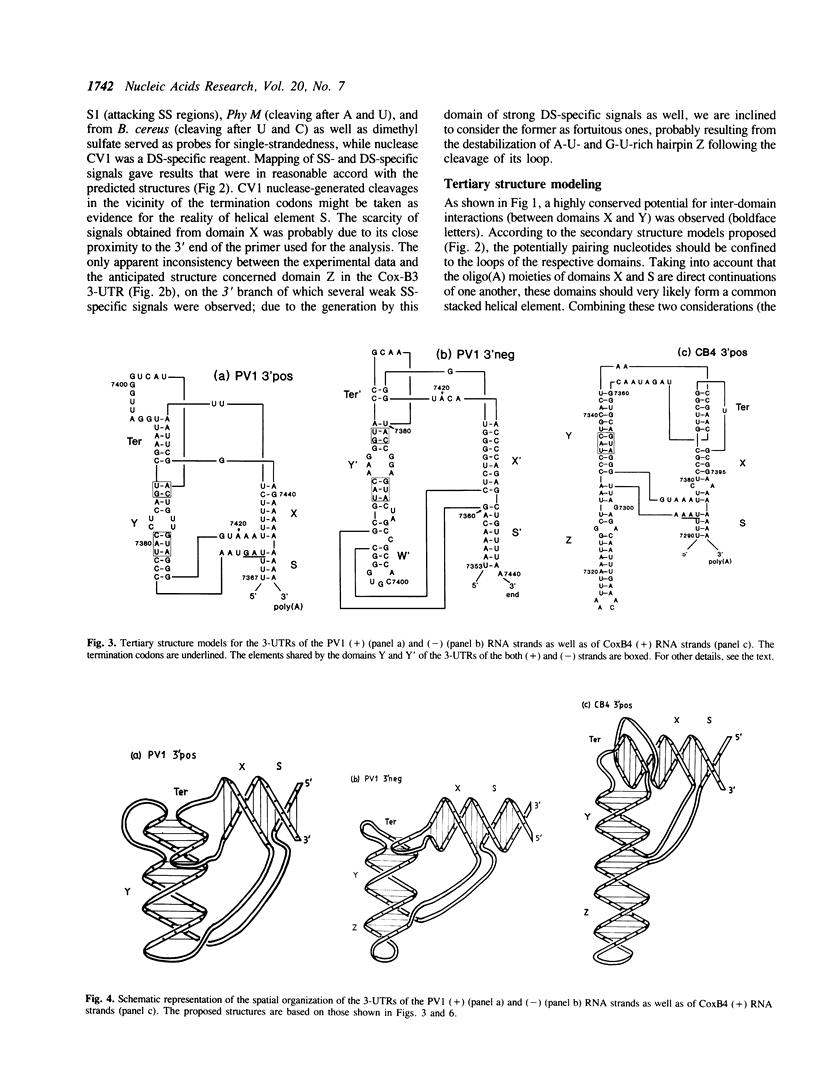
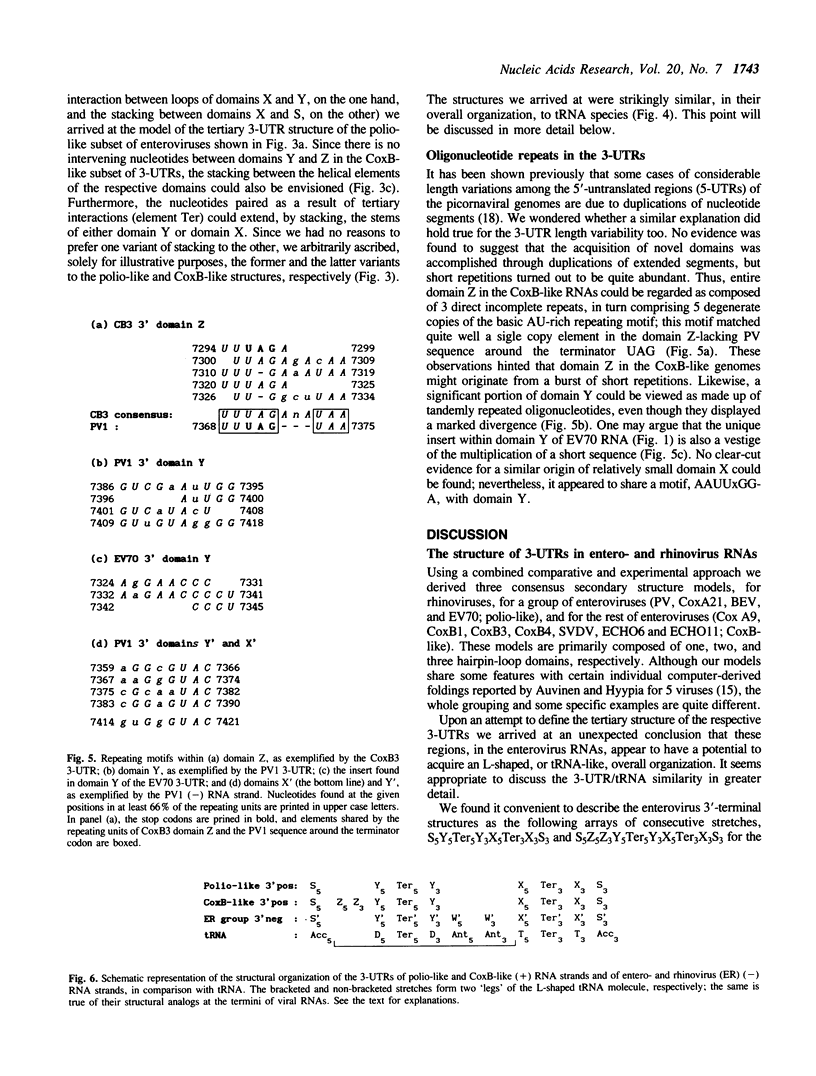
On the basis of a comparative analysis of published sequences, models for the secondary structure of the 3'-terminal [poly(A)-preceding] untranslated region of the entero- and rhinovirus RNAs were worked out. The models for all these viruses share a common core element, but there are an extra enterovirus-specific element and still an additional element characteristic of a subset of enterovirus RNAs. The two latter models were verified for poliovirus and coxsackievirus B genomes by testing with single-strand and double-strand specific enzymatic and chemical probes. A tRNA-like tertiary structure model for the 3'-terminal folding of enterovirus RNAs was proposed. A similar folding was proposed for the 3' termini of the negative RNA strands as well as for the 5' termini of the positive strand of all entero- and rhinovirus RNAs. Implications of these data for template recognition during negative and positive RNA strands synthesis and for the evolution of the picornavirus genomes are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhin M. R., Alblas J., van Duin J. Secondary structure at the 3' terminal region of RNA coliphages: comparison with tRNA. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):110–118. doi: 10.1016/0167-4781(90)90150-z. [DOI] [PubMed] [Google Scholar]
- Andino R., Rieckhof G. E., Baltimore D. A functional ribonucleoprotein complex forms around the 5' end of poliovirus RNA. Cell. 1990 Oct 19;63(2):369–380. doi: 10.1016/0092-8674(90)90170-j. [DOI] [PubMed] [Google Scholar]
- Auvinen P., Hyypiä T. Echoviruses include genetically distinct serotypes. J Gen Virol. 1990 Sep;71(Pt 9):2133–2139. doi: 10.1099/0022-1317-71-9-2133. [DOI] [PubMed] [Google Scholar]
- Auvinen P., Stanway G., Hyypiä T. Genetic diversity of enterovirus subgroups. Arch Virol. 1989;104(3-4):175–186. doi: 10.1007/BF01315541. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Carmichael G. G. RNA replication: function and structure of Qbeta-replicase. Annu Rev Biochem. 1979;48:525–548. doi: 10.1146/annurev.bi.48.070179.002521. [DOI] [PubMed] [Google Scholar]
- Chang K. H., Auvinen P., Hyypiä T., Stanway G. The nucleotide sequence of coxsackievirus A9; implications for receptor binding and enterovirus classification. J Gen Virol. 1989 Dec;70(Pt 12):3269–3280. doi: 10.1099/0022-1317-70-12-3269. [DOI] [PubMed] [Google Scholar]
- Earle J. A., Skuce R. A., Fleming C. S., Hoey E. M., Martin S. J. The complete nucleotide sequence of a bovine enterovirus. J Gen Virol. 1988 Feb;69(Pt 2):253–263. doi: 10.1099/0022-1317-69-2-253. [DOI] [PubMed] [Google Scholar]
- Haenni A. L., Joshi S., Chapeville F. tRNA-like structures in the genomes of RNA viruses. Prog Nucleic Acid Res Mol Biol. 1982;27:85–104. doi: 10.1016/s0079-6603(08)60598-x. [DOI] [PubMed] [Google Scholar]
- Hughes P. J., North C., Minor P. D., Stanway G. The complete nucleotide sequence of coxsackievirus A21. J Gen Virol. 1989 Nov;70(Pt 11):2943–2952. doi: 10.1099/0022-1317-70-11-2943. [DOI] [PubMed] [Google Scholar]
- Inoue T., Suzuki T., Sekiguchi K. The complete nucleotide sequence of swine vesicular disease virus. J Gen Virol. 1989 Apr;70(Pt 4):919–934. doi: 10.1099/0022-1317-70-4-919. [DOI] [PubMed] [Google Scholar]
- Le S. Y., Zuker M. Common structures of the 5' non-coding RNA in enteroviruses and rhinoviruses. Thermodynamical stability and statistical significance. J Mol Biol. 1990 Dec 5;216(3):729–741. doi: 10.1016/0022-2836(90)90395-3. [DOI] [PubMed] [Google Scholar]
- Peritz A. E., Kierzek R., Sugimoto N., Turner D. H. Thermodynamic study of internal loops in oligoribonucleotides: symmetric loops are more stable than asymmetric loops. Biochemistry. 1991 Jul 2;30(26):6428–6436. doi: 10.1021/bi00240a013. [DOI] [PubMed] [Google Scholar]
- Pilipenko E. V., Blinov V. M., Agol V. I. Gross rearrangements within the 5'-untranslated region of the picornaviral genomes. Nucleic Acids Res. 1990 Jun 11;18(11):3371–3375. doi: 10.1093/nar/18.11.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilipenko E. V., Blinov V. M., Chernov B. K., Dmitrieva T. M., Agol V. I. Conservation of the secondary structure elements of the 5'-untranslated region of cardio- and aphthovirus RNAs. Nucleic Acids Res. 1989 Jul 25;17(14):5701–5711. doi: 10.1093/nar/17.14.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilipenko E. V., Blinov V. M., Romanova L. I., Sinyakov A. N., Maslova S. V., Agol V. I. Conserved structural domains in the 5'-untranslated region of picornaviral genomes: an analysis of the segment controlling translation and neurovirulence. Virology. 1989 Feb;168(2):201–209. doi: 10.1016/0042-6822(89)90259-6. [DOI] [PubMed] [Google Scholar]
- Richards O. C., Ehrenfeld E. Poliovirus RNA replication. Curr Top Microbiol Immunol. 1990;161:89–119. doi: 10.1007/978-3-642-75602-3_4. [DOI] [PubMed] [Google Scholar]
- Rivera V. M., Welsh J. D., Maizel J. V., Jr Comparative sequence analysis of the 5' noncoding region of the enteroviruses and rhinoviruses. Virology. 1988 Jul;165(1):42–50. doi: 10.1016/0042-6822(88)90656-3. [DOI] [PubMed] [Google Scholar]
- Ryan M. D., Jenkins O., Hughes P. J., Brown A., Knowles N. J., Booth D., Minor P. D., Almond J. W. The complete nucleotide sequence of enterovirus type 70: relationships with other members of the picornaviridae. J Gen Virol. 1990 Oct;71(Pt 10):2291–2299. doi: 10.1099/0022-1317-71-10-2291. [DOI] [PubMed] [Google Scholar]
- Sarnow P., Bernstein H. D., Baltimore D. A poliovirus temperature-sensitive RNA synthesis mutant located in a noncoding region of the genome. Proc Natl Acad Sci U S A. 1986 Feb;83(3):571–575. doi: 10.1073/pnas.83.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. A., Racaniello V. R., Dunn G., Cooper J., Minor P. D., Almond J. W. New model for the secondary structure of the 5' non-coding RNA of poliovirus is supported by biochemical and genetic data that also show that RNA secondary structure is important in neurovirulence. J Mol Biol. 1989 May 20;207(2):379–392. doi: 10.1016/0022-2836(89)90261-1. [DOI] [PubMed] [Google Scholar]
- Stanway G. Structure, function and evolution of picornaviruses. J Gen Virol. 1990 Nov;71(Pt 11):2483–2501. doi: 10.1099/0022-1317-71-11-2483. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Maizels N. tRNA-like structures tag the 3' ends of genomic RNA molecules for replication: implications for the origin of protein synthesis. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7383–7387. doi: 10.1073/pnas.84.21.7383. [DOI] [PMC free article] [PubMed] [Google Scholar]



