Abstract
Mycobacterium avium subsp. paratuberculosis causes paratuberculosis (Johne's disease) in ruminants in most countries. Historical data suggest substantial differences in culturability of M. avium subsp. paratuberculosis isolates from small ruminants and cattle; however, a systematic comparison of culture media and isolates from different countries and hosts has not been undertaken. Here, 35 field isolates from the United States, Spain, Northern Ireland, and Australia were propagated in Bactec 12B medium and Middlebrook 7H10 agar, genomically characterized, and subcultured to Lowenstein-Jensen (LJ), Herrold's egg yolk (HEY), modified Middlebrook 7H10, Middlebrook 7H11, and Watson-Reid (WR) agars, all with and without mycobactin J and some with sodium pyruvate. Fourteen genotypes of M. avium subsp. paratuberculosis were represented as determined by BstEII IS900 and IS1311 restriction fragment length polymorphism analysis. There was no correlation between genotype and overall culturability, although most S strains tended to grow poorly on HEY agar. Pyruvate was inhibitory to some isolates. All strains grew on modified Middlebrook 7H10 agar but more slowly and less prolifically on LJ agar. Mycobactin J was required for growth on all media except 7H11 agar, but growth was improved by the addition of mycobactin J to 7H11 agar. WR agar supported the growth of few isolates. The differences in growth of M. avium subsp. paratuberculosis that have historically been reported in diverse settings have been strongly influenced by the type of culture medium used. When an optimal culture medium, such as modified Middlebrook 7H10 agar, is used, very little difference between the growth phenotypes of diverse strains of M. avium subsp. paratuberculosis was observed. This optimal medium is recommended to remove bias in the isolation and cultivation of M. avium subsp. paratuberculosis.
INTRODUCTION
Mycobacterium avium subsp. paratuberculosis is the causative agent of Johne's disease of ruminants, which occurs in most countries. Usually it is an enteric infection with a long incubation period and progressively worsening granulomatous infiltration of the small and large intestines leading to weight loss and death. It is economically significant in farmed cattle, goats, sheep, and deer and is known to be capable of infecting a wide range of domesticated and free-living herbivores and occasionally also the carnivores that prey upon them (2, 4, 6, 15). Like many pathogenic mycobacteria, M. avium subsp. paratuberculosis is not host specific, and cross-species transmission is common where there are high contact rates. Although tissue colonization and histological lesions may occur, clinical disease in response to infection is rarely reported for nonruminant species, except for rabbits (2, 4). There is a long-standing debate about the role of M. avium subsp. paratuberculosis in chronic inflammatory bowel disease of humans, specifically Crohn's disease. Consequently, the isolation and cultivation of M. avium subsp. paratuberculosis are attempted in both veterinary and medical diagnostic and research laboratories worldwide (20, 33, 46).
Isolation of M. avium subsp. paratuberculosis from feces, which is the most relevant and common clinical sample, is not straightforward because the organism grows extremely slowly and cultures are prone to contamination. A primary requirement for in vitro culture of M. avium subsp. paratuberculosis is inclusion of mycobactin in media to enable iron acquisition; mycobactin dependence is used as a discriminatory test for confirmation of M. avium subsp. paratuberculosis in cultures. Apart from these considerations, M. avium subsp. paratuberculosis has been cultivated on a wide range of media since its original isolation in 1910 (46).
M. avium subsp. paratuberculosis was once believed to be a relatively homogenous taxon with 98% identity to Mycobacterium avium subsp. avium (serovars 2 and 5) based on comparisons by DNA hybridization (27–28). However, the discovery of the multicopy IS900 element (14) and its use as a probe in restriction fragment length polymorphism (RFLP) analysis revealed more than 20 genotypes of M. avium subsp. paratuberculosis in screens of isolates gathered from around the world. The patterns were due to mutations at restriction endonuclease cut sites and variation in the numbers of copies and the sites of insertion in the genome of IS900. Two primary fingerprint patterns were identified, leading to classification of isolates as S strains (derived from sheep), C strains (derived from cattle and other species), and intermediate strains (9); these are also known as type I, II, and III strains, respectively (12, 39). Immediately, a correlation was noticed between strain type and ease of isolation, as the S strains were notoriously difficult to grow. Greater genetic diversity was later identified, particularly between S and C strains, as small and large genomic deletions and insertions and single nucleotide polymorphisms were found (13, 24–26). The so-called S and C strains probably represent the extremes of a taxon; a continuum may exist, and this will be determined when representative genomes have been sequenced and annotated. Cross-species transmission under sufficiently high contact rates can cloud this basic separation (50). Of interest in the context of culture is the so-called M. avium subsp. paratuberculosis bison (B) strain. This was identified using IS1311 polymorphism analysis of isolates from one population of bison in the United States, and it too is difficult to isolate from clinical samples (17, 45, 49).
At the phenotypic level, isolates of M. avium subsp. paratuberculosis can be distinguished by several criteria, including the degree of pigmentation of colonies (rare orange-pigmented versus common nonpigmented varieties) (39–40), culturability (S strains are not easily cultivable, but C strains are more readily cultivable), and degree of clumping of cells in saline suspensions (35). While M. avium subsp. paratuberculosis is defined as being mycobactin dependent during in vitro cultivation (42, 44), Spanish researchers reported that M. avium subsp. paratuberculosis from clinical cases of paratuberculosis in sheep could be isolated on some media without inclusion of mycobactin (1). Although this property was observed using Middlebrook 7H11 medium, the isolates required mycobactin for growth on Lowenstein-Jensen (LJ) medium. It remains unclear whether these observations were due to idiosyncrasy in the types of media used or the growth capacities of the particular strains. Although there is evidence for a beneficial effect of sodium pyruvate (18, 29), it is not always included in culture media and may be inhibitory to some strains (19).
Despite the economic importance of M. avium subsp. paratuberculosis in livestock and its potential role in Crohn's disease in humans, there has never been a systematic international comparison to determine the suitability of culture media for different strains of this organism or consideration of possible geographic variants of M. avium subsp. paratuberculosis with different cultural requirements. Consequently, the aims of this study were to investigate phenotypic differences among isolates from different species and countries, to correlate phenotypic characteristics with genotypic characteristics, and to evaluate the suitability of media for cultivation of M. avium subsp. paratuberculosis. Specifically, is there a universally applicable culture medium?
MATERIALS AND METHODS
Isolates of M. avium subsp. paratuberculosis.
To obtain a diverse range of M. avium subsp. paratuberculosis isolates, a collection was compiled from four widely separated geographic regions, among which there had been no recent animal host contact: Australia, Northern Ireland (United Kingdom), Spain, and the United States. Isolates were obtained for this study as cultures on either Herrold's egg yolk medium with mycobactin J (HEY+MJ), modified Middlebrook 7H10 medium with MJ (7H10+MJ), or Middlebrook 7H9 broth (Table 1), following their primary isolation on HEY+MJ (Australia, United States, Northern Ireland, Spain), Lowenstein-Jensen medium (Spain), or modified Middlebrook 7H9-based media (Bactec) (Australia) (Table 1). Alternatively, some isolates that had been isolated originally in this way were lyophilized and stored; they were reconstituted in 0.5 ml of sterile water. All isolates were considered to be field isolates and had not been subjected to repeated laboratory subcultures. A volume of 0.1 ml of colony suspension, broth, or reconstituted lyophilized suspension was inoculated into modified Bactec 12B with mycobactin J medium and incubated at 37°C, and the growth index (GI) was measured weekly. By 4 weeks, the GI for all isolates was >400 and a 0.1-ml aliquot was taken and inoculated onto modified 7H10+MJ slopes, which were incubated at 37°C. After 6 weeks, there was very heavy growth of tiny colonies on all slopes. From each slope, a suspension was prepared from multiple colonies by dragging a plastic microbiology loop over a linear field 1 cm in length and emulsifying the colonies so obtained in sterile saline with 0.5% (vol/vol) Tween 80 (ST) (29). These undiluted suspensions were mixed thoroughly. A 50-fold dilution of each suspension was made in ST. The identity of each isolate as M. avium subsp. paratuberculosis was confirmed by examination of a smear stained with Ziehl-Neelsen stain and by PCR and restriction endonuclease analysis of the IS900 and IS1311 elements (see below).
Table 1.
Source of 35 M. avium subsp. paratuberculosis isolates and one M. avium subsp. avium isolate, their cultural characteristics, and molecular characterizationh
| Isolate | Accession no.a | Primary mediumb | Formc | Country | Isolate source | Incubation period (wk), colony size on indicated culture mediumd |
Incubation period (wk), colony size on indicated culture mediumd |
PCR-REA result with probe for: |
RFLPg with probe for: |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WR+MJ | LJ+MJ | LJ+P+MJ | HEY+MJ | HEY+P+MJ | 7H10+MJ | 7H11+MJ | WR | LJ | LJ+P | HEY | HEY+P | 7H10 | 7H11 | IS900e | IS1311f | IS900 | IS1311 | ||||||
| 4 | Telford 9-2 | Bactec | SL | Australia | Sheep feces | 13, m | 14, m | 4 (1) | 2, p | 10, m | MP | S | S1 | O1 | |||||||||
| 5 | Telford 9-2 | Bactec | FD | Australia | Sheep feces | 13, m | 13, m | 4, m | 2, m | 7, m | MP | S | S1 | O1 | |||||||||
| 6 | VRS1065 (Vanderstock) | HEY+MJ | SL | Australia | Sheep | 13, m | 13, m | 6, p | 11, p | 3, p | 2, m | 7, m | MP | S | S1 | O1 | |||||||
| 7 | VRS1068 (Wrigley) | HEY+MJ | SL | Australia | Sheep | 13, m | 13, m | 7, m | 7, m | 3, m | 2, m | 7, m | MP | S | S1 | O1 | |||||||
| 8 | FD1104 (Sharwood) | HEY+MJ | SL | Australia | Sheep | 3, m | 6, p | 8, p | 3, p | 2, m | 7, m | MP | S | S1 | O2 | ||||||||
| 12 | 99/145-10 | Bactec | SL | Australia | Alpaca feces | 13, m | 8 (1) | 6, p | 7, m | MP | S | S1 | O1 | ||||||||||
| 13 | 99/454-10 | Bactec | SL | Australia | Sheep feces | 6 (1) | 5, p | 7, m | MP | S | S1 | O1 | |||||||||||
| 23 | 98/91-1 | Bactec | FD | Australia | Llama | 13, m | 11, m | 6, p | 6, p | 3, p | 2, p | 2, m | MP | C | C3 | B1 | |||||||
| 24 | Telford poly | Bactec | SL | Australia | Sheep feces | 13, m | 10, m | 3, p | 2, p | 5, m | MP | S | S1 | O1 | |||||||||
| 25 | 98/101 | Bactec | SL | Australia | Nematode larvae | 13, m | 11, m | 3, p | 2, p | 2, m | MP | S | S1 | O1 | |||||||||
| 26 | PC98/86-1 | Bactec | SL | Australia | Sheep feces | 13, m | 3, m | 11, m | 3, p | 2, p | 2, m | MP | S | S1 | O1 | ||||||||
| 27 | 116/1 (Parfett) | Bactec | SL | Australia | Cow feces | 13, m | 9, m | 11, m | 3, p | 2, p | 5, m | MP | S | S1 | O1 | ||||||||
| 35 | CM00/416 | HEY+MJ | SL | Australia | Cow lymph node | 13, m | 3, m | 7, p | 7, p | 3, p | 2, p | 5, p | MP | C | C3 | B1 | |||||||
| 36 | CM00/426 | HEY+MJ | SL | Australia | Cow feces | 13, m | 13, m | 7, p | 7, p | 3, p | 2, m | 2, p | MP | C | CU8 | B1 | |||||||
| 20 | 99/21-1 | HEY+MJ | SL | Northern Ireland | Sheep feces | 9, p | 13, m | 9, m | 6, p | 7, p | 3, p | 2, p | 5 (3) | MP | C | C5 | B1 | ||||||
| 21 | 99/21-34 | HEY+MJ | SL | Northern Ireland | Sheep feces | 13, m | 3, m | 7, p | 7 (1) | 3, p | 2, p | 2, p | MP | C | CU7 | B1 | |||||||
| 22 | 99/21-G5 | HEY+MJ | FD | Northern Ireland | Sheep milk | 13, m | 13, m | 6, p | 6, p | 3 (4) | 2 (1) | 5, p | MP | C | C5 | B1 | |||||||
| 2 | JD98/107-3 (938i ovi) | LJ+MJ | BR | Spain | Sheep | 13, m | 13, m | 6, p | 4, m | 3, p | 2, p | 2, m | MP | C | CU5 | B1 | |||||||
| 1 | JD98/107-1 (17G ovi) | LJ+MJ | BR | Spain | Sheep | 13, m | 13, m | 12, p | 4, p | 3, p | 14, m | MP | S | I8 | O3 | ||||||||
| 3 | JD98/107-6 (22G ovi) | LJ+MJ | BR | Spain | Sheep | 13, m | 13, m | 7, p | 13, p | 4, m | 5, p | 6, p | MP | S | I9 | O1 | |||||||
| 9 | 99/233 (19I) | LJ+MJ | BR | Spain | Sheep | 13, m | 12, p | 4 (1) | 5 (0.5) | 6, p | MP | S | I10 | O3 | |||||||||
| 10 | 99/233 (18I) | LJ+MJ | BR | Spain | Sheep | 13, m | 13, m | 8, m | 4 (1) | 5 (2) | 6 (0.5) | MP | S | I11 | In1 | ||||||||
| 14 | 99/87-78 (2) | HEY+MJ | FD | United States | Bison | 2 (1) | 2 | 2, p | 1 | 3 (4) | 1 (1) | 2, p | 2 | 2 | 2 | 1 | 3 | 1 | 2, p | Neg | A | ||
| 15 | 99/87-87 (4) | HEY+MJ | FD | United States | Bison | 13, m | 3, m | 7, p | 7, p | 3, p | 2, p | 2, m | MP | B | C1 | B1 | |||||||
| 16 | 99/87-33 (10) | HEY+MJ | FD | United States | Goat | 13, m | 13, m | 7, p | 6, p | 3, p | 3, p | 13, m | 8, p | MP | C | CU6 | B1 | ||||||
| 17 | 99/87-91 (14) | HEY+MJ | FD | United States | Cow | 13, m | 3, m | 7, p | 7, p | 3, p | 2, p | 2, p | MP | C | C1 | B1 | |||||||
| 18 | 99/87-174 (18) | HEY+MJ | FD | United States | Cow | 13, m | 3, m | 7, p | 7, p | 3, p | 2, p | 2, p | MP | C | CU4 | B1 | |||||||
| 34 | JD99/87-36 (30) | HEY+MJ | FD | United States | Bison | 13, m | 7, p | 10, m | 3, p | 2, p | 5, m | MP | B | C5 | B1 | ||||||||
| 31 | JD00/41 −1 | HEY+MJ | SL | United States | Gibbon | 13, m | 7, p | 7, p | 3, p | 2, p | 12, m | MP | C | C1 | B1 | ||||||||
| 32 | JD00/41-12 | HEY+MJ | FD | United States | Tule elk | 13, m | 3, m | 7, p | 7, p | 3, p | 2, p | 2, p | MP | C | C3 | B1 | |||||||
| 33 | JD00/41-13 | HEY+MJ | FD | United States | Elk | 13, m | 9, p | 10, p | 6 (1) | 2 (0.5) | 2, m | MP | C | C1 | B1 | ||||||||
| 39 | JD00/41-21 | HEY+MJ | SL | United States | Springbok | 13, m | 13, m | 7, p | 7, m | 3, p | 2, p | 2, m | MP | C | C1 | B1 | |||||||
| 40 | JD00/41-24 | HEY+MJ | SL | United States | Tule elk | 13, m | 13, m | 6, m | 6, m | 3, p | 2, p | 6, m | MP | C | C3 | B1 | |||||||
| 41 | JD00/41-25 | HEY+MJ | SL | United States | Oryx | 13, m | 3, m | 7, p | 7, m | 3, p | 2, p | 6, m | MP | C | C1 | B1 | |||||||
| 42 | JD00/41-14 | HEY+MJ | SL | United States | Elk | 13, m | 3, m | 7, p | 8, p | 3, p | 2, p | 2, m | MP | C | C1 | B1 | |||||||
| 43 | JD00/41-26 | HEY+MJ | SL | United States | Springbok | 13, m | 8, m | 6, p | 6, p | 3, p | 2, p | 2, m | MP | C | C1 | B1 | |||||||
Original accession names/codes are provided in parentheses.
The medium used for primary isolation from the clinical sample. Abbreviations are as described in Table 2, but medium constituents may vary from those in the present study and also between laboratories.
Cultures were supplied as slopes (SL), as broth cultures in Middlebrook 7H9 medium (BR), or as freeze-dried ampoules (FD).
Incubation period in weeks for the appearance of colonies and the maximum colony size recorded at 14 weeks, as follows: p, pinpoint colonies visible to the naked eye; m, minute colonies visible only with a hand lens; and number in parentheses, diameter of a representative colony in millimeters. WR, Watson-Reid agar; P, sodium pyruvate.
MP, M. avium subsp. paratuberculosis; Neg, negative.
A, M. avium; B, bison strain; C, cattle strain; S, sheep strain.
Isolates are grouped by country of origin.
Culture media and inoculation.
Each isolate was subcultured onto 14 different solid media (Table 2). The media were prepared in 35-ml polycarbonate culture tubes (Techno-Plas; catalog no. C8027SU) using 10 to 11 ml medium per tube, which was allowed to form a slant.
Table 2.
Culture media used in this study
| Medium | Base medium | Abbreviation | Egg | MJ concn | Additive(s) |
|---|---|---|---|---|---|
| 1 | Herrold's egg yolk | HEY+MJ+P | Yolk | 2 mg/liter | Sodium pyruvate |
| 2 | Herrold's egg yolk | HEY+P | Yolk | Nil | Sodium pyruvate |
| 3 | Herrold's egg yolk | HEY+MJ | Yolk | 2 mg/liter | Nil |
| 4 | Herrold's egg yolk | HEY | Yolk | Nil | Nil |
| 5 | Lowenstein-Jensen | LJ+MJ+P | Whole | 2 mg/liter | Sodium pyruvate |
| 6 | Lowenstein-Jensen | LJ+P | Whole | Nil | Sodium pyruvate |
| 7 | Lowenstein-Jensen | LJ+MJ | Whole | 2 mg/liter | Nil |
| 8 | Lowenstein-Jensen | LJ | Whole | Nil | Nil |
| 9 | Middlebrook 7H11 | 7H11+MJ | Nil | 940 μg/liter | Oleic acid, albumin, dextrose, catalase |
| 10 | Middlebrook 7H11 | 7H11 | Nil | Nil | Oleic acid, albumin, dextrose, catalase |
| 11 | Watson-Reid (pH 5.5) | WR+MJ | Nil | 2 mg/liter | Nil |
| 12 | Watson-Reid (pH 5.5) | WR | Nil | Nil | Nil |
| 13 | Middlebrook 7H10 | 7H10+MJ | Yolk | 940 μg/liter | Albumin, dextrose, catalase |
| 14 | Middlebrook 7H10 | 7H10 | Yolk | Nil | Albumin, dextrose, catalase |
Watson-Reid agar, with the addition of ammonium citrate, was prepared according to the method of Merkal and Curran (29) and consisted of (per liter) l-asparagine (5 g), d-glucose (10 g), glycerol (63 ml), ammonium hydrogen citrate (2 g), ferric ammonium citrate (75 mg), potassium dihydrogen phosphate (2 g), sodium chloride (2 g), magnesium sulfate (1 g), zinc sulfate (10 mg), calcium chloride (20 mg), cobalt chloride (2 mg), Noble agar (1 g; Difco), and, if required, mycobactin J (2 mg; Allied Monitor). The pH was adjusted to 5.5 with 1 M NaOH. The medium was autoclaved at 121°C for 20 min.
Lowenstein-Jensen medium contained (per liter) potassium dihydrogen phosphate (1.5 g), magnesium sulfate (310 mg), magnesium citrate (375 mg), l-asparagine (2.25 g), malachite green (12.5 mg), homogenized whole egg (625 ml), and, if required, sodium pyruvate (4 g) and mycobactin J (2 mg). The medium was both sterilized and set in the culture tubes by steaming them at 85°C for 30 min.
Herrold's egg yolk medium was prepared by the method of Merkal and Curran (29) and contained (per liter) peptone (9 g; Difco, BD), sodium chloride (4.5 g), Noble agar (15.3 g), beef extract (2.7 g; Oxoid), glycerol (27 ml), malachite green (100 mg), egg yolk (120 ml), and, if required, mycobactin J (2 mg). The medium was autoclaved prior to the addition of egg yolk and, if required, sodium pyruvate (4 g/liter). No antibiotics were added to this medium.
Middlebrook 7H10 medium was based on that of Whittington et al. (48) and contained (per liter) Middlebrook 7H10 agar (14.3 g; Difco), Casitone (750 mg; Difco), glycerol (3.76 ml), an antibiotic mixture (polymyxin B at 37,600 U, amphotericin B at 3.8 mg, nalidixic acid at 15 mg, trimethoprim at 3.8 mg, azlocillin at 3.8 mg, and polyoxyethylene stearate at 150 mg [Panta Plus]; Becton Dickinson), a supplement (bovine albumin at 3.8 g, dextrose at 1.5 g, and catalase at 3 mg [ADC enrichment]; Difco), egg yolk (188 ml), and, if required, mycobactin J (940 μg). The antibiotic mixture, supplement, egg yolk, and mycobactin J were added after the medium was autoclaved.
Middlebrook 7H11 medium was based on that of Whittington et al. (48) and contained (per liter) Middlebrook 7H11 agar (14.3 g; Difco), glycerol (3.76 ml), an antibiotic mixture (polymyxin B at 37,600 U, amphotericin B at 3.8 mg, nalidixic acid at 15 mg, trimethoprim at 3.8 mg, azlocillin at 3.8 mg, polyoxyethylene stearate at 150 mg [Panta Plus]; Becton Dickinson), a supplement (oleic acid at 37.6 mg, bovine albumin at 3.8 g, dextrose at 1.5 g, catalase at 3 mg [OADC]; Difco), and, if required, mycobactin J (940 μg). The antibiotic mixture, supplement, and mycobactin J were added after the medium was autoclaved.
A volume of 40 μl of each undiluted and diluted bacterial suspension was inoculated at the top of the slope and allowed to run down the surface and to spread out. Cultures were incubated at 37°C. Each tube was inspected weekly for 14 weeks under bright transverse illumination using magnification. The time to appearance of colonies was recorded, as was colony size. Colonies were called minute where they were clearly visible only with magnification and pinpoint where tiny colonies were observed with the naked eye; the colony diameter was estimated when colonies were large enough to be observed easily with the naked eye.
Extraction and purification of DNA.
DNA extraction was performed as described by Choy et al. (7). Briefly, 0.4- to 0.5-g (wet weight) bacterial cell pellets were resuspended in 1 ml of 10 mM Tris, 1 mM EDTA, pH 8.0 (TE), after which DNA extraction was undertaken using lysozyme and mutanolysin in conjunction with proteinase K. Following this, cetyltrimethylammonium bromide (CTAB) and a standard chloroform-isoamyl alcohol technique was used to purify the DNA. The purified DNA was resuspended in sterile TE (pH 8.0) and stored at 4°C. The concentration of the DNA was calculated by spectrophotometry (Pharmacia; GeneQuant II) using the formula (μg/ml = A260 × DF × 50), where DF is the dilution factor.
PCR and restriction endonuclease analysis.
IS900 and IS1311 PCR and restriction endonuclease analyses were performed as described previously to confirm the identities of isolates of M. avium subsp. paratuberculosis and to classify them as C, S, or B strains (10, 23, 30).
IS900 and IS1311 RFLP analysis.
Substrain determination was undertaken by restriction fragment length polymorphism (RFLP) analysis using probes for IS900 and IS1311 according to the methods described previously (7–8). Briefly, the DNA sample was digested with the restriction endonuclease BstEII (Roche). The digested DNA samples were separated on 1% (wt/vol) agarose gels by electrophoresis at 35 V for 20 h and transferred under vacuum (Hybaid) to positively charged nylon membranes (Amersham). A 229-bp IS900 PCR-based digoxigenin-labeled probe was hybridized with the membrane overnight. After being washed, the membrane was then developed with a digoxigenin chemiluminescence kit (Roche) and exposed to X-ray film for 2 h. The films were then developed using standard procedures. At the completion of the IS900 RFLP process, the membranes were stripped and reprobed using a 268-bp IS1311 digoxigenin-labeled probe (23). The RFLP process was completed as described above.
The IS900 RFLP profiles identified in this study were compared with published profiles (8, 31, 34, 47). The IS1311 RFLP profiles identified in this study were also compared with published RFLP profiles (8). However, in the absence of a naming system in that study, one is proposed here: B for bovine profiles, O for ovine profiles, and In for intermediate profiles (In is used to differentiate this profile from IS900 RFLP intermediate [I] profiles).
Microscopic appearance.
The morphology of the isolates was assessed by preparing smears from a saline suspension made from colonies grown on HEY medium plus MJ (HEY+MJ) and Middlebrook 7H10 medium plus MJ (7H10+MJ) for 8 weeks. Smears were stained by a Ziehl-Neelsen method and examined at a ×1,000 magnification using oil immersion.
Ease of suspension of colonies in saline.
A sample of several colonies was taken using a bacteriological loop and mixed with 20 μl of saline on a glass microscope slide to form a suspension. The suspension was examined with the naked eye using bright illumination to assess its uniformity.
RESULTS
Identification and genomic characterization of isolates.
After exclusion of four contaminated cultures, all but one of the remaining 36 field isolates were identified as M. avium subsp. paratuberculosis; the single isolate that was not confirmed to be M. avium subsp. paratuberculosis by IS900 PCR and restriction endonuclease analysis (REA) originated from a bison in the United States and produced an M. avium subsp. avium profile in the IS1311 PCR-REA (isolate 14 in Table 1). It was retained in the study as a control, as it would likely grow on all media.
The strain type of M. avium subsp. paratuberculosis was established using IS1311 PCR-REA. The majority of the IS1311 types matched those expected based on the host species of origin, with the following exceptions. Three isolates originating from sheep samples from Northern Ireland produced C profiles, as did a single isolate from a sheep sample from Spain. Of the 35 field isolates of M. avium subsp. paratuberculosis, there were 18 C, 15 S, and 2 B isolates (Table 1). The C isolates were derived from cattle, sheep, goats, a primate, wild ruminants, and a camelid. The S isolates were derived from cattle, sheep, and a camelid, while the B isolates were from bison only.
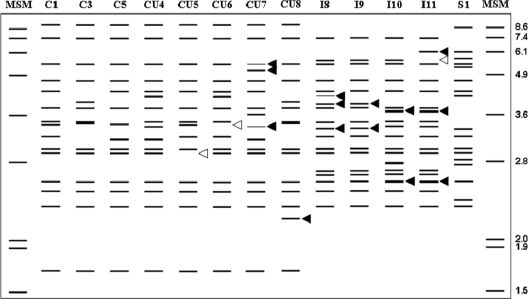
RFLP analysis was used to subtype the isolates. Four previously unknown BstEII IS900 RFLP profiles were identified (Fig. 1). By following the nomenclature of Whittington et al. (47), these were named CU5, CU6, CU7, and CU8 and originated from Spain, the United States, Northern Ireland, and Australia, respectively. All four profiles correlated with C strain profiles generated from the IS1311 PCR-REA typing. C1 profiles dominated the cattle types, and all of the IS900 RFLP C subtypes had the same pattern, B1, with the IS1311 probe. The genomic DNA of bison (B) strains subtyped by IS1311 PCR-REA as C1-B1 and C5-B1 with IS900 and IS1311 probes.
Fig. 1.
IS900 RFLP profiles observed in this study. New profiles are characterized by differing band intensities and the presence (◀) and/or absence (◁) of bands responsible for the new profile. MSM, molecular size markers. Numbers at the right are molecular sizes (in kilobase pairs).
No new IS900 S strain RFLP profiles were observed; all had S1 profiles, but they divided into two subtypes (S1-O1 and S1-O2) with the IS1311 probe.
Four new IS900 RFLP intermediate (I) profiles were observed; all were from sheep samples originating from Spain. By following the most recent identification of new I profiles generated from BstEII IS900 RFLP typing (31), these were named I8 to I11. One of these, I11, had a unique IS1311 RFLP profile, the first to be described as an intermediate profile (In1). The profile lacked the uppermost band that is typical of IS1311 RFLP S profiles and the lowermost band that is typical of the IS1311 RFLP B profiles (Fig. 2).
Fig. 2.
IS1311 RFLP profiles observed in this study. New profiles are characterized by the presence (◀) and absence (◁) of bands responsible for the new profile. Numbers at the right are molecular sizes (in kilobase pairs).
Based on RFLP typing with the IS900 and IS1311 probes, there were a total of 14 genotypes of M. avium subsp. paratuberculosis: eight subtypes of C isolates (profiles C1-B1, C3-B1, C5-B1, CU4-B1, CU5-B1, CU6-B1, CU7-B1, and CU8-B1), two subtypes of S isolates (S1-O1 and S1-O2), and four subtypes of I isolates (I8-O3, I9-O1, I10-O3, and I11-In1) (Table 1).
Growth of M. avium subsp. paratuberculosis on a range of culture media.
The isolates of M. avium subsp. paratuberculosis grew satisfactorily on a narrow range of media (Table 3). Middlebrook 7H10 and 7H11 media with mycobactin J (MJ) supported the growth of all isolates, most commonly by week 2 or 3, and the most common colony size was pinpoint. More isolates had colonies with a diameter of >0.5 mm on Middlebrook 7H10 medium than on any other medium. With the exception of Middlebrook 7H11 medium, MJ was an essential component of all media for the cultivation of field isolates of M. avium subsp. paratuberculosis. On Middlebrook 7H11 medium without MJ, colonies of all 35 field isolates were apparent in 2 to 14 weeks, but almost all were minute even after 14 weeks of incubation, which is not optimal growth (Table 3). The addition of MJ to this medium improved the growth of M. avium subsp. paratuberculosis, as the colonies appeared sooner in many cases and were often larger.
Table 3.
Patterns of growth of 35 field isolates of M. avium subsp. paratuberculosis on media supporting growth
| Mediuma | Abbreviation | No. of field isolates that grew | No. of wks to appearance of coloniesb | Colony sizea | Comment(s) on type of isolates |
|---|---|---|---|---|---|
| 1 | HEY+P+MJ | 23 | 4–11 (7) | Minute to 1 mm (pinpoint) | Isolates which failed to grow were S strain isolates |
| 3 | HEY+MJ | 29 | 6–12 (7) | Minute to pinpoint (pinpoint) | Isolates which failed to grow were S strain isolates |
| 5 | LJ+P+MJ | 32 | 3–14 (13) | Minute to pinpoint (minute) | |
| 6 | LJ+P | 1 | 13 | Minute | Isolate 16, United States, goat, C strain |
| 7 | LJ+MJ | 30 | 13 | Minute | |
| 9 | 7H11+MJ | 35 | 2–6 (2) | Minute to 2 mm (pinpoint) | |
| 10 | 7H11 | 35 | 2–14 (2) | Minute to 3 mm (minute) | |
| 11 | WR+MJ | 1 | 9 | Pinpoint | Isolate 20, Northern Ireland, sheep feces, C strain |
| 13 | 7H10+MJ | 35 | 3–8 (3) | Minute to 4 mm (pinpoint) |
Numbers correspond to numbers in Table 2.
Data are ranges (modes).
Lowenstein-Jensen medium (LJ) with MJ and with or without sodium pyruvate supported the growth of many isolates, but most required prolonged incubation to yield only minute colonies. The isolates that did not grow on these media were mostly S strains.
Herrold's egg yolk medium with MJ (HEY+MJ) supported the growth of most isolates. Colonies appeared usually at about 7 weeks and, for most isolates, were pinpoint in size. The isolates that did not grow were all S strains. However, some S strains did grow on this medium, including several from Australia that were originally recovered from sheep before 1986 and one from cattle, as well as several of Spanish origin. More isolates grew on HEY+MJ without sodium pyruvate than on HEY+MJ with sodium pyruvate.
Watson-Reid medium with MJ was unsuitable for cultivation of field isolates of M. avium subsp. paratuberculosis, suggesting that it is suitable only for laboratory-adapted strains. Only one field isolate grew on this medium.
The colony morphologies of M. avium subsp. paratuberculosis isolates did not vary greatly between isolates or media. All isolates formed small domed colonies with a shiny surface and entire edges on all media on which they grew. However, there was a difference between the colors of colonies grown on HEY+MJ and 7H10+MJ. Colonies were mostly white on HEY+MJ. On 7H10+MJ, colonies were initially cream colored, but after 7 to 10 weeks, the colonies of some isolates became white. On 7H11+MJ, they were translucent, white, or occasionally yellow.
M. avium subsp. avium (isolate 14) grew on all media and had cream-colored colonies which became yellow after about week 6.
Ziehl-Neelsen staining observations.
The morphologies of the isolates in smears stained by a Ziehl-Neelsen method were similar for colonies grown on HEY+MJ and 7H10+MJ for most isolates: short acid-fast bacilli. However, morphologies differed between media for C strain isolates 2, 3, 20, 21, 23, 31, 32, 33, 34, 35, and 36 and S strain isolate 3. For these isolates, the bacteria grown on 7H10+MJ appeared shorter and wider than those grown on HEY+MJ. On the latter medium, there were sometimes relatively long, slender bacilli.
Ease of emulsification of colonies in saline.
When mixed with a bacteriological loop in a drop of saline on a glass slide, all isolates grown on 7H10+MJ were able to be suspended in saline. In contrast, isolates that grew on HEY+MJ tended to remain in small but macroscopically visible clumps when the bacteria were mixed with saline on a slide.
DISCUSSION
The primary finding of this study with respect to the suitability of culture media for the growth of diverse M. avium subsp. paratuberculosis isolates was that media based on Middlebrook 7H9 agar supported the growth of all isolates. None of the other media that were evaluated did this, and none resulted in such large colonies (the most common size was pinpoint colonies visible to the naked eye) within reasonable incubation periods. MJ was an essential component for growth on Middlebrook 7H10 agar and greatly improved growth on Middlebrook 7H11 agar; that is, MJ was not required for growth on egg-free Middlebrook 7H11 medium but did stimulate growth. All of the isolates evaluated were propagated first in Bactec medium and then in 7H10+MJ to obtain uniform suspensions for inoculation of solid media. Thus, it could be argued that there was selection bias toward isolates that grew best on such media. In fact, except for the Australian isolates, which were derived mostly from primary Bactec 12B (Middlebrook 7H9) broth cultures, the isolates had negative selection bias, as most isolates were originally recovered from clinical samples using HEY+MJ or LJ+MJ (Spain), and all of these retained their ability to grow on these media (Table 2).
Testing for mycobactin dependency is a fundamental method for identification of M. avium subsp. paratuberculosis. The data presented here suggest that it is possible to conduct this test using microbial suspensions grown on Middlebrook 7H10 agar, HEY medium, or LJ medium. Strict protocols have been suggested for determination of mycobactin dependency due to attachment of mycobactin from culture media to bacteria prior to their subculture to mycobactin-free medium (21). Although some carryover of mycobactin was possible from the 7H10+MJ that was used to prepare suspensions in this study, it did not appear to be of practical significance, because none of the isolates grew on Middlebrook 7H10 medium or HEY medium unless mycobactin was included and only one grew on LJ medium. An important observation from this study is that mycobactin dependency should not be assessed on egg-free Middlebrook 7H11 medium.
Sodium pyruvate was reported to stimulate the growth of some isolates of M. avium subsp. paratuberculosis in HEY+MJ or LJ+MJ, compensating for the inhibitory effects of antibiotics that were included in the latter medium (18, 29). Inclusion of pyruvate may have been based on findings from culture of the tubercle bacillus (22). However, when included in HEY+MJ in this study, three S strain isolates from sheep from Spain failed to grow. These results were consistent with original reports from Spain in which pyruvate appeared to stimulate the growth of isolates of M. avium subsp. paratuberculosis from cattle but inhibited the isolation of M. avium subsp. paratuberculosis from sheep and goats (19).
Consistent with findings of previous studies, S strains from various sources grew poorly on HEY+MJ. The exceptions were S strains from Spain and three S strain isolates from sheep in Australia, which were cultured from clinical samples in the early 1980s, lyophilized, and archived (isolates 6, 7, and 8 in Table 1). Apart from these isolates, there are no known S strains from Australia with the capacity to grow on HEY+MJ; in fact, until the development of media suitable for the cultivation of S strains in 1998 (48), a presumptive diagnosis of paratuberculosis due to the S strain was made when cultures on HEY+MJ from animals that had typical histopathological lesions of paratuberculosis were negative. It is unknown why these isolates from the very early stages of the paratuberculosis epizootic in sheep in Australia (37) and not isolates obtained later in the epizootic can grow on HEY+MJ. It is possible that there was no selective advantage in the biochemistry underlying this growth capacity, and it was lost during microbial evolution in the sheep population. Alternatively, the alternative phenotype had a selective advantage in vivo and now predominates. Genomic studies are warranted to compare isolates 6, 7, and 8 with later isolates, such as isolate 4, which has been more fully characterized (24) to determine the genetic basis of the growth differences.
The growth of all five Spanish isolates on HEY media is not consistent with previous results from Spain, where only a small proportion of isolates from sheep grew on this type of medium (19). Possibly the isolates used in this study were not representative of those used in the earlier study, or the HEY media used in the two laboratories differed in some important way. Additionally, it might be possible that after primary isolation on LJ+MJ, which was common practice in Spain when the isolates were obtained, subculture on other egg-based types of media is facilitated. In a separate study in Spain, tissue homogenates from cattle and goats yielded colonies of M. avium subsp. paratuberculosis on a range of media, but only C strains grew readily on HEY media (11).
Lowenstein-Jensen medium has been popular for the routine isolation of M. avium subsp. paratuberculosis from clinical samples in northern Europe for decades (18). However, in studies of sheep paratuberculosis in other parts of Europe, colonies on this medium were tiny and took longer to grow than on Middlebrook 7H11 agar (1). These findings were confirmed in this study and were independent of strain type; overall, LJ medium did not support the growth of M. avium subsp. paratuberculosis as well as other media did.
Watson-Reid medium is a chemically defined medium with a high iron level, and there is one report suggesting that at low pH, mycobactin is not required for cultivation of M. avium subsp. paratuberculosis in this medium (32). However, as found in the present study, others have reported that M. avium subsp. paratuberculosis does not grow well on this medium (29, 43).
Fourteen genotypes of M. avium subsp. paratuberculosis originating from a range of species from four geographically separated countries were included in this study, thereby providing a robust assessment of M. avium subsp. paratuberculosis diversity in the context of culturability. Differences in culturability were broadly defined in terms of IS1311 PCR-REA genotype, in that S strains tended to grow poorly on media except Middlebrook 7H9 and related solid media (7H10, 7H11) with mycobactin. The few Australian S strains which grew on HEY+MJ were not distinguished by RFLP analysis from ones which did not grow on this medium, while the S strains from Spain had unique RFLP profiles. Collectively, these data suggest that RFLP profiles alone are not predictive of growth requirements. Exceptions exist to the general rule of thumb that S strains do not grow on HEY+MJ. Therefore, there must be another genomic feature which is correlated with but not linked to IS900 loci. Furthermore, although there have been many reports that show an association between host ruminant species, isolate type, and culturability (3, 9, 19, 38), the host ruminant species does not determine culturability. For example, isolation of S strains from cattle was difficult (50), isolation of C strains from several bison populations was easily accomplished, and isolation of B strains from the original infected herd and later from a dairy cow was mostly unsuccessful on HEY+MJ (17).
A prior observation that, compared to colonies of an S strain, colonies of the laboratory-adapted C strain 316V grown on 7H10+MJ were difficult to emulsify in saline (35) does not extend to field strains of M. avium subsp. paratuberculosis. C strain 316V has other properties which differ from those of field strains, such as the capacity to grow on Watson-Reid medium (unpublished data). In the present study, the ease of emulsification between S and C field strains grown on 7H10+MJ was less different than between C field strains grown on HEY+MJ and S field strains grown on 7H10+MJ. The tendency to clump appears to be a feature of the culture medium upon which the organisms are grown rather than the strain of M. avium subsp. paratuberculosis per se. Presumably, organisms grown on these two media utilize different growth factors and have different cell surface characteristics as a result. Differences observed in cell morphology for some isolates grown on different media were interesting but require quantification in a future study.
The rate of growth of M. avium subsp. paratuberculosis is an important practical consideration in diagnostic laboratories and should be assessed when selecting a culture medium for routine use. It is well recognized that incubation periods for some solid media often need to be prolonged; for example, 70% of S strain isolates took more than 3 months to grow, and 50% took 5 to 7 months on LJ or Middlebrook 7H11 medium (11). The results of the present study indicate that very prolonged incubation periods can be avoided by use of a more suitable medium: the interval to the appearance of colonies on 7H10+MJ was 3 weeks (mode) compared to 13 weeks on LJ+MJ.
It may be unwise to make direct comparisons between data presented here for certain culture media and those in previous studies because media with the same name may not be formulated identically across studies; the compositions of media were often not described in earlier studies. To avoid problems with future interpretation of the data in this study, the culture media are described in detail herein. Importantly, the 7H10+MJ used in this study contained egg yolk, a modification introduced specifically to enable the culture of S strains (48). While modified 7H10+MJ may be a universally applicable culture medium for M. avium subsp. paratuberculosis, for diagnostic applications which require greater analytical sensitivity, liquid culture media with a Middlebrook 7H9 base are recommended (46).
In summary, the differences in growth that have been reported historically among species of hosts and M. avium subsp. paratuberculosis strain types, particularly the difficulty of culture of M. avium subsp. paratuberculosis from sheep and sometimes other small ruminants in diverse settings, such as Australia (5), New Zealand (9), England (41), Iceland (16), Spain (11, 19), and the United States (36), have been strongly determined by the type of culture medium used. When using an optimal culture medium, such as modified 7H10+MJ, there is very little difference between growth phenotypes of S, C, and B strains of M. avium subsp. paratuberculosis. Since one solid medium formulation supported the growth of all isolates in this study, it is logical for diagnosticians to request the use of this medium alongside other solid media that may be in routine use to maximize the chances of isolation of M. avium subsp. paratuberculosis. This optimal medium is recommended to remove bias in the isolation and cultivation of M. avium subsp. paratuberculosis.
Footnotes
Published ahead of print on 23 March 2011.
REFERENCES
- 1. Aduriz J. J., Juste R. A., Cortabarria N. 1995. Lack of mycobactin dependence of mycobacteria isolated on Middlebrook 7H11 from clinical cases of ovine paratuberculosis. Vet. Microbiol. 45:211–217 [DOI] [PubMed] [Google Scholar]
- 2. Anderson J. L., et al. 2007. Mycobacterium avium subsp. paratuberculosis in scavenging mammals in Wisconsin. J. Wildl. Dis. 43:302–308 [DOI] [PubMed] [Google Scholar]
- 3. Bauerfeind R., et al. 1996. Molecular characterization of Mycobacterium paratuberculosis isolates from sheep, goats, and cattle by hybridization with a DNA probe to insertion element IS900. J. Clin. Microbiol. 34:1617–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beard P. M., et al. 2001. Paratuberculosis infection of nonruminant wildlife in Scotland. J. Clin. Microbiol. 39:1517–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carrigan M. J., Seaman J. T. 1990. The pathology of Johne's disease in sheep. Aust. Vet. J. 67:47–50 [DOI] [PubMed] [Google Scholar]
- 6. Chiodini R. J., van Kruiningen H. J. 1983. Eastern white-tailed deer as a reservoir of ruminant paratuberculosis. J. Am. Vet. Med. Assoc. 182:168–169 [PubMed] [Google Scholar]
- 7. Choy E., Whittington R. J., Marsh I., Marshall J., Campbell M. T. 1998. A method for purification and characterisation of Mycobacterium avium subsp. paratuberculosis from the intestinal mucosa of sheep with Johne's disease. Vet. Microbiol. 64:51–60 [DOI] [PubMed] [Google Scholar]
- 8. Collins D. M., Cavaignac S., de Lisle G. W. 1997. Use of four DNA insertion sequences to characterize strains of the Mycobacterium avium complex isolated from animals. Mol. Cell. Probes 11:373–380 [DOI] [PubMed] [Google Scholar]
- 9. Collins D. M., Gabric D. M., de Lisle G. W. 1990. Identification of two groups of Mycobacterium paratuberculosis strains by restriction endonuclease analysis and DNA hybridization. J. Clin. Microbiol. 28:1591–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cousins D. V., et al. 1999. Mycobacteria distinct from Mycobacterium avium subsp. paratuberculosis isolated from the faeces of ruminants possess IS900-like sequences detectable by IS900 polymerase chain reaction: implications for diagnosis. Mol. Cell. Probes 13:431–442 [DOI] [PubMed] [Google Scholar]
- 11. de Juan L., et al. 2006. Comparison of four different culture media for isolation and growth of type II and type I/III Mycobacterium avium subsp. paratuberculosis strains isolated from cattle and goats. Appl. Environ. Microbiol. 72:5927–5932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Juan L., Mateos A., Dominguez L., Sharp J. M., Stevenson K. 2005. Genetic diversity of Mycobacterium avium subspecies paratuberculosis isolates from goats detected by pulsed-field gel electrophoresis. Vet. Microbiol. 106:249–257 [DOI] [PubMed] [Google Scholar]
- 13. Dohmann K., et al. 2003. Characterization of genetic differences between Mycobacterium avium subsp. paratuberculosis type I and type II isolates. J. Clin. Microbiol. 41:5215–5223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Green E. P., et al. 1989. Sequence and characteristics of IS900, an insertion element identified in a human Crohn's disease isolate of Mycobacterium paratuberculosis. Nucleic Acids Res. 17:9063–9073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greig A., et al. 1999. Epidemiological study of paratuberculosis in wild rabbits in Scotland. J. Clin. Microbiol. 37:1746–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gunnarsson E. 1979. Isolation of Mycobacterium paratuberculosis from sheep and cattle in Iceland. Acta Vet. Scand. 20:191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris B., Robbe-Austerman S. 2010. 2010 Johne's disease fecal proficiency panel general summary. United States Department of Agriculture, Animal and Plant Health Inspection Service, http://www.aphis.usda.gov/animal_health/lab_info_services/proficiency.shtml
- 18. Jorgensen J. B. 1982. An improved medium for culture of Mycobacterium paratuberculosis from bovine faeces. Acta Vet. Scand. 23:325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Juste R. A., Marco J. C., Saez-de-Ocariz C., Aduriz J. J. 1991. Comparison of different media for the isolation of small ruminant strains of Mycobacterium paratuberculosis. Vet. Microbiol. 28:385–390 [DOI] [PubMed] [Google Scholar]
- 20. Kirkwood C. D., et al. 2009. Mycobacterium avium subspecies paratuberculosis in children with early-onset Crohn's disease. Inflamm. Bowel Dis. 15:1643–1655 [DOI] [PubMed] [Google Scholar]
- 21. Lambrecht R. S., Collins M. T. 1992. Mycobacterium paratuberculosis. Factors that influence mycobactin dependence. Diagn. Microbiol. Infect. Dis. 15:239–246 [DOI] [PubMed] [Google Scholar]
- 22. Marks J. 1963. Pyruvic acid in the cultivation of tubercle bacilli. Mon. Bull. Min. Health Publ. Health Lab. Serv. 22:150–152 [PubMed] [Google Scholar]
- 23. Marsh I., Whittington R., Cousins D. 1999. PCR-restriction endonuclease analysis for identification and strain typing of Mycobacterium avium subsp. paratuberculosis and M. avium subsp. avium based on polymorphisms in IS1311. Mol. Cell. Probes 13:115–126 [DOI] [PubMed] [Google Scholar]
- 24. Marsh I. B., et al. 2006. Genomic comparison of Mycobacterium avium subsp. paratuberculosis sheep and cattle strains by microarray hybridization. J. Bacteriol. 188:2290–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marsh I. B., Whittington R. J. 2005. Deletion of an mmpL gene and multiple associated genes from the genome of the S strain of Mycobacterium avium subsp. paratuberculosis identified by representational difference analysis and in silico analysis. Mol. Cell. Probes 19:371–384 [DOI] [PubMed] [Google Scholar]
- 26. Marsh I. B., Whittington R. J. 2007. Genomic diversity in Mycobacterium avium: single nucleotide polymorphisms between the S and C strains of M. avium subsp. paratuberculosis and with M. a. avium. Mol. Cell. Probes 21:66–75 [DOI] [PubMed] [Google Scholar]
- 27. McFadden J. J., Butcher P. D., Chiodini R., Hermon-Taylor J. 1987. Crohn's disease-isolated mycobacteria are identical to Mycobacterium paratuberculosis, as determined by DNA probes that distinguish between mycobacterial species. J. Clin. Microbiol. 25:796–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McFadden J. J., Butcher P. D., Thompson J., Chiodini R., Hermon-Taylor J. 1987. The use of DNA probes identifying restriction-fragment-length polymorphisms to examine the Mycobacterium avium complex. Mol. Microbiol. 1:283–291 [DOI] [PubMed] [Google Scholar]
- 29. Merkal R. S., Curran B. J. 1974. Growth and metabolic characteristics of Mycobacterium paratuberculosis. Appl. Microbiol. 28:276–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Millar D., et al. 1996. IS900 PCR to detect Mycobacterium paratuberculosis in retail supplies of whole pasteurized cow's milk in England and Wales. Appl. Environ. Microbiol. 62:3446–3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mobius P., Fritsch I., Luyven G., Hotzel H., Kohler H. 2009. Unique genotypes of Mycobacterium avium subsp. paratuberculosis strains of type III. Vet. Microbiol. 139:398–404 [DOI] [PubMed] [Google Scholar]
- 32. Morrison N. E. 1965. Circumvention of the mycobactin requirement of Mycobacterium paratuberculosis. J. Bacteriol. 89:762–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Naser S. A., Ghobrial G., Romero C., Valentine J. F. 2004. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet 364:1039–1044 [DOI] [PubMed] [Google Scholar]
- 34. Pavlik I., et al. 1999. Standardisation of restriction fragment length polymorphism analysis for Mycobacterium avium subspecies paratuberculosis. J. Microbiol. Methods 38:155–167 [DOI] [PubMed] [Google Scholar]
- 35. Reddacliff L. A., Nicholls P. J., Vadali A., Whittington R. J. 2003. Use of growth indices from radiometric culture for quantification of sheep strains of Mycobacterium avium subsp. paratuberculosis. Appl. Environ. Microbiol. 69:3510–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robbe-Austerman S., Stokes K., Fett K., Myers D., Harris B. 2009. Evaluation of direct PCR and culture using spiked cattle, sheep and goat feces for different strains of Mycobacterium avium subsp. paratuberculosis, p. 180 In Proceedings of the American Association of Veterinary Laboratory Diagnosticians 53rd Annual Conference, November 10-17, 2010 American Association of Veterinary Laboratory Diagnosticians, San Diego, CA [Google Scholar]
- 37. Sergeant E. S. 2001. Ovine Johne's disease in Australia—the first 20 years. Aust. Vet. J. 79:484–491 [DOI] [PubMed] [Google Scholar]
- 38. Sevilla I., Garrido J. M., Geijo M., Juste R. A. 2007. Pulsed-field gel electrophoresis profile homogeneity of Mycobacterium avium subsp. paratuberculosis isolates from cattle and heterogeneity of those from sheep and goats. BMC Microbiol. 7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stevenson K., et al. 2002. Molecular characterization of pigmented and nonpigmented isolates of Mycobacterium avium subsp. paratuberculosis. J. Clin. Microbiol. 40:1798–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stuart P. 1965. A pigmented M. johnei strain of bovine origin. Brit. Vet. J. 121:332–334 [DOI] [PubMed] [Google Scholar]
- 41. Taylor A. W. 1945. Ovine paratuberculosis (Johne's disease of sheep). J. Comp. Pathol. 55:41–44 [Google Scholar]
- 42. Thorel M. F. 1984. Review of the occurrence of mycobactin dependence among mycobacteria species. Ann. Rech. Vet. 15:405–409 [PubMed] [Google Scholar]
- 43. Thorel M. F., Haagsma J. 1987. Components of media used for isolation of certain slow growing mycobacteria. Ann. Inst. Pasteur Microbiol. 138:745–749 [DOI] [PubMed] [Google Scholar]
- 44. Thorel M. F., Krichevsky M., Levy-Frebault V. V. 1990. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int. J. Syst. Bacteriol. 40:254–260 [DOI] [PubMed] [Google Scholar]
- 45. Whitlock R. H., et al. 1999. Paratuberculosis in bison: a comparison of PCR, culture, serology and histopathology, p. 424–438 In Manning E. J. B., Collins M. T. (ed.), Proceedings of the Sixth International Colloquium on Paratuberculosis International Association for Paratuberculosis, Madison, WI [Google Scholar]
- 46. Whittington R. J. 2010. Cultivation of Mycobacterium avium subsp. paratuberculosis, p. 244–266 In Behr M. A., Collins D. M. (ed.), Paratuberculosis. Organism, disease, control. CABI, Wallingford, United Kingdom [Google Scholar]
- 47. Whittington R. J., Hope A. F., Marshall D. J., Taragel C. A., Marsh I. 2000. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis: IS900 restriction fragment length polymorphism and IS1311 polymorphism analyses of isolates from animals and a human in Australia. J. Clin. Microbiol. 38:3240–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Whittington R. J., et al. 1999. Evaluation of modified Bactec 12B radiometric medium and solid media for culture of Mycobacterium avium subsp. paratuberculosis from sheep. J. Clin. Microbiol. 37:1077–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Whittington R. J., Marsh I. B., Whitlock R. H. 2001. Typing of IS 1311 polymorphisms confirms that bison with paratuberculosis in Montana are infected with a strain of Mycobacterium avium subsp. paratuberculosis distinct from that occurring in cattle and other domesticated livestock. Mol. Cell. Probes 15:139–145 [DOI] [PubMed] [Google Scholar]
- 50. Whittington R. J., et al. 2001. Molecular epidemiological confirmation and circumstances of occurrence of sheep (S) strains of Mycobacterium avium subsp. paratuberculosis in cases of paratuberculosis in cattle in Australia and sheep and cattle in Iceland. Vet. Microbiol. 79:311–322 [DOI] [PubMed] [Google Scholar]