Abstract
Although mumps virus is still causing annual epidemics in Mongolia, very few epidemiological and virological data have been reported. We describe here the first phylogenetic analysis data on the mumps viruses circulated in Mongolia in 2009. We detected 21 mumps virus cDNAs and obtained a virus isolate from 32 throat swabs of mumps patients in Ulaanbaatar, the capital of Mongolia. The phylogenetic analyses based on the 316 nucleotides of the small hydrophobic gene show that these sequences form a single cluster, with the closest relatedness to the viruses belonging to genotype H. According to the recommendation of the World Health Organization, Mongolian mumps viruses could be classified into a novel genotype because the divergence between new sequences and genotype H reference viruses is >5% (6.3 to 8.2%). However, additional analyses based on the fusion gene, the hemagglutinin-neuraminidase gene, and the whole-genome indicate that the divergences between the Mongolian isolate and other genotype H strains never exceed the within-genotype divergences of other genotypes. These results suggest that Mongolia strains should be included in genotype H and that the current criteria for mumps virus genotyping should be revised. We propose here that the Mongolian viruses should be classified as a new subgenotype termed H3. Since previous epidemiological studies suggested that genotypes H may be associated with central nervous system diseases, we evaluated the neurovirulence of the Mongolian isolate in the neonatal rat system. However, the virus does not exhibit prominent neurovirulence in rats.
INTRODUCTION
Mumps is a common and highly contagious viral disease characterized by fever and swelling of the salivary glands. Although mumps infections are benign and usually not fatal, they rarely cause more serious neurological complications, such as aseptic meningitis, encephalitis, and deafness.
The mumps virus (MuV) belongs to a member of the genus Rubulavirus of the family Paramyxoviridae (8). It has a single-stranded, negative-sense, nonsegmented RNA genome of 15,384 nucleotides (nt). The genome contains seven transcription units that encode open reading frames for the nucleocapsid (N), phospho (P), matrix (M), fusion (F), small hydrophobic (SH), hemagglutinin-neuraminidase (HN), and large (L) proteins. Two envelope glycoprotein genes, F and HN, are 1,617 and 1,749 nt in length and encode 538 and 582 amino acids, respectively (11, 39, 40). These proteins are located on the virion surface and play cooperatively essential roles in virus entry into the host cells by causing viral attachment to the cell surface receptor molecules and subsequent membrane fusion (33, 40). The HN protein, in addition, is the major target for the humoral immune response in MuV infection and bears several neutralizing epitopes (10, 17, 21). The SH gene, which contains 316 nt and encodes 57 amino acids, is nonessential for viral replication, and its function is unclear (9, 31). Since the SH gene is the most genetically divergent region among whole MuV genes (4, 32), its sequence data have been mainly used as the minimum amount of information for the phylogenetic analyses of MuVs (2, 15, 23). However, it may not necessarily reflect the antigenic properties of MuVs. Although it is generally believed that MuV is serologically monotypic, 13 genotypes (A to M) have been proposed thus far (2, 4, 12–14, 18, 22, 29, 35–38, 42, 43). Because of its high divergence, SH gene-based genotyping of the circulating virus is useful for tracing the viral transmission and investigating the chronological and geographical distribution of MuV strains. Several MuV genotypes exhibit a differential geographical distribution. For instance, genotypes A, C, D, E, and H are mainly observed in the Western Hemisphere (1, 2, 13, 34), whereas genotypes B, F, and I are solely detected in Asia (16, 28, 30, 42). Some studies have reported the potential associations between genotypes C, D, H, I, or J and neuropathogenicity (28, 35, 36, 38).
In Mongolia, since no mumps vaccine is available, mumps outbreaks occur repeatedly. However, very few data on molecular epidemiology have been reported. We describe here the results of phylogenetic analyses on MuVs circulating in Mongolia. The present study is the first report of the current epidemiological situation of MuVs in Mongolia.
MATERIALS AND METHODS
Viruses.
Wild-type MuV 02-49 strain (genotype J) was obtained from Niigata Prefectural Institute of Public Health and Environmental Sciences (Niigata, Japan). Odate-3 strain (genotype I) was obtained from Akita Prefectural Institute of Public Health (Akita, Japan). Viruses were propagated and titrated in Vero cells.
Clinical samples.
Throat swabs were collected on days 0 to 6 after onset (2.4 days on average) from 32 mumps patients hospitalized in the Airborne Infection Ward of the National Center for Communicable Diseases (NCCD), Ulaanbaatar, from May to July 2009 (Table 1). All cases were confirmed by enzyme immunoassay (EIA). Serum samples were collected from all patients except one. These specimens were stored at −70°C temporarily and then transported to the National Institute of Infectious Diseases (NIID), Tokyo, Japan, and were stored at −80°C until analyzed.
Table 1.
Clinical specimens described in this study
| Case | Age (yr) | Gendera | Specimenb | ELISA |
RT-PCR | Virus isolation | MuV strain | Accession no. | |
|---|---|---|---|---|---|---|---|---|---|
| IgM | IgG | ||||||||
| 1 | 21 | M | TS, Se | 19.3 | 4.0 | + | – | MuVs-MNG09-016 | AB598760 |
| 2 | 27 | F | TS, Se | 8.6 | 3.9 | + | – | MuVs-MNG09-017 | AB598761 |
| 3 | 19 | F | TS, Se | 13.1 | 3.9 | + | – | MuVs-MNG09-018 | AB598762 |
| 4 | 1 | M | TS, Se | 28.5 | 3.9 | – | – | ||
| 5 | 25 | M | TS, Se | 24.4 | 4.0 | – | – | ||
| 6 | 14 | F | TS, Se | 19.6 | 3.9 | + | – | MuVs-MNG09-021 | AB598763 |
| 7 | 19 | M | TS, Se | 27.5 | 3.8 | + | – | MuVs-MNG09-022 | AB598764 |
| 8 | 10 | F | TS, Se | 27.7 | 3.8 | + | – | MuVs-MNG09-023 | AB598765 |
| 9 | 28 | F | TS, Se | 13.6 | 3.1 | + | + | MuVi-MNG09-024 | AB600843 |
| 10 | 15 | M | TS, Se | 26.1 | 4.0 | + | – | MuVs-MNG09-025 | AB598766 |
| 11 | 15 | M | TS, Se | 17.0 | 2.0 | + | – | MuVs-MNG09-026 | AB598767 |
| 12 | 6 | M | TS, Se | 23.2 | 3.5 | + | – | MuVs-MNG09-027 | AB598768 |
| 13 | 10 | F | TS, Se | 6.4 | 4.0 | – | – | ||
| 14 | 17 | F | TS, Se | 16.2 | 3.6 | – | – | ||
| 15 | 22 | M | TS, Se | 23.4 | 4.0 | – | – | ||
| 16 | 15 | M | TS, Se | 26.5 | 3.3 | + | – | MuVs-MNG09-244 | AB598769 |
| 17 | 20 | M | TS, Se | 25.6 | 4.0 | – | – | ||
| 18 | 17 | M | TS, Se | 3.0 | 3.3 | – | – | ||
| 19 | 11 | M | TS, Se | 19.8 | 3.8 | + | – | MuVs-MNG09-247 | AB598770 |
| 20 | 31 | M | TS, Se | 21.0 | 3.8 | + | – | MuVs-MNG09-248 | AB598771 |
| 21 | 34 | M | TS, Se | 20.3 | 4.0 | – | – | ||
| 22 | 16 | F | TS, Se | 8.2 | 3.3 | + | – | MuVs-MNG09-250 | AB598772 |
| 23 | 33 | F | TS, Se | 8.5 | 2.4 | – | – | ||
| 24 | 35 | M | TS, Se | 7.0 | 3.8 | + | – | MuVs-MNG09-252 | AB598773 |
| 25 | 23 | M | TS, Se | 15.9 | 4.0 | + | – | MuVs-MNG09-253 | AB598774 |
| 26 | 5 | M | TS, Se | 0.4 | 1.3 | – | – | ||
| 27 | 14 | M | TS, Se | 14.2 | 3.8 | + | – | MuVs-MNG09-255 | AB598775 |
| 28 | 15 | F | TS, Se | 8.0 | 3.5 | + | – | MuVs-MNG09-256 | AB598776 |
| 29 | 22 | F | TS, Se | 24.8 | 4.0 | – | – | ||
| 30 | 13 | M | TS, Se | 23.3 | 3.5 | + | – | MuVs-MNG09-258 | AB598777 |
| 31 | 17 | M | TS, Se | 1.7 | 2.3 | + | – | MuVs-MNG09-259 | AB598778 |
| 32 | 16 | M | TS | NDc | ND | + | – | MuVs-MNG09-260 | AB598779 |
F, female; M, male.
TS, throat swab; Se, serum.
ND, not done.
Measurement of mumps specific IgG and IgM.
A commercially available capture IgM EIA kit and a commercially available indirect IgG EIA kit (Denka Seiken Co., Ltd., Niigata, Japan) were used to determine mumps specific IgM and IgG levels of mumps patient sera. Both assays were performed and interpreted according to the manufacturer's instructions. The IgM antibody titer was designated as an antibody index by calculating the ratio of the optical density (OD) of the sample to the OD of the weakly positive control serum provided with the kit. An antibody index exceeding 1.20 was determined as positive. IgG antibody titers were calculated from the sample absorbance and the reference standard curve generated from the reference sera provided with the kit. IgG antibody titers of ≥4.0 were defined as positive.
Virus isolation.
Throat swabs soaked in Eagle minimum essential medium were centrifuged at 1,500 × g for 10 min, and the supernatant was supplemented with 3% fetal bovine serum, 200 μg of streptomycin/ml, and 200 U of penicillin/ml and then inoculated onto Vero cells. The cells were cultured at 37°C, observed for 14 days, and harvested when the cytopathic effects (CPE) became prominent. Two blind passages were performed on all CPE-negative tissue cultures.
RNA extraction and RT-PCR.
Viral genomic RNA was extracted from 200 μl of throat swab samples for SH gene sequencing or the same volume of MuVi-MNG09-024 strain culture fluid for full-genome sequencing by using a QIAamp viral RNA minikit (Qiagen, Tokyo, Japan) according to the manufacturer's instruction manual. To amplify the SH gene region, one-step reverse transcription-PCR (RT-PCR) was performed with the primers SHfw (5′-TCAAGTAGTGTCGATGATCTC-3′) and HNrv (5′-CATAGGGCCTCCCGCGGCATTCACTATGAC-3′) by using a One-Step RNA-PCR kit (AMV) (TaKaRa, Kyoto, Japan) according to the manufacturer's instructions. A second PCR primer set, SHfw and SHrv (5′-AGCCTTGATCATTGATCATCC-3′), was designed to amplify genome positions 6130 to 6803 to sequence a 632-nt region including the entire SH gene.
In order to determine the entire genome sequence of the Mongolian isolate, RT-PCR was performed on extracted viral RNA with random hexamers by using a Transcriptor high-fidelity cDNA synthesis kit (Roche Applied Science, Tokyo, Japan) and seven sets of genome specific primers spanning the entire MuV genome. The second-round nested PCR was repeated using another seven sets of primer pairs. The resulting RT-PCR products were gel purified by using a QIAquick gel extraction kit (Qiagen) and then subjected to sequence analyses.
Sequence analysis.
Nucleotide sequences were determined by using a BigDye Terminator v3.1 cycle sequencing kit using a 3130xl Genetic Analyzer (Applied Biosystems, Tokyo, Japan).
Nucleotide alignments and phylogenetic analysis were performed by CLUSTALW (version 1.83) using neighbor-joining and Kimura two-parameter methods. The statistical significance of a particular tree topology was evaluated by bootstrap resampling of the sequences 1,000 times. To determine the genotype of the Mongolia mumps sequences, we used the criteria that Jin et al. proposed and that the World Health Organization (WHO) recommended on the basis of the reference strain sequences that they assigned (15, 41). Subgenotyping for registered genotype H sequences conformed to the previous reports (23, 38).
The GenBank accession numbers of the nucleotide sequences obtained in the present study are found in Table 1.
Neurovirulence test in neonatal rats.
We carried out a neurovirulence test in neonatal rats as previously described (24). Briefly, a litter of Lewis neonatal rats (<24 h old) (Charles River Japan Co., Ltd., Japan) were inoculated intracerebrally with 100 PFU of each MuV strain. Each litter consisted of six to seven pups. We administered the MuV isolates MuVi-MNG09-024, 02-49, and Odate-3 in this test. After a month, magnetic resonance imaging was performed in the injected animals under anesthesia by using MRmini SA (DS Pharma Biomedical Co., Ltd., Osaka, Japan). On the basis of the scanned images, the neurovirulence test (NVT) score was calculated as the ratio of the cross-sectional area of the lateral ventricle to that of the entire brain in pixel units by using the image analysis software ImageJ (National Institutes of Health, Bethesda, MD). The differences in the scores were determined by using the Student t test, with P < 0.05 being the criterion for statistical significance.
RESULTS
Mumps epidemics in Mongolia.
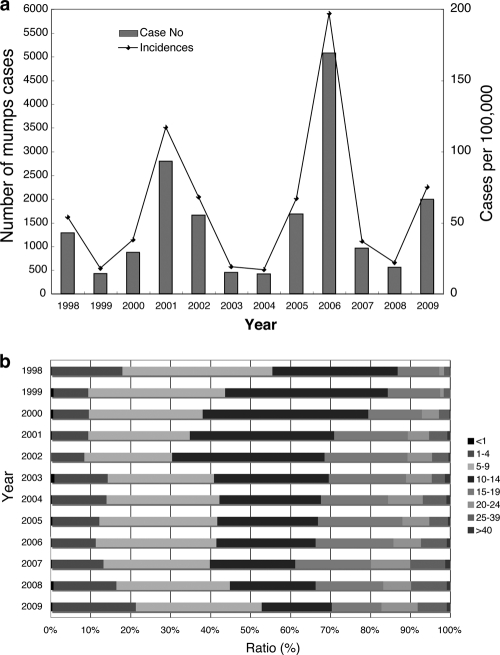
Since mumps vaccine is not available yet in Mongolia, mumps epidemics occur repeatedly with a periodicity of ∼5 years (Fig. 1a). The most recent outbreak occurred in 2006 and was the largest in the past 11 years in Mongolia, i.e., 5,073 annual cases (an incidence of 197/100,000) were reported countrywide. The number of reported mumps cases in 2009, from the latest data, was 1,990 annual cases (an incidence of 75/100,000), which is an average outbreak, and most patients resided in Ulaanbaatar, the capital of Mongolia. According to age-specific incidence, the 5- to 14-year age group is the predominant group for all reported mumps cases (ca. 50 to 70%) (Fig. 1b). In contrast, the latest percentage of the 10- to 14-year age group had decreased to half of the ratio in 1998. In contrast, the >20-year age group has increased ∼6 times over the past 11 years (Fig. 1b). However, the reasons for these phenomena are unclear.
Fig. 1.
Mumps activity in Mongolia from 1998 to 2009. (a) Annual reported mumps cases (bars) and incidence (solid line). (b) Component ratios (%) of age-specific incidence.
Phylogenetic analysis of MuV sequences from clinical samples.
We collected 32 throat swabs and 31 serum samples from 32 mumps patients (21 males and 11 females; age range, 1 to 35 years; mean age, 18 years 4 months) who were hospitalized in NCCD, Ulaanbaatar, from May to July 2009 (Table 1). Almost all serum samples (30 of 31 [96.7%]) were positive for IgM in an enzyme-linked immunosorbent assay, and their titers were very high (average, 16.9) (Table 1). In contrast, their IgG antibody titers were quite low (average, 3.5), and only nine samples (29.0%) were positive (Table 1). These results suggest that these mumps cases were primary infections.
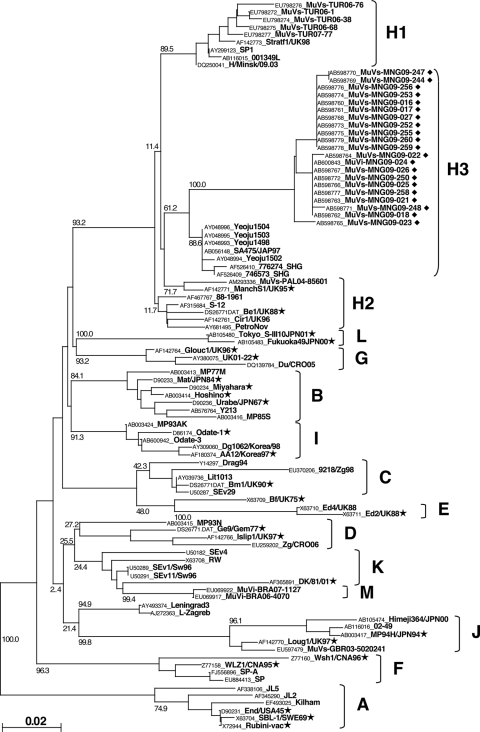
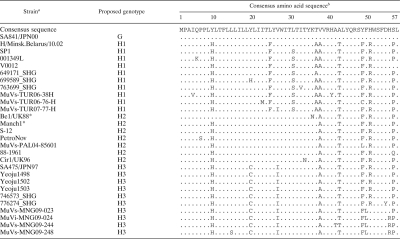
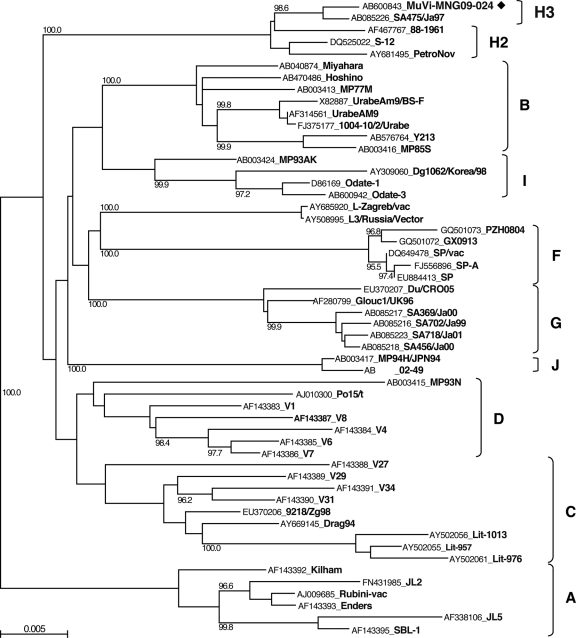
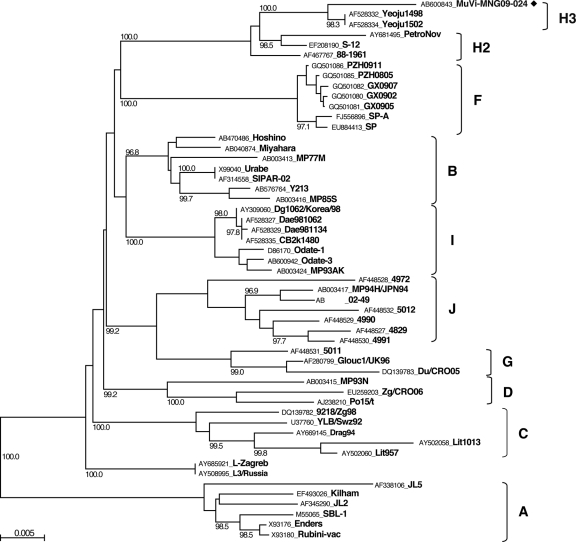
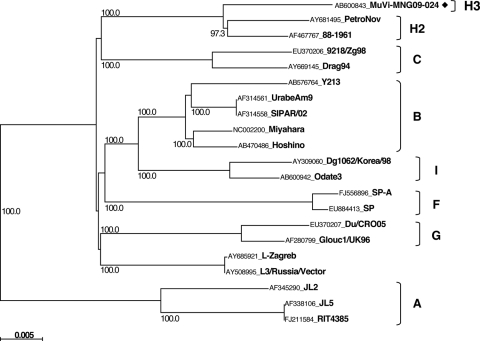
We detected 21 MuV cDNAs and obtained one virus isolate (MuVi-MNG09-024) from the 32 mumps patient throat swabs (Table 1). No associations were found between epidemiological factors (age, gender, IgM antibody titer, and IgG antibody titer) and the MuV cDNA detection (data not shown). Although the detected sequences included seven different sequences (MuVi-MNG09-024, MuVs-MNG09-016, MuVs-MNG09-022, MuVs-MNG09-023, MuVs-MNG09-244, MuVs-MNG09-247, and MuVs-MNG09-248), as a result of phylogenetic analysis of the SH region (316 nt), these viruses were classified into an identical unique cluster (Fig. 2), and their divergence is within 2.2%. Among the all genotypes thus far identified, genotype H sequences exhibit the closest relatedness to Mongolian viruses. However, the genetic divergences between the new sequences and genotype H established reference virus sequences (Be1/UK88 and ManchS1/UK95) are more than 5% (6.3 to 8.2%). Since this result fulfills the proposed criteria for assigning a new MuV genotype (15, 41), it is plausible that Mongolian MuV sequences might be classified into a novel genotype. Meanwhile, the genotype H sequences of MuVs isolated in the late 1990s from Japan (SA475/JPN97) (37), Korea (Yeoju1498, Yeoju1502, Yeoju1503, and Yeoju1504) (19), and Switzerland (776273SHG and 776274SHG) (38), which were classified as members of subgenotype H2 (23, 38), are included in the same branch with our new sequences, and the divergences among some of these sequences are not necessarily >5% (4.1 to 6.3%) (Fig. 2). In addition, this cluster of viruses share the characteristic two conserved amino acid sequences at positions 19 and 26 (cysteine and isoleucine, respectively) in the SH gene (18) (Table 2). Therefore, in order to define more precisely the phylogenetic position of the Mongolian MuVs, we determined the whole-genome sequence of the isolated virus MuVi-MNG09-024, and additional analyses were performed on the sequences of the F gene (Fig. 3), HN gene (Fig. 4), and the whole genome (Fig. 5) of the isolate by comparison to the sequences available in the DDBJ/EMBL/GenBank databases. According to these results, the phylogenetic trees constructed from the F or HN or whole-genome sequences branch in the same manner mutually and compared to that based on the SH gene (Fig. 3 and 4). However, former divergences are much restricted compared to that of the SH gene. In particular, the divergences between the Mongolian sequences and other genotype H sequences are relatively low (0.5 to 1.6% in F genes, 1.3 to 3.1% in HN genes, and 2.0 to 2.3% in whole genomes) compared to that of the same set of the SH gene (5.4 to 7.9%). It should be noted that those divergences are equivalent to the within-genotype variability of other genotypes, (e.g., up to 4.9% in F, up to 4.9% in HN, and up to 2.7% in whole genomes). In addition, a high bootstrap value (e.g., >95%) is also another important index to confirm the reliability of a phylogenetic relatedness. For instance, the bootstrap value of the SH gene-based lineage group that includes the Mongolian sequences SA475/JPN97, Yeoju1498, Yeoju1502, Yeoju1503, Yeoju1504, 776273SHG, and 776274SHG is relatively low (61.2%, Fig. 2). Meanwhile, the F gene-based lineage group, including the Mongolian sequence and SA475/JPN97 (Fig. 3), or the HN gene-based lineage group, including the Mongolian sequence, Yeoju1498, and Yeoju1502 (Fig. 4), is isolated from the other members of genotype H with high bootstrap values (98.6 and 100%, respectively). These results strongly suggest that Mongolian MuVs may be classified into genotype H and into an individual subgenotype, along with SA475/JPN97, Yeoju1498, Yeoju1502, Yeoju1503, Yeoju1504, and probably 776273SHG and 776274SHG. We propose to name this novel lineage subgenotype H3. The subclassification is also supported by the amino acid sequences of SH genes (Table 2) because all members of this cluster share a specific amino acid pattern at positions 19 and 26 in the SH gene.
Fig. 2.
Phylogenetic relationship between mumps virus strains obtained in the present study and previously published sequences based on the entire SH gene. Genotype A sequences were used as the outgroup. The bar scale indicates the nucleotide substitutions per site. ◆, Mumps virus strains obtained in the present study; ★, reference strains proposed by Jin et al. (15, 41).
Table 2.
Alignment of deduced amino acid sequences of SH gene of Mongolian strains and genotype H mumps viruses
*, Reference strain of genotype H.
The sequences representing each subgenotype strains are listed in this table. In order to highlight the type-specific differences in genotype H sequences, a genotype G sequence (SA841/JPN00 strain, AB056141) was adopted as a consensus amino acid sequence. The numbers above the consensus amino acid sequence show the amino acid residue numbers. Dots in each alignment exhibit amino acids identical to those to the consensus sequence.
Fig. 3.
Phylogenetic tree based on F gene sequences. ◆, Mumps virus strains obtained in the present study.
Fig. 4.
Phylogenetic tree based on HN gene sequences. ◆, Mumps virus strains obtained in the present study.
Fig. 5.
Phylogenetic tree based on whole-genome sequences. ◆, Mumps virus strains obtained in the present study.
Neurovirulence of Mongolian isolate.
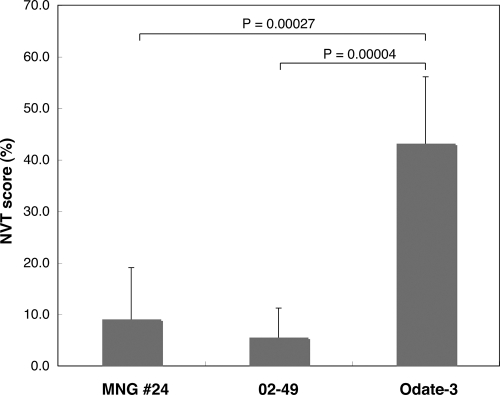
Several epidemiological studies reported that some genotypes (C, D, H, I, or J) were able to cause central nervous system (CNS) diseases (20, 28, 34–36, 38). Mongolian MuVs belong to genotype H, and all originated from the hospitalized mumps patients. Meanwhile, reliable animal models using common marmosets or neonatal rats to evaluate the neurovirulence of MuV have been proposed (24–27). Therefore, we assessed the neurovirulence of the Mongolian isolate, MuVi-MNG09-024, in a rat model (Fig. 6). As control viruses, we used the Odate-3 (genotype I) and 02-49 (genotype J) strains. Odate-3 is a variant of Odate-1 that caused meningitis with quite high incidence (>70%) in Akita Prefecture, Japan, in 1993 and was isolated during the same period in the same area (28). Odate-3 causes prominent meningitis in common marmosets by peripheral infection (27). The 02-49 strain was isolated in 2001 from the throat swab of a mumps patient with unilateral parotitis in Niigata Prefecture, Japan, and causes moderate hydrocephalus in rats. As expected, Odate-3 caused the most severe hydrocephalus in rats and exhibited the highest NVT score (43.2%) among the three strains. In contrast, the 02-49 strain showed the mildest hydrocephalus (NVT score, 5.6%). MuVi-MNG09-024 shows a moderate result (NVT score, 9.1%). Although the NVT score of MuVi-MNG09-024 is higher than that of 02-49, its scores are below 10%, and there is no significant difference between them (P = 0.476). Meanwhile, the NVT score of Odate-3 is significantly different from that of 02-49 and the Mongolian isolate (P = 0.00004 and 0.00027, respectively). These results suggest that the Mongolian isolate does not necessarily have highly neurovirulent properties.
Fig. 6.
Neurovirulence of MuV isolates in newborn rats. Each bar represents the average neurovirulence score with the corresponding standard deviation from six to seven animals.
DISCUSSION
This is the first report describing the mumps epidemic situation and molecular epidemiological analyses of the circulating MuVs in Mongolia. Interestingly, teenagers comprised the majority of mumps patients by age group during the period of study, and the age distribution of mumps onset is much older than that in Japan, where the <9-year population accounts for more than 90% of all mumps patients. In addition, the ratios of mumps patients in the >20-year age groups have also been consistently increasing over the period. One possible reason for mumps epidemics among the older age groups is due to low probabilities of exposure to MuV because of the low population density in Mongolia (two persons per square kilometer). This situation may resemble rural areas of the prevaccine period in the United States (7).
We detected 21 MuV cDNAs from 32 throat swabs of mumps patients in Mongolia. Although the positive ratio of RT-PCR was reasonable (21 of 32), the virus isolation rate was unexpectedly low (1 of 32). The reason for this low frequency of isolation is probably due to freeze-thawing of specimens resulting from insufficient cooling during 5-day transportation from Mongolia to Japan.
By phylogenetic analyses, the SH gene sequences (316 nt) obtained from these Mongolia mumps clinical specimens formed a single cluster, which consists of seven different sequences, and their genetic divergences are >6% (6.3 to 8.2%) compared to the reference strains of the most closely related genotype H. According to the criteria proposed by Jin et al. and the WHO (15, 41), it is plausible that these sequences should be classified into a new genotype based on these results. However, the results of analyses based on F or HN genes or whole-genome sequence data show that the divergences between the Mongolian isolate and other genotype H strains never exceed the within-genotype divergences of other genotypes. Therefore, we concluded that the Mongolian MuVs should be classified into genotype H. Because of its high divergence, the SH gene has been adopted for MuV genotyping. Primarily, phylogenetic analyses results based on F and HN genes are consistent with the SH gene. However, when there would be discrepancies between the phylogenetic results based on the SH gene and that based on F or HN genes or the whole-genome, and latter results were consistent with each other, the latter results should be adopted. From these results, we propose that F or HN gene sequences should be used in combination with SH gene data for confirming a new MuV genotype. By taking this additional information into the criteria for genotyping, it would be possible to reflect the antigenicities of MuVs to the phylogenetic results and to clarify the phylogenetic positions of outliers such as the strains RW(X63708), Tay-UK50(AF142774), and UK02-19(AY380077) (23). In order to establish the new criteria, we need to accumulate more F or HN gene sequence data of existing MuV strains.
Molecular phylogenetic analyses show that the Mongolia MuV sequences form a single cluster, and that these viruses are most closely related to a cluster of genotype H viruses, such as Japanese isolate (SA475/JPN97, isolated in 1997) (37), Korean isolates (Yeoju1498, Yeoju1502, Yeoju1503, and Yeoju1504, isolated in 1999) (18, 19) and Swiss isolates (776273SHG and 776274SHG, isolated in 1999 and 2000) (38). These strains were all isolated within a relatively short time period, from 1997 to 2000, and their SH gene sequences are almost homologous at the amino acid level (Table 2). The alignment of their SH gene nucleotide sequences suggests that SA475/JPN97 is phylogenetically closest to the ancestral virus of all of these viruses and that Mongolia viruses could be derived from the same ancestral virus because this cluster of viruses share the characteristic two conserved amino acid sequences at positions 19 and 26 in the SH gene (18) (Table 2). This amino acid motif characterizes this cluster of viruses and is never observed among other genotype H viruses. For these reasons and based on the results of phylogenetic analyses on F and HN genes, these viruses should be classified into a unique subgenotype, H3. The other two subgenotypes, H1 and H2, also have characteristic amino acid motifs within the SH region (Table 2). For instance, H1 viruses share a phenylalanine and alanine at positions 24 and 36. H2 viruses have no specific motif, other than a threonine residue at position 42, which is common in all genotype H viruses (Table 2). Meanwhile, all H2 viruses except Be1/UK88 strain share a histidine at position 9, which is also common in H1 and H2 viruses. These amino acid sequence properties suggest that subgenotype H2 may be an ancestral group of genotype H viruses and that the Be1/UK88 strain may be phylogenetically closest to an ancestral virus of genotype H.
Genotype H viruses mainly circulated in the western hemisphere (3, 5, 6, 20). However, since both the ancestral virus (SA475/JPN97) and the highly differentiated progenies (the Mongolian MuVs) were isolated from East Asia, genotype H3 viruses may have been indigenous to this area for more than 10 years. Although 776273SHG and 776274SHG were isolated in Switzerland, they might originate from the Asian origin virus because of their phylogenetic position and the time at which the viruses were isolated (38). In the present study, we did not detect any other genotypes, such as F, which is the dominant genotype in China. A major reason for this may be the restricted time period and region for collecting specimens in this study. Our samples were collected within 6 months from the patients residing in Ulaanbaatar and surrounding areas. Accordingly, it may be possible to detect other genotypes if we extended our study to cover larger areas in Mongolia for longer periods. In any case, this is the first report of MuVs circulated in Mongolia. Molecular epidemiological research on MuVs in this area has just started.
Several studies have suggested that there may be an association between several genotypes (C, D, H, I, or J) and neurovirulence (34, 36, 38). Meanwhile, Utz has reported that there is no statistical relationship between neurovirulence and genotype H (38). In order to clarify this ambiguity, we evaluated the neurovirulence of the Mongolia isolate by using a newborn rat system. As a result, the Mongolia isolate was found to only exhibit mild neuropathogenic property comparable with genotype J isolate, 02-49 strain. In contrast, genotype I Japanese isolate Odate-3 shows the highest neuropathogenicity among the three isolates, and faithfully reflects its highly neurovirulent properties in humans and in marmosets. Although these three strains are all classified in genotypes possibly associated with neurovirulence, their pathogenicities in rats are divided. In addition, genotype I strains isolated in Korea were not necessarily highly pathogenic (18). These facts suggest that there is no association between genotypes and neurovirulence.
We describe here the molecular epidemiology of the MuVs circulating in Mongolia. It is important to continuously investigate genotype distribution in this area in order to develop an understanding of not merely the domestic but the global epidemiology of mumps infection.
ACKNOWLEDGMENTS
All animal procedures were approved by the Committees on Animal Handling and Ethical Regulations of NIID.
We thank T. Kageyama (NIID) and T. Oka (NIID) for helpful advice on phylogenetic analyses and A. Kato (NIID), T. Saito (Kanagawa Prefectural Institute of Public Health), and K. Uchida (Saitama Prefectural Institute of Public Health) for donating MuV isolates.
This study was supported by a grant from the Ministry of Health, Labor, and Welfare of Japan.
Footnotes
Published ahead of print on 16 March 2011.
REFERENCES
- 1. Afzal M. A., et al. 1997. RT-PCR based diagnosis and molecular characterisation of mumps viruses derived from clinical specimens collected during the 1996 mumps outbreak in Portugal. J. Med. Virol. 52:349–353 [DOI] [PubMed] [Google Scholar]
- 2. Afzal M. A., Buchanan J., Heath A. B., Minor P. D. 1997. Clustering of mumps virus isolates by SH gene sequence only partially reflects geographical origin. Arch. Virol. 142:227–238 [DOI] [PubMed] [Google Scholar]
- 3. Akcali A., Yilmaz N., Uyar Y., Ertek M., Buzgan T. 2009. Genotyping of mumps virus circulating in Turkey in the 2006–2007 winter season. Arch. Virol. 154:1807–1812 [DOI] [PubMed] [Google Scholar]
- 4. Amexis G., Rubin S., Chatterjee N., Carbone K., Chumakov K. 2003. Identification of a new genotype H wild-type mumps virus strain and its molecular relatedness to other virulent and attenuated strains. J. Med. Virol. 70:284–286 [DOI] [PubMed] [Google Scholar]
- 5. Atrasheuskaya A. V., et al. 2007. Investigation of mumps vaccine failures in Minsk, Belarus, 2001–2003. Vaccine 25:4651–4658 [DOI] [PubMed] [Google Scholar]
- 6. Atrasheuskaya A. V., Kulak M. V., Rubin S., Ignatyev G. M. 2007. Mumps vaccine failure investigation in Novosibirsk, Russia, 2002–2004. Clin. Microbiol. Infect. 13:670–676 [DOI] [PubMed] [Google Scholar]
- 7. Barskey A. E., Glasser J. W., LeBaron C. W. 2009. Mumps resurgences in the United States: a historical perspective on unexpected elements. Vaccine 27:6186–6195 [DOI] [PubMed] [Google Scholar]
- 8. Carbone K. M., Rubin S. (ed.). 2007. Mumps virus, 5th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, PA [Google Scholar]
- 9. Cui A., Myers R., Xu W., Jin L. 2009. Analysis of the genetic variability of the mumps SH gene in viruses circulating in the UK between 1996 and 2005. Infect. Genet. Evol. 9:71–80 [DOI] [PubMed] [Google Scholar]
- 10. Cusi M. G., et al. 2001. Localization of a new neutralizing epitope on the mumps virus hemagglutinin-neuraminidase protein. Virus Res. 74:133–137 [DOI] [PubMed] [Google Scholar]
- 11. Elango N., Varsanyi T. M., Kovamees J., Norrby E. 1988. Molecular cloning and characterization of six genes, determination of gene order and intergenic sequences and leader sequence of mumps virus. J. Gen. Virol. 69(Pt. 11):2893–2900 [DOI] [PubMed] [Google Scholar]
- 12. Inou Y., et al. 2004. Molecular epidemiology of mumps virus in Japan and proposal of two new genotypes. J. Med. Virol. 73:97–104 [DOI] [PubMed] [Google Scholar]
- 13. Jin L., Beard S., Brown D. W. 1999. Genetic heterogeneity of mumps virus in the United Kingdom: identification of two new genotypes. J. Infect. Dis. 180:829–833 [DOI] [PubMed] [Google Scholar]
- 14. Jin L., Brown D. W., Litton P. A., White J. M. 2004. Genetic diversity of mumps virus in oral fluid specimens: application to mumps epidemiological study. J. Infect. Dis. 189:1001–1008 [DOI] [PubMed] [Google Scholar]
- 15. Jin L., et al. 2005. Proposal for genetic characterisation of wild-type mumps strains: preliminary standardisation of the nomenclature. Arch. Virol. 150:1903–1909 [DOI] [PubMed] [Google Scholar]
- 16. Kim S. H., et al. 2000. Phylogenetic analysis of the small hydrophobic (SH) gene of mumps virus in Korea: identification of a new genotype. Microbiol. Immunol. 44:173–177 [DOI] [PubMed] [Google Scholar]
- 17. Kovamees J., Rydbeck R., Orvell C., Norrby E. 1990. Hemagglutinin-neuraminidase (HN) amino acid alterations in neutralization escape mutants of Kilham mumps virus. Virus Res. 17:119–129 [DOI] [PubMed] [Google Scholar]
- 18. Lee J. Y., et al. 2003. Molecular characterization of two genotypes of mumps virus circulated in Korea during 1998–2001. Virus Res. 97:111–116 [DOI] [PubMed] [Google Scholar]
- 19. Lee J. Y., et al. 2004. Regional outbreak of mumps due to genotype H in Korea in 1999. J. Med. Virol. 73:85–90 [DOI] [PubMed] [Google Scholar]
- 20. Muhlemann K. 2004. The molecular epidemiology of mumps virus. Infect. Genet. Evol. 4:215–219 [DOI] [PubMed] [Google Scholar]
- 21. Orvell C., Alsheikhly A. R., Kalantari M., Johansson B. 1997. Characterization of genotype-specific epitopes of the HN protein of mumps virus. J. Gen. Virol. 78(Pt. 12):3187–3193 [DOI] [PubMed] [Google Scholar]
- 22. Orvell C., Kalantari M., Johansson B. 1997. Characterization of five conserved genotypes of the mumps virus small hydrophobic (SH) protein gene. J. Gen. Virol. 78(Pt. 1):91–95 [DOI] [PubMed] [Google Scholar]
- 23. Palacios G., et al. 2005. Molecular identification of mumps virus genotypes from clinical samples: standardized method of analysis. J. Clin. Microbiol. 43:1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rubin S. A., et al. 2005. The rat-based neurovirulence safety test for the assessment of mumps virus neurovirulence in humans: an international collaborative study. J. Infect. Dis. 191:1123–1128 [DOI] [PubMed] [Google Scholar]
- 25. Rubin S. A., Pletnikov M., Carbone K. M. 1998. Comparison of the neurovirulence of a vaccine and a wild-type mumps virus strain in the developing rat brain. J. Virol. 72:8037–8042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rubin S. A., et al. 2000. Evaluation of a neonatal rat model for prediction of mumps virus neurovirulence in humans. J. Virol. 74:5382–5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saika S., Kidokoro M., Ohkawa T., Aoki A., Suzuki K. 2002. Pathogenicity of mumps virus in the marmoset. J. Med. Virol. 66:115–122 [DOI] [PubMed] [Google Scholar]
- 28. Saito H., et al. 1996. Isolation and characterization of mumps virus strains in a mumps outbreak with a high incidence of aseptic meningitis. Microbiol. Immunol. 40:271–275 [DOI] [PubMed] [Google Scholar]
- 29. Santos C. L., et al. 2008. Detection of a new mumps virus genotype during parotitis epidemic of 2006–2007 in the state of Sao Paulo, Brazil. J. Med. Virol. 80:323–329 [DOI] [PubMed] [Google Scholar]
- 30. Takahashi M., et al. 2000. Single genotype of measles virus is dominant, whereas several genotypes of mumps virus are co-circulating. J. Med. Virol. 62:278–285 [PubMed] [Google Scholar]
- 31. Takeuchi K., Tanabayashi K., Hishiyama M., Yamada A. 1996. The mumps virus SH protein is a membrane protein and not essential for virus growth. Virology 225:156–162 [DOI] [PubMed] [Google Scholar]
- 32. Takeuchi K., Tanabayashi K., Hishiyama M., Yamada A., Sugiura A. 1991. Variations of nucleotide sequences and transcription of the SH gene among mumps virus strains. Virology 181:364–366 [DOI] [PubMed] [Google Scholar]
- 33. Tanabayashi K., Takeuchi K., Okazaki K., Hishiyama M., Yamada A. 1993. Identification of an amino acid that defines the fusogenicity of mumps virus. J. Virol. 67:2928–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tecle T., Bottiger B., Orvell C., Johansson B. 2001. Characterization of two decades of temporal co-circulation of four mumps virus genotypes in Denmark: identification of a new genotype. J. Gen. Virol. 82:2675–2680 [DOI] [PubMed] [Google Scholar]
- 35. Tecle T., Johansson B., Jejcic A., Forsgren M., Orvell C. 1998. Characterization of three co-circulating genotypes of the small hydrophobic protein gene of mumps virus. J. Gen. Virol. 79(Pt. 12):2929–2937 [DOI] [PubMed] [Google Scholar]
- 36. Tecle T., Mickiene A., Johansson B., Lindquist L., Orvell C. 2002. Molecular characterisation of two mumps virus genotypes circulating during an epidemic in Lithuania from 1998 to 2000. Arch. Virol. 147:243–253 [DOI] [PubMed] [Google Scholar]
- 37. Uchida K., Shinohara M., Shimada S., Segawa Y., Hoshino Y. 2001. Characterization of mumps virus isolated in Saitama Prefecture, Japan, by sequence analysis of the SH gene. Microbiol. Immunol. 45:851–855 [DOI] [PubMed] [Google Scholar]
- 38. Utz S., et al. 2004. Phylogenetic analysis of clinical mumps virus isolates from vaccinated and non-vaccinated patients with mumps during an outbreak, Switzerland 1998–2000. J. Med. Virol. 73:91–96 [DOI] [PubMed] [Google Scholar]
- 39. Waxham M. N., et al. 1988. Sequence determination of the mumps virus HN gene. Virology 164:318–325 [DOI] [PubMed] [Google Scholar]
- 40. Waxham M. N., Server A. C., Goodman H. M., Wolinsky J. S. 1987. Cloning and sequencing of the mumps virus fusion protein gene. Virology 159:381–388 [DOI] [PubMed] [Google Scholar]
- 41. World Health Organization 2005. Global status of mumps immunization and surveillance. Wkly. Epidemiol. Rec 80:418–424 [PubMed] [Google Scholar]
- 42. Wu L., Bai Z., Li Y., Rima B. K., Afzal M. A. 1998. Wild-type mumps viruses circulating in China establish a new genotype. Vaccine 16:281–285 [DOI] [PubMed] [Google Scholar]
- 43. Yeo R. P., Afzal M. A., Forsey T., Rima B. K. 1993. Identification of a new mumps virus lineage by nucleotide sequence analysis of the SH gene of ten different strains. Arch. Virol. 128:371–377 [DOI] [PubMed] [Google Scholar]