Abstract
We describe the first case of hip prosthetic infection due to Lactococcus garvieae. The patient, a 71-year-old woman fishmonger, developed a hip infection 7 years after total hip arthroplasty. The origin of infection was possibly due to the manipulation or intake of seafood or fish contaminated with Lactococcus garvieae.
CASE REPORT
A 71-year-old woman was admitted for orthopedic consultation at Nantes University Hospital because of pain in the left hip she had suffered for about 1 year. Her medical history included obesity (body mass index [BMI] of 41), arterial hypertension, type 2 diabetes mellitus, hemochromatosis, ischemic cardiopathy, and chronic alcoholism. Osteonecrosis of the femoral head had been treated with total hip arthroplasty 7 years before. Infection of the hip prosthesis was suspected on clinical examination. Radiographs of the left hip revealed signs of unsealing of the prosthesis, with serious modifications to the cortical bones. The patient was admitted for aspiration of the hip, using fluoroscopic guidance, 1 month later. Laboratory data included a blood leukocyte count (6.34 × 109 cells liter−1), with 74% polymorphonuclear leukocytes and a C-reactive protein level of 50 mg liter−1 (normal range is <5 mg liter−1). Serial blood cultures remained negative despite the absence of antibiotic treatment. A purulent fluid was aspirated from the hip. Direct examination showed Gram-positive cocci arranged in pairs, short chains, and clusters. Antibiotics were not prescribed until the final microbiological result from the joint fluid aspiration was completed.
A two-stage exchange arthroplasty was planned. The first stage involved removal of the infected prosthesis and insertion of a gentamicin spacer (Biomet Orthopaedics Switzerland GmbH, Dietikon, Switzerland) for six weeks. Seven out of eight perioperative samples (five tissue specimens from around the prosthesis, one bone specimen, and two joint fluids) were positive in culture. An antibiotic therapy was started, including a combination of ceftriaxone (2 g per day) and levofloxacin (750 mg per day) for 3 months. Intravenous administration of drugs was preferred to improve patient compliance and tissue diffusion of antibiotics. Ceftriaxone was chosen as an alternative to high-dose amoxicillin, which is more efficacious when patients have a high BMI.
Cultures from three samples (two tissue specimens and one from joint fluid) performed during the second stage of arthroplasty (6 weeks after the removal of the infected prosthesis) remained sterile after 14 days of incubation. Treatment was well tolerated, and the clinical outcome was favorable at 1 year after the hip replacement. The patient could walk again without any difficulty.
Culture of the joint fluid was positive on blood agar after 1 day of aerobic incubation (bioMérieux, Marcy l'Etoile, France). The bacterium was catalase and oxidase negative. An accurate identification (probability, 99%; typicity, 100%), using an IDGP N052 card (bioMérieux, Marcy-l'Etoile, France), led to Lactococcus garvieae. Sequencing of the partial 16S rRNA gene was performed, as previously described (7, 16, 21), to confirm identification of this rarely encountered bacterium in clinical specimens. The 500-bp fragment obtained was compared with NCBI GenBank entries using the BLAST algorithm (http://www.ncbi.nlm.nih.gov/BLAST) and the BIBI database (http://umr5558-sud-str1.univ-lyon1.fr/lebibi/lebibi.cgi). The sequence of the bacterium showed 100% identity with the sequence of the L. garvieae type strain (16S rRNA gene, GenBank accession number no. AB362690).
After 24 or 48 h of incubation at 37°C in aerobic conditions, the seven positive perioperative specimen cultures yielded Gram-positive cocci identified as L. garvieae, which allowed us to identify this bacterium as the origin of infection (1). In this case, the prosthetic and bone infections seem to have been acquired by hematogenous spread from a distant source.
Testing for in vitro susceptibility was performed using the disk diffusion and the Etest diffusion methods on Mueller-Hinton medium with 5% sheep blood (bioMérieux). The breakpoints described for general bacteria allowed us to consider this bacterium susceptible to amoxicillin (MIC, 1 μg/ml), ceftriaxone (MIC, 0.38 μg/ml), levofloxacin (MIC, 0.50 μg/ml), moxifloxacin (MIC, 0.25 μg/ml), and tetracycline but resistant to lincomycin, fosfomycin, cotrimoxazole, and rifampin, according to the French committee guidelines (3).
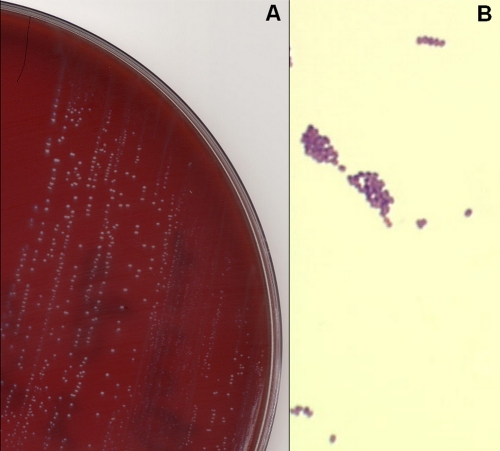
Lactococcus garvieae is a Gram-positive bacterium with nonmotile and nonsporulating cocci. Colonies on blood agar or nutrient agar are circular, smooth, and entire (Fig. 1). The small nonhemolytic gray colonies were able to grow in aerobic as well as anaerobic atmospheres. Ovoid cells were elongated in the direction of the chain and were mostly in pairs or short chains. The L. garvieae isolate showed the following phenotypic characteristics: PYR (pyrrolidonylarylamidase) positive, LAP (leucine aminopeptidase) positive, 6.5% salt positive growth culture, arginine dihydrolase positive, positive Voges-Proskauer reaction, and acidification in mannitol, trehalose, ribose, and saccharose broths but not in sorbitol, arabinose, or raffinose broths.
Fig. 1.
(A) Growth of small gray colonies of Lactococcus garvieae on a horse blood agar plate after 48 h of incubation in aerobic conditions. (B) Gram stain of Lactococcus garvieae with clusters and short chains of Gram-positive cocci (oil immersion, ×1,000).
L. garvieae is responsible for septicemia in various fish species (yellow tail, rainbow trout, gray mullet, giant freshwater prawns) and for mastitis in ruminants (2, 17, 18). Emergence of L. garvieae zoonotic diseases has been partly attributed to intensive aquaculture practice (18, 20). However, the role of L. garvieae in human infections has not been clearly established, with few reported cases. Nevertheless, microbiological diagnosis has been improved by molecular identification, such as PCR for 16S rRNA gene amplification. The most common infection is infective endocarditis, occurring in seven out of 11 reported L. garvieae-infected patients (6, 8, 10, 12, 19, 20, 21), followed by bacteremia in two out of 11 patients (20), osteomyelitis in one out of 11 (10), liver abscess in one out of 11 (13), and peritonitis in one out of 11 cases reported (20). However, L. garvieae had not yet been described as a cause of prosthetic joint infection. Analysis of these 11 cases of L. garvieae infection highlights the role of debilitating host factors: five patients out of the 11 suffered from heart disease, as did our patient, and six suffered from digestive tract disease.
One of the taxonomic changes in the classification of streptococci in the past few years has been the establishment of the Lactococcus genus (11, 16). These bacteria, formerly known as the lactic group of streptococci, consisted of Streptococcus lactis, Streptococcus cremoris, and, in many texts, Streptococcus diacetylactis. The Lactococcus genus was separated from the Streptococcus genus on the basis of genetic analysis, including DNA-DNA relatedness and 16S rRNA sequencing data (16). The Lactococcus genus comprises seven species and subspecies (L. lactis subsp. lactis, L. lactis subsp. cremoris, L. lactis subsp. hordniae, L. garvieae, L. piscium, L. plantarum, and L. raffinolactis) identified by phenotypic analysis (5, 14, 15). These species are facultative anaerobic, catalase-negative, Gram-positive cocci that primarily produce lactic acid from the fermentation of carbohydrates. Lactococcus garvieae, originally described as Streptococcus garvieae in 1981 by Garvie et al., was one of the new species of this genus, and the identification was confirmed by Collins et al. in 1983 and Elliott et al. in 1991 (2, 4, 9).
L. garvieae is not a fastidious-growing microorganism, but its diagnosis is difficult when bacteriologists are not aware of its possible links with animal contact or food contamination. Indeed, this species is rarely found in clinical routine samples, and its Gram morphology leads to suspicion of Streptococcus. To the best of our knowledge, this is the first report of prosthetic infection caused by L. garvieae. Despite several investigations, the source of contamination in this case was not found. However, fish consumption and its manipulation are the most probable sources of bacteremia, as has been reported in the literature (20). In fact, as was found from our patient's medical story, this former fishmonger and immunosuppressed woman lived near the sea and prepared and ate fish and shellfish frequently. A skin wound could be the gateway for asymptomatic bacteremia with secondary localization at the prosthesis.
This case illustrates the ability of L. garvieae to cause a late prosthetic joint infection in a patient with multiple comorbidities. The propensity for a commensal bacterium to cause unapparent bloodstream infections with septic metastasis and joint infections is of special concern for patients with a severely suppressed immune system.
Footnotes
Published ahead of print on 2 March 2011.
REFERENCES
- 1. Atkins B. L., et al. 1998. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J. Clin. Microbiol. 36:2932–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collins M. D., Farrow J. A., Phillips B. A., Kandler O. 1983. Streptococcus garvieae sp. nov. and Streptococcus plantarum sp. nov. J. Gen. Microbiol. 129:3427–3431 [DOI] [PubMed] [Google Scholar]
- 3. Comité de l'Antibiogramme de la Société Française de Microbiologie 2010. Recommandations 2010. Société Française de Microbiologie, Paris, France: http://www.sfm-microbiologie.org/UserFiles/file/casfm_2010.pdf [Google Scholar]
- 4. Elliott J. A., Collins M. D., Pigott N. E., Facklam R. R. 1991. Differentiation of Lactococcus lactis and Lactococcus garvieae from humans by comparison of whole-cell protein patterns. J. Clin. Microbiol. 29:2731–2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Facklam R., Elliott J. A. 1995. Identification, classification, and clinical relevance of catalase-negative, gram-positive cocci, excluding the streptococci and enterococci. Clin. Microbiol. Rev. 8:479–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fefer J. J., Ratzan K. R., Sharp S. E., Saiz E. 1998. Lactococcus garvieae endocarditis: report of a case and review of the literature. Diagn. Microbiol. Infect. Dis. 32:127–130 [DOI] [PubMed] [Google Scholar]
- 7. Fihman V., et al. 2006. Lactococcus garvieae endocarditis: identification by 16S rRNA and sodA sequence analysis. J. Infect. 52:e3–e6 [DOI] [PubMed] [Google Scholar]
- 8. Furutan N. P., et al. 1991. Lactococcus garvieae infection in humans: a cause of prosthetic valve endocarditis, p.109 Abstr. 91st Gen. Meet. Am. Soc. Microbiol American Society for Microbiology, Washington, DC [Google Scholar]
- 9. Garvie E. I., Farrow J. A. E., Phillips B. A. 1981. A taxonomic study of some strains of streptococci which grow at 10°C but riot at 45°C, including Streptococcus lactis and Streptococcus cremoris. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. 1 Orig. C 2:151–165 [Google Scholar]
- 10. James P. R., Hardman S. M., Patterson D. L. 2000. Osteomyelitis and possible endocarditis secondary to Lactococcus garvieae: a first case report. Postgrad. Med. J. 76:301–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kilpper-Balz R., Fischer G., Schleifer K. H. 1982. Nucleic acid hybridization of group N and group D streptococci. Curr. Microbiol. 7:245–250 [Google Scholar]
- 12. Li W. K., Chen Y. S., Wann S. R., Liu Y. C., Tsai H. C. 2008. Lactococcus garvieae endocarditis with initial presentation of acute cerebral infarction in a healthy immunocompetent man. Intern. Med. 47:1143–1146 [DOI] [PubMed] [Google Scholar]
- 13. Mofredj A., Baraka D., Kloeti G., Dumont J. L. 2000. Lactococcus garvieae septicemia with liver abscess in an immunosuppressed patient. Am. J. Med. 109:513–514 [DOI] [PubMed] [Google Scholar]
- 14. Pu Z. Y., Dobos M., Limsowtin G. K., Powell I. B. 2002. Integrated polymerase chain reaction-based procedures for the detection and identification of species and subspecies of the Gram-positive bacterial genus Lactococcus. J. Appl. Microbiol. 93:353–361 [DOI] [PubMed] [Google Scholar]
- 15. Schleifer K. H. 1987. Recent changes in the taxonomy of lactic acid bacteria. FEMS Microbiol. Rev. 46:201–203 [Google Scholar]
- 16. Schleifer K. H., et al. 1985. Transfer of Streptococcus lactis and related streptococci to the genus Lactococcus gen. nov. Syst. Appl. Microbiol. 6:183–195 [Google Scholar]
- 17. Teixeira L. M., et al. 1996. Phenotypic and genotypic characterization of atypical Lactococcus garvieae strains isolated from water buffalos with subclinical mastitis and confirmation of L. garvieae as a senior subjective synonym of Enterococcus seriolicida. Int. J. Syst. Bacteriol. 46:664–668 [DOI] [PubMed] [Google Scholar]
- 18. Vela A. I., et al. 2000. Phenotypic and genetic characterization of Lactococcus garvieae isolated in Spain from lactococcosis outbreaks and comparison with isolates of other countries and sources. J. Clin. Microbiol. 38:3791–3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vinh D. C., Nichol K. A., Rand F., Embil J. M. 2006. Native-valve bacterial endocarditis caused by Lactococcus garvieae. Diagn. Microbiol. Infect. Dis. 56:91–94 [DOI] [PubMed] [Google Scholar]
- 20. Wang C. Y., et al. 2007. Lactococcus garvieae infections in humans: possible association with aquaculture outbreaks. Int. J. Clin. Pract. 61:68–73 [DOI] [PubMed] [Google Scholar]
- 21. Yiu K. H., et al. 2007. A rare cause of infective endocarditis; Lactococcus garvieae. Int. J. Cardiol. 114:286–287 [DOI] [PubMed] [Google Scholar]