Abstract
Three phenotypic methods (modified Hodge test, chromogenic agar, and meropenem discs combined with specific inhibitors) used for the detection of carbapenemase activity were tested on a panel of characterized Enterobacteriaceae expressing various β-lactamase mechanisms. Overall, the meropenem-plus-inhibitor approach was more sensitive and specific than the other methods, despite its limitation of being unable to detect class D carbapenemases.
TEXT
The identification of carbapenemase production in Enterobacteriaceae that results in resistance or intermediate resistance to one or more carbapenems has serious implications in hospital infection control and/or epidemiological investigations. Several approaches for the detection of these microorganisms have been described, including the modified Hodge test (MHT) (3), chromogenic agars (1, 13, 18), and the use of carbapenem discs with the addition of specific β-lactamase inhibitors (7, 9, 10, 15, 16, 19, 22). In this study, we compared these 3 approaches with a panel of characterized Enterobacteriaceae. The goal of this comparison was to find the most accurate method for the detection of carbapenemase activity in clinical isolates when these enzymes are suspected in laboratories after initial antimicrobial susceptibility screening.
A panel of 77 genotypically characterized strains harboring different mechanisms of β-lactam resistance was used for this study (Table 1). Carbapenem hydrolysis was measured by spectrophotometer analysis as described previously (15). MICs were determined by the agar dilution method and Etest (bioMérieux) and interpreted using Clinical and Laboratory Standards Institute guidelines (3). The imipenem-EDTA Etest (metallo-β-lactamase [MBL]-EDTA hereinafter) was used when necessary. The sensitivities and specificities of the inhibitor assay (7), MHT (3), and KPC chromogenic agar (Colorex KPC; Inverness Medical, Ottawa, Canada) in the detection of carbapenemase activity were compared. MHT was performed using both meropenem and ertapenem discs (Oxoid). Since our previous tests showed similar results for both meropenem and imipenem discs combined with dipicolinic acid (DPA), aminophenylboronic acid (BA), or cloxacillin (CLX) (data not shown), the discs used in this study (meropenem discs [Oxoid] supplemented with DPA [M-DPA], BA [M-BA], and CLX [M-CLX] [Sigma]) and the interpretation of the assays were based on a recent study by Giske et al. (7). Briefly, an increase of 4 mm in the inhibition zone for M-BA and 5 mm for M-CLX or M-DPA compared with the zone when the meropenem disc was used alone were considered to be positive results. A positive result for only M-BA indicates the presence of a serine carbapenemase; a combination of M-BA and M-CLX inhibitions indicates AmpC hyperproduction plus impermeability (AmpC+imp); an M-DPA-positive result indicates the inhibition of a metallo-β-lactamase. The in-laboratory-prepared discs were kept at −20°C; their stability was tested once a week during 5 weeks by using the same positive controls selected from the panel studied and comparing the results to the results obtained with freshly prepared discs. Extended-spectrum β-lactamase (ESBL) and carbapenemase gene screening were performed when necessary (4, 20).
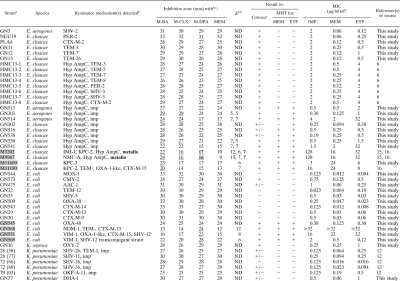
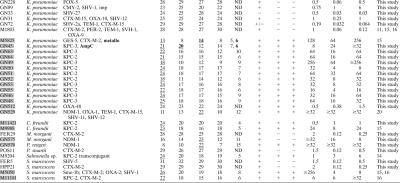
Table 1.
Comparison of the inhibitor method, Colorex KPC selective agar, and the modified Hodge test, and MICs for imipenem, meropenem, and ertapenem
All of the carbapenemase-producing strains are highlighted in gray boxes.
Previously unknown metallo-β-lactamase and AmpC activities are indicated in boldface. Hyp AmpC, hyperproduced chromosomal AmpC; imp, impermeability.
The halo zones affected by the inhibitors are underlined. M-BA, meropenem-aminophenylboronic acid; M-DPA, meropenem-dipicolinic acid; M-CLX, meropenem-cloxacillin; MEM, meropenem alone.
Δ, difference between inhibition zones with meropenem alone and meropenem-inhibitor; ND, no difference.
Interpretation of Colorex KPC selective agar results: +, positive growth; +/−, few colonies; −, no growth.
IMP, imipenem; MEM, meropenem; ETP, ertapenem.
Enterobacter spp. can display a carbapenemase-resistant phenotype that is commonly due to the combination of chromosomal AmpC+imp (8, 17, 21, 23). In our panel, we included 26 Enterobacter spp. with different β-lactam resistance mechanisms (Table 1). MHT detected all serine carbapenemase producers but also the strains with AmpC+imp, thus reducing the specificity (Table 2). All 26 Enterobacter spp. showed positive results on the Colorex KPC plates. The inhibitor approach was able to detect all 4 serine carbapenemase-producing Enterobacter cloacae. Interestingly, synergy with the M-CLX disc (Δ of 6 to 7 mm [where Δ is the difference between the inhibition zones with meropenem alone and meropenem-inhibitor], suggesting AmpC hyperproduction) was observed in 2 of them, but the increase in the inhibition zones was much lower than that observed with M-BA (Δ of 12 to 15 mm, KPC-2/IMI-1 and NMC-A producers), showing a clear difference from the results for the 4 isolates displaying only AmpC+imp (see below). The inhibitor method also allowed us to detect previously unknown MBL activity (M-DPA positive, Δ of 7 mm) in 2 of these 4 serine carbapenemase-producing isolates. The MBL-EDTA confirmed these results (isolate M3202, imipenem MIC ≥ 256 μg/ml and imipenem-EDTA MIC of 12 μg/ml; isolate M9967, imipenem ≥ 256 μg/ml and imipenem-EDTA MIC of 64 μg/ml). PCR did not detect the most common MBL genes (blaIMP, blaVIM, and blaNDM), and these 2 isolates remain under study. The phenotype AmpC+imp was detected in 4 of 8 cases (M-BA and M-CLX positives, Δ of 5 to 7 mm). This differential detection (in only 4 isolates with that phenotype) could be attributed to different levels of expression of the chromosomal blaAMP-C gene.
Table 2.
Sensitivity, specificity, and positive and negative predictive values of the methods testeda
| Strain group | Method | SN | SP | PPV | NPV |
|---|---|---|---|---|---|
| Enterobacter spp. | MHT | 1 | 0.73 | 0.33 | 1 |
| Colorex | 1 | 0.50 | 0.15 | 1 | |
| IM | 1 | 1 | 1 | 1 | |
| E. coli | MHT | 1 | 1 | 1 | 1 |
| Colorex | 1 | 0.82 | 0.67 | 1 | |
| IM | 0.80 | 1 | 1 | 0.90 | |
| Klebsiella spp. | MHT | 1 | 1 | 1 | 1 |
| Colorex | 1 | 0.54 | 0.56 | 1 | |
| IM | 0.93 | 1 | 1 | 0.93 | |
| Other | MHT | 0.88 | 1 | 1 | 0.80 |
| Colorex | 1 | 0.50 | 0.64 | 1 | |
| IM | 1 | 1 | 1 | 1 |
All values are relative to the genotypic characterization. MHT, modified Hodge test; Colorex, Colorex KPC selective agar; IM, inhibitor method; SN, sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value.
Thirteen Escherichia coli isolates were also studied (Table 1). MHT and Colorex KPC plates detected 4 carbapenemase producers (Table 1), but 2 plasmid-mediated AmpC producers also grew on chromogenic agar. Using the inhibitor approach, strong potentiation with M-DPA (Δ of 6 to 12 mm) was observed among 3 MBL producers (2 VIM-1 and 1 NDM-1), but the OXA-48-producing strain could not be detected with this method; this limitation was also previously reported (7).
One Klebsiella oxytoca and 26 Klebsiella pneumoniae isolates with different mechanisms of β-lactam resistance were included (Table 1). MHT detected all carbapenemase producers, while the Colorex KPC also detected most other Klebsiella isolates (hence reducing specificity) (Table 2). The inhibitor approach clearly detected all the KPC- and GES-5-producing isolates (potentiation with M-BA only, Δ of 5 to 10 mm), as well as one NDM-1 strain (positive result with M-DPA, Δ of 12 mm) but was not able to detect OXA-48. One isolate also displayed potentiation with M-CLX (Δ of 6 mm), suggesting the expression of a plasmid-mediated AmpC. Another strain was positive for M-DPA (Δ of 6 mm), and the MBL activity was confirmed by MBL-EDTA (isolate M5825, imipenem MIC of 32 μg/ml and imipenem-EDTA MIC of 1.5 μg/ml). However, no plasmid-mediated AmpC- or carbapenemase-encoding genes were detected in these last 2 isolates, and they remain under investigation.
The remaining 11 isolates in the collection included 1 Providencia stuartii, 1 Providencia rettgeri, 1 Salmonella sp., 2 Citrobacter freundii, 2 Morganella morganii, and 4 Serratia marcescens (Table 1). MHT could detect all serine carbapenemases, but it failed to detect the NDM-1-producing M. morganii. All 11 isolates were positive on the Colorex KPC agar. The inhibitor test detected all carbapenemase activity correctly (M-BA, Δ of 4 to 8 mm, and M-DPA, Δ of 11 to 15 mm, detecting serine and metallo carbapenemase activity, respectively).
The stability of in-laboratory-prepared discs, stored at −20°C, was tested weekly for 5 weeks, using 3 controls expressing MBL (NDM-1, to test M-DPA discs), serine carbapenemase (KPC-3, for M-BA discs), and derepressed AmpC plus porin loss (for M-BA and M-CLX discs). The activities of discs prepared in week 1 were compared with those of freshly made discs, and similar inhibition zones were observed for all meropenem-inhibitor combinations during the period studied, indicating that these discs can be prepared and kept at −20°C without losing activity.
Although new CLSI guidelines have minimized the importance of phenotypic tests for clinical purposes (3), accurate identification of carbapenem resistance mechanisms in the clinical laboratory could help guide proper antibiotic therapy against these Enterobacteriaceae. For example, clinical isolates with a combination of mechanisms causing carbapenem resistance (e.g., impermeability plus ESBLs and/or hyperproduced AmpC) could still respond to carbapenem treatment (5), whereas carbapenemase-producing Enterobacteriaceae would rule out the use of β-lactams to treat patients, thus significantly limiting treatment options for life-threatening infections. Also, the use of reliable tests for the detection of carbapenemases in microbiology laboratories has great impact on infection control and epidemiological surveillance of this resistance. Accurate results obtained at this stage followed by proper infection control measures could prevent the dissemination of these carbapenem-resistant microorganisms in nosocomial settings. MHT is the current method recommended by CLSI for these purposes. In the analysis of different Enterobacteriaceae species, we found that MHT was sensitive and able to detect carbapenemase activity in K. pneumoniae and E. coli. However, this study was limited by the lack of isolates expressing ESBLs (like CTX-Ms) plus impermeability, a combination that has been shown to display false-positive results (2, 16). Many false positives were observed in Enterobacter spp. with this test. Most alarming, a false negative was found in one NDM-1-producing M. morganii isolate. Laboratories using the MHT should be aware of these test limitations.
In this study, we found Colorex KPC to be a poor predictor of carbapenemase activity, as our results have shown that all 29 carbapenemase producers and 39 non-carbapenemase producers grew on this selective medium. In previous studies, high sensitivity and specificity have been assigned to a similar commercial KPC chromogenic agar (CHROMagar KPC), but Enterobacteriaceae with different β-lactam resistance mechanisms were not included (13, 18). Considering that the Colorex KPC is designed to detect bacteria with carbapenem resistance (Inverness Medical technical sheet) and all isolates tested in this study expressed reduced susceptibility/resistance to at least one carbapenem, their detection was correct but most of the cases were carbapenemase false positives.
The inhibitor method could specifically detect carbapenemase activity (i.e., KPC-2/3, GES-5, IMI-1, NMC-A, NDM-1, and VIM-1 producers included in the bacterial collection) and differentiate it from AmpC+imp. Using this approach, we could distinguish serine carbapenemase activity in 2 of the E. cloacae isolates with derepressed AmpC, comparing different Δ values obtained for M-BA and M-CLX (the Δ of M-BA was double the Δ of M-CLX) (Table 1). The method allowed us to identify previously unknown MBL activity in 3 isolates and AmpC activity in 1 K. pneumoniae isolate. However, since there are no currently known specific inhibitors for class D carbapenemases, this method was unable to detect 2 OXA-48-producing isolates, and their detection in suspected Enterobacteriaceae negative by this approach has to be performed by molecular methods.
In conclusion, the inhibitor approach appears to be the most accurate method in detecting all carbapenemases from class A and B but not from class D (Table 2). Despite this limitation, this method has proven to be best of the 3 tested in this study. With this test, carbapenemase-producing Enterobacter spp. could be differentiated from the isolates expressing combinations of other mechanisms. Based on the specificity of the inhibitors used (BA, CLX, and DPA), this approach could also be used to differentiate Klebsiella spp. isolates expressing ESBLs (e.g., CTX-M) plus impermeability, as has been reported in part by Pasteran and collaborators using ertapenem-BA and ertapenem-oxacillin discs (16). Regardless, molecular tests must be used to confirm the phenotypic results, which are an important tool for rapid epidemiologic or infection control purposes.
Acknowledgments
We are indebted to Prasad Rawte, Stephen Lo, and Heather Siebert for their technical support. We are grateful to Fernando Pasteran and Alejandra Corso for supplying some reference strains for this study.
Footnotes
Published ahead of print on 23 March 2011.
REFERENCES
- 1. Carrër A., Fortineau N., Nordmann P. 2010. Use of ChromID extended-spectrum β-lactamase medium for detecting carbapenemase-producing Enterobacteriaceae. J. Clin. Microbiol. 48:1913–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carvalhaes C. G., Picao R. C., Nicoletti A. G., Xavier D. E., Gales A. C. 2010. Cloverleaf test (modified Hodge test) for detecting carbapenemase production in Klebsiella pneumoniae: be aware of results. J. Antimicrob. Chemother. 65:249–251 [DOI] [PubMed] [Google Scholar]
- 3. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing: 19th informational supplement. CLSI document M100-S20. CLSI, Wayne, PA [Google Scholar]
- 4. Dallenne C., Da Costa A., Decre D., Favier C., Arlet G. 2010. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 65:490–495 [DOI] [PubMed] [Google Scholar]
- 5. Elliott E., et al. 2006. In vivo development of ertapenem resistance in a patient with pneumonia caused by Klebsiella pneumoniae with an extended-spectrum β-lactamase. Clin. Infect. Dis. 42:e95–e98 [DOI] [PubMed] [Google Scholar]
- 6.Galas M., et al. Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. D-896.1999. [Google Scholar]
- 7. Giske C. G., et al. 2010. A sensitive and specific phenotypic assay for detection of metallo-β-lactamases and KPC in Klebsiella pneumoniae with the use of meropenem disks supplemented with aminophenylboronic acid, dipicolinic acid and cloxacillin. Clin. Microbiol. Infect. 17:552–556 [DOI] [PubMed] [Google Scholar]
- 8. Jacoby G. A., Mills D. M., Chow N. 2004. Role of β-lactamases and porins in resistance to ertapenem and other β-lactams in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:3203–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kimura S., Ishii Y., Yamaguchi K. 2005. Evaluation of dipicolinic acid for detection of IMP- or VIM-type metallo-β-lactamase-producing Pseudomonas aeruginosa clinical isolates. Diagn. Microbiol. Infect. Dis. 53:241–244 [DOI] [PubMed] [Google Scholar]
- 10. Lee K., Lim Y. S., Yong D., Yum J. H., Chong Y. 2003. Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-β-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J. Clin. Microbiol. 41:4623–4629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Melano R., et al. 2003. Multiple antibiotic-resistance mechanisms including a novel combination of extended-spectrum β-lactamases in a Klebsiella pneumoniae clinical strain isolated in Argentina. J. Antimicrob. Chemother. 52:36–42 [DOI] [PubMed] [Google Scholar]
- 12. Melano R. G., Davidson R. J., Musgrave H. L., Forward K. R. 2006. Cephalosporin resistance in Klebsiella pneumoniae from Nova Scotia, Canada. Diagn. Microbiol. Infect. Dis. 56:197–205 [DOI] [PubMed] [Google Scholar]
- 13. Panagea T., et al. 2011. Evaluation of CHROMagar™ KPC for the detection of carbapenemase-producing Enterobacteriaceae in rectal surveillance cultures. Int. J. Antimicrob. Agents. 37:124–128 [DOI] [PubMed] [Google Scholar]
- 14. Pasteran F. G., et al. 2008. Klebsiella pneumoniae carbapenemase-2, Buenos Aires, Argentina. Emerg. Infect. Dis. 14:1178–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pasteran F., Mendez T., Guerriero L., Rapoport M., Corso A. 2009. Sensitive screening tests for suspected class A carbapenemase production in species of Enterobacteriaceae. J. Clin. Microbiol. 47:1631–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pasteran F., Mendez T., Rapoport M., Guerriero L., Corso A. 2010. Controlling the false positive results of the Hodge and Masuda assays for class A carbapenemase detection in species of Enterobacteriaceae. J. Clin. Microbiol. 48:1323–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raimondi A., Traverso A., Nikaido H. 1991. Imipenem- and meropenem-resistant mutants of Enterobacter cloacae and Proteus rettgeri lack porins. Antimicrob. Agents Chemother. 35:1174–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Samra Z., et al. 2008. Evaluation of CHROMagar KPC for rapid detection of carbapenem-resistant Enterobacteriaceae. J. Clin. Microbiol. 46:3110–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samuelsen Ø., et al. 2008. Evaluation of phenotypic tests for the detection of metallo-β-lactamase-producing Pseudomonas aeruginosa in a low prevalence country. J. Antimicrob. Chemother. 61:827–830 [DOI] [PubMed] [Google Scholar]
- 20. Tijet N., et al. 2011. New Delhi metallo-β-lactamase, Ontario, Canada. Emerg. Infect. Dis. 17:306–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woodford N., et al. 2007. Ertapenem resistance among Klebsiella and Enterobacter submitted in the UK to a reference laboratory. Int. J. Antimicrob. Agents. 29:456–459 [DOI] [PubMed] [Google Scholar]
- 22. Yagi T., et al. 2005. Practical methods using boronic acid compounds for identification of class C β-lactamase-producing Klebsiella pneumoniae and Escherichia coli. J. Clin. Microbiol. 43:2551–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yigit H., et al. 2002. Carbapenem resistance in a clinical isolate of Enterobacter aerogenes is associated with decreased expression of OmpF and OmpC porin analogs. Antimicrob. Agents Chemother. 46:3817–3822 [DOI] [PMC free article] [PubMed] [Google Scholar]