Abstract
This article describes positive (1→3)-β-d-glucan levels in serum from infants with primary Pneumocystis infection and from immunosuppressed patients with Pneumocystis pneumonia (PCP) and negative levels in serum from patients colonized by Pneumocystis jirovecii. Glucan detection is a complementary tool for the diagnosis of the diverse clinical presentations of P. jirovecii infection.
TEXT
Pneumocystis jirovecii (the human-specific Pneumocystis species) is an atypical fungus that has been recognized for a long time as a cause of severe pneumonia (Pneumocystis pneumonia [PCP]) in immunocompromised individuals. More recently, the use of PCR assays for P. jirovecii detection in pulmonary samples has revealed that immunocompromised patients and patients with lung diseases can be infected by only a small number of microorganisms, which are usually not detected by microscopy (13). The term colonization is frequently used in this context. It has also been established that P. jirovecii can be detected by PCR in nasopharyngeal aspirates (NPA) from immunocompetent infants who develop primary Pneumocystis infection contemporaneously with acute respiratory syndromes (18, 19).
(1→3)-β-d-Glucan (BG) represents a major structural component of the cell wall of most fungi and is abundant in Pneumocystis cysts (4). Previous studies have reported high levels of BG in serum samples from patients with PCP (2, 3, 5–9, 11, 12, 14, 15, 17, 20). In contrast, this marker has been investigated in only one study concerning pulmonary colonization with P. jirovecii (16) and has not yet been studied in primary Pneumocystis infection. In this study, we retrospectively investigated BG levels in serum samples from infants developing primary Pneumocystis infection, adults colonized by P. jirovecii, and adults developing PCP, who were followed up at Amiens University Hospital (France).
Fourteen immunocompetent term infants (mean age, 6 months [range, 1.7 to 15.7]; 9 boys and 5 girls) with primary Pneumocystis infection were enrolled. They were hospitalized between November 1999 and April 2001. NPA and serum samples were initially collected from all infants to investigate an acute respiratory syndrome. Serum samples were collected over an interval ranging from 2 days before to 3 days after NPA retrieval. No infants presented immunodeficiency and risk factors or clinical signs of invasive fungal infection. No infants had received antibiotics before sampling. P. jirovecii was detected by a real-time PCR assay targeting the mitochondrial large subunit rRNA (mtLSU rRNA) gene, as previously reported (18). The diagnosis of primary Pneumocystis infection was based on positive results of P. jirovecii detection and the low mean age of the infants (6 months), compatible with first contact with the fungus. Clinical improvement was obtained in all infants in response to respiratory physiotherapy, despite the absence of any specific treatment for the fungus. Eight patients colonized by P. jirovecii (mean age, 50.8 years [range, 23 to 77]; 5 men and 3 women) hospitalized between February 2008 and June 2009 were enrolled. Bronchoalveolar lavage (BAL) and serum samples were initially collected from patients to investigate pulmonary symptoms (abnormal chest X-ray, cough) or fever. Serum samples were collected over an interval ranging from 2 days before to 10 days after BAL fluid retrieval. None of the patients presented clinical or laboratory signs of invasive fungal infection. Detection of Aspergillus galactomannan and Candida mannan antigens in serum samples using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Platelia Candida Ag plus and Platelia Aspergillus Ag; Bio-Rad, Marnes-la-Coquette, France) and blood cultures (Bactec Mycosis; Becton, Dickinson and Company, Sparks, MD) were negative. P. jirovecii was not detected in BAL specimens by microscopic examination but was detected by a real-time PCR assay directed at the mtLSU rRNA gene, as previously described (10). Alternative diagnoses of PCP were bacterial pneumonia (six patients), pulmonary sarcoidosis (one patient), and bronchial carcinoma (one patient). Clinical improvement was observed in all patients except for the patient with bronchial carcinoma, despite the absence of any specific treatment for the fungus. Patients with bacterial pneumonia and pulmonary sarcoidosis were successfully treated by extended-spectrum beta-lactam antibiotics and corticosteroids, respectively. The follow-up with no PCP occurrence ranged from 2 to 21 months. The patient with lung cancer died 6 weeks after P. jirovecii detection in a context of terminal cancer with no evidence of a contribution of P. jirovecii to death. A diagnosis of PCP was therefore excluded by the physicians, and all eight patients were considered to be colonized by P. jirovecii.
Six patients diagnosed with PCP (mean age, 53 years [range, 31 to 69]; 5 men and 1 woman) hospitalized between August 2008 and April 2009 were enrolled and were used as positive controls for BG detection. The diagnosis of PCP was based on the criteria described by the Centers for Disease Control and Prevention for HIV-infected patients (1). BAL and serum samples were initially collected from all patients to investigate pulmonary symptoms. Serum samples were collected on the same day as BAL fluid retrieval. None of the patients presented any clinical or laboratory signs of invasive fungal infection other than PCP. Serum samples were negative for Aspergillus galactomannan and Candida mannan antigens. Blood cultures were also negative. None of the patients had received antibiotics before sampling. The BAL specimens tested positive for P. jirovecii by microscopic examination with Giemsa stain and an immunofluorescence assay (MonofluoKit Pneumocystis; Bio-Rad, Marnes-la-Coquette, France) and by a real-time PCR assay directed at the mtLSU rRNA gene. Underlying conditions were HIV infection, bronchial carcinoma, colonic adenocarcinoma, acute alcoholic hepatitis treated with high-dose corticosteroids (one patient each), and kidney transplantation (two patients). All patients received anti-Pneumocystis treatment immediately after the diagnosis of PCP was established. Clinical improvement was obtained after 3 weeks of treatment in five patients. One patient died from stroke 4 days after PCP diagnosis.
Serum samples from the three populations were stored at −80°C. BG levels in stored serum samples were determined using the Fungitell test kit (Associates of Cape Cod, Inc., Cape Cod, MA) according to the manufacturer's instructions. A BG level of ≥80 pg/ml was considered to be positive. The results of BG detection in the three populations were compared using Student's test and Wilcoxon's test. Statistical significance was defined as a P value of <0.05.
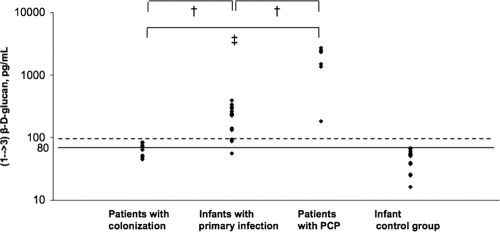
The results of BG detection in infants ranged from 56 to 394 pg/ml, with a median value of 217.6 pg/ml. Thirteen of the 14 infants had a positive result, i.e., ≥80 pg/ml. The results in colonized patients ranged from 45 to 84 pg/ml, with a median value of 69.5 pg/ml. Six of the 8 colonized patients had a negative result. BG levels in the remaining two patients were 82.7 and 84 pg/ml. Patients with PCP had positive BG results, with high values ranging from 184 to 2,710 pg/ml and a median value of 1,768.5 pg/ml.
BG levels in infants and colonized patients were significantly lower than those in the PCP control group (P < 0.05). BG levels in infants were significantly higher than those in colonized adults (P < 0.05). Results are shown in Fig. 1.
Fig. 1.
Serum (1→3)-β-d-glucan levels in infants with primary Pneumocystis infection, patients with Pneumocystis colonization, patients with Pneumocystis pneumonia (PCP), and an infant control group. Serum BG levels were significantly higher in the PCP control group than in infants with primary infection (†, Student's test, P < 0.05) and colonized patients (‡, Wilcoxon's test, P < 0.05). Serum BG levels were significantly higher in infants with primary infection than in colonized patients (†, Student's test, P < 0.05).
This study reports high BG levels for patients with PCP. Similar results have previously been reported (2, 3, 5–9, 11, 12, 14–17, 20). None of these patients presented any factors that interfere with BG levels, especially antibiotics prior to sampling or invasive fungal infection. These BG levels can then be correlated with the presence of P. jirovecii cysts in the lungs, which were effectively numerous and easily observed on microscopic examination of BAL samples.
Although the number of infants is limited, this is the first study to report serum BG levels during primary Pneumocystis infection. Positive serum BG levels were observed in 93% (13/14) of infants, with a median value of 217.6 pg/ml. None of these infants presented any factors that interfere with serum BG levels. Furthermore, BG was not detected in a control group that consisted of 14 infants hospitalized for an acute respiratory syndrome and negative for P. jirovecii detection (results are shown in Fig. 1). These results are therefore consistent with the presence of P. jirovecii cysts in the lungs of infants with primary Pneumocystis infection, as was established in adults with PCP. However, none of the infants presented BG levels higher than 400 pg/ml, in contrast with adult patients with PCP. The burden of P. jirovecii cysts in the lungs may be limited during the course of primary infection. Indeed, although infants are immune naive for P. jirovecii, they are not immunocompromised and their immune response is sufficiently effective to finally clear the fungus without the need for specific treatment.
In contrast, 6/8 colonized patients had negative serum BG levels, i.e., less than 80 pg/ml. The two remaining patients presented positive BG results, but with values clearly lower than 100 pg/ml, i.e., 82.7 and 84 pg/ml. These results may be explained by the absence or rarity of P. jirovecii cysts in pulmonary alveoli.
Taking into account the results of BG detection, Pneumocystis infection in immunocompetent infants appears to be a clinical entity which may be closer to PCP than to pulmonary colonization. Moreover, serum BG levels combined with PCR assay may discriminate between PCP and pulmonary colonization with P. jirovecii. A serum BG assay is a noninvasive test which appears to be a complementary tool for the diagnosis of the diverse presentations of P. jirovecii infection.
Acknowledgments
This study was supported by the Agence Française de la Sécurité Sanitaire de l'Environnement et du Travail (conventions EST/2006/1/41).
This study was approved by the Ethics Commission of Picardy, France. The project was registered in France with the Direction Générale de la Santé (no. 990440).
Footnotes
Published ahead of print on 23 March 2011.
REFERENCES
- 1. Centers for Disease Control and Prevention 1992. Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb. Mortal. Wkly. Rep. 41:1–19 [PubMed] [Google Scholar]
- 2. Cuetara M. S., et al. 2008. Use of a serum (1→3)-beta-D-glucan assay for diagnosis and follow-up of Pneumocystis jirovecii pneumonia. Clin. Infect. Dis. 47:1364–1366 [DOI] [PubMed] [Google Scholar]
- 3. de Boer M. G., et al. 2011. β-D-Glucan and s-adenosylmethionine serum levels for the diagnosis of Pneumocystis pneumonia in HIV-negative patients: a prospective study. J. Infect. 63:93–100 [DOI] [PubMed] [Google Scholar]
- 4. Dei-Cas E., Aliouat E. M., Caillez J. C. 2005. Pneumocystis cellular structure, p. 61–94 In Walzer P. D., Cushion M. (ed.), Pneumocystis pneumonia, 3rd ed. Marcel Dekker, New York, NY [Google Scholar]
- 5. Del Bono V., et al. 2009. Clinical evaluation of a (1,3)-beta-D-glucan assay for presumptive diagnosis of Pneumocystis jiroveci pneumonia in immunocompromised patients. Clin. Vaccine Immunol. 16:1524–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Desmet S., et al. 2009. Serum (1–3)-beta-D-glucan as a tool for diagnosis of Pneumocystis jirovecii pneumonia in patients with human immunodeficiency virus infection or hematological malignancy. J. Clin. Microbiol. 47:3871–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finkelman M. A. 2010. Pneumocystis jirovecii infection: Cell wall (1→3)-β-D-glucan biology and diagnostic utility. Crit. Rev. Microbiol. 36:271–281 [DOI] [PubMed] [Google Scholar]
- 8. Held J., Koch M., Reischl U., Danner T., Serr A. 2010. Serum (1→3)-beta-D-glucan measurement as early indicator for Pneumocystis jirovecii pneumonia and evaluation of its prognostic value. Clin. Microbiol. Infect. doi:10.1111/j.1469-0691.2010.03318.x [DOI] [PubMed] [Google Scholar]
- 9. Marty F. M., Koo S., Bryar J., Baden L. R. 2007. (1→3) beta-D-glucan assay positivity in patients with Pneumocystis (carinii) jiroveci pneumonia. Ann. Intern. Med. 147:70–72 [DOI] [PubMed] [Google Scholar]
- 10. Meliani L., et al. 2003. Real time quantitative PCR assay for Pneumocystis jirovecii detection. J. Eukaryot. Microbiol. 50(Suppl.):651. [DOI] [PubMed] [Google Scholar]
- 11. Nakamura H., et al. 2009. Clinical utility of serum beta-D-glucan and KL-6 levels in Pneumocystis jirovecii pneumonia. Intern. Med. 48:195–202 [DOI] [PubMed] [Google Scholar]
- 12. Persat F., et al. 2008. Contribution of the (1→3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 46:1009–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peterson J. C., Cushion M. T. 2005. Pneumocystis: not just pneumonia. Curr. Opin. Microbiol. 8:393–398 [DOI] [PubMed] [Google Scholar]
- 14. Pisculli M. L., Sax P. E. 2008. Use of a serum beta-glucan assay for diagnosis of HIV-related Pneumocystis jiroveci pneumonia in patients with negative microscopic examination results. Clin. Infect. Dis. 46:1928–1930 [DOI] [PubMed] [Google Scholar]
- 15. Shimizu A., Oka H., Matsuda T., Ozaki S. 2005. (1→3)-Beta-D glucan is a diagnostic and negative prognostic marker for Pneumocystis carinii pneumonia in patients with connective tissue disease. Clin. Exp. Rheumatol. 23:678–680 [PubMed] [Google Scholar]
- 16. Shimizu Y., et al. 2009. Serum markers in interstitial pneumonia with and without Pneumocystis jirovecii colonization: a prospective study. BMC Infect. Dis. 9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tasaka S., et al. 2007. Serum indicators for the diagnosis of Pneumocystis pneumonia. Chest 131:1173–1180 [DOI] [PubMed] [Google Scholar]
- 18. Totet A., et al. 2003. Immunocompetent infants as a human reservoir for Pneumocystis jirovecii: rapid screening by non-invasive sampling and real-time PCR at the mitochondrial large subunit rRNA gene. J. Eukaryot. Microbiol. 50(Suppl.):668–669 [DOI] [PubMed] [Google Scholar]
- 19. Vargas S. L., et al. 2001. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin. Infect. Dis. 32:855–861 [DOI] [PubMed] [Google Scholar]
- 20. Watanabe T., et al. 2009. Serum (1→3) beta-D-glucan as a noninvasive adjunct marker for the diagnosis of Pneumocystis pneumonia in patients with AIDS. Clin. Infect. Dis. 49:1128–1131 [DOI] [PubMed] [Google Scholar]