Abstract
“Candidatus Neoehrlichia mikurensis” is a new intracellular pathogen associated with human infection and death. “Candidatus Neoehrlichia mikurensis” infection in a chronically neutropenic dog from Germany was confirmed by DNA sequencing. The same organism was previously described from ticks and two sick human beings from Germany.
CASE REPORT
In March 2007, an 8-year-old, female, spayed Irish Setter was seen at a private practice in Munich, Germany, due to multiple nodules in her mammary glands. The dog had lived in California from August 1999 until August 2003. While residing in the United States, the dog had traveled within California and Mexico. Between 2003 and 2007, the dog had not left Germany. Preoperatively, a complete blood cell count (CBC) was normal. Due to a presumptive diagnosis of mammary carcinoma, a complete unilateral mastectomy was performed, and the dog recovered uneventfully. In May 2007, an ovariohysterectomy and a mastectomy of the opposite mammary chain were performed. Postoperatively, the dog was lethargic and developed profuse subcutaneous hemorrhage around the surgery site. Vitamin K was empirically administered by the primary veterinarian. Three days after the surgery, the owner noted severe swelling of the left hind limb, which was diagnosed as a subcutaneous hematoma by the primary veterinarian. CBC revealed mild anemia (red blood cell count, 5.47 × 106/μl; reference range, 5.5 × 106 to 8.5 × 106/μl) and thrombocytopenia (platelets, 143 × 103/μl; reference range, 200 × 103 to 560 × 103/μl). Numbers of leukocytes (8.4 ×103/μl; reference range, 3.0 × 103 to 10.0 ×103/μl) and the differential cell count were within the reference ranges. A serum chemistry profile and coagulation panel (prothrombin time and activated partial thromboplastin time) were normal. Thoracic computed tomography (CT) scanning, abdominal ultrasound, and bone marrow cytology did not detect metastasis or any other abnormalities.
Because of the travel history, screening for vector-borne organisms was performed. Antibodies to Ehrlichia canis, Babesia canis, Anaplasma phagocytophilum, or Leishmania species antigens (by an immunofluorescent antibody [IFA] test) were not detected. A Dirofilaria immitis (antigen detection) enzyme-linked immunosorbent assay (ELISA) was negative. A PCR test for Ehrlichia spp. and Anaplasma spp. performed at a private diagnostic laboratory in Germany, approximately 5 days after the second surgery, was reported as positive (species were not provided, and a DNA sequence was not obtained). Based upon the PCR result, treatment was initiated 10 days after the second surgery (May 2007) with doxycycline (10 mg/kg of body weight every 12 h orally [p.o.] for 4 weeks) and meloxicam (0.1 mg/kg p.o. for 4 days). This therapy resulted in clinical stabilization of the patient and resolution of thrombocytopenia. In July 2007, the dog was neutropenic and the platelet count was at the lower end of the reference range, and these hematological findings persisted in several follow-up CBCs over the next 2 months (neutrophils, 1.0 × 103 to 1.4 × 103/μl; reference range, 3.0 × 103 to 9.0 × 103/μl). A Coombs test was positive, whereas antigranulocytic antibodies were not detected. A second Ehrlichia/Anaplasma species PCR, performed in August 2007, was again positive for a DNA sequence later identified as “Candidatus Neoehrlichia mikurensis” (the gene targeted and sequence similarity were not provided by the private laboratory). A second course of doxycycline (10 mg/kg every 12 h p.o.) was prescribed for 4 weeks. Due to persistent neutropenia, despite doxycycline therapy, the dog was referred to the Veterinary Teaching Hospital of the University of Munich in September 2007. Upon presentation, the physical examination was unremarkable, although the owner reported a slightly decreased level of activity and episodic mild serous nasal discharge. CBC confirmed neutropenia (neutrophils, 1.55 × 103/μl; reference range, 3.0 × 103 to 9.0 × 103/μl), but the remaining blood cell counts were within reference ranges. The previously extracted DNA from the blood sample collected in August 2007 and new blood samples from September 2007 (before and after the second course of doxycycline therapy) were shipped to the Intracellular Pathogens Research Laboratory at North Carolina State University for attempted characterization or confirmation of the organism identified by the commercial laboratory.
Due to the very limited volume of the extracted DNA from the blood sample collected in August 2007, a suicide PCR was designed to target the groEL gene of “Candidatus Neoehrlichia mikurensis” using GenBank database sequences available in December 2007. Primers NM-128s (5′-AACAGGTGAAACACTAGATAAGTCCAT-3′) and NM-1152as (5′-TTCTACTTTGAACATTTGAAGAATTACTAT-3′) were manually designed. Amplification was performed in a 25-μl-final-volume reaction mixture containing 1× PCR Premix Ex Taq (which included TaKaRa Ex Taq HS, a deoxynucleoside triphosphate [dNTP] mixture, and MgCl2), 12.5 pmol of each primer, and 5 μl of DNA template. Conventional PCR was performed in an Eppendorf Mastercycler (EP gradient) under the following conditions: a single hot-start cycle at 95°C for 1 min, followed by 55 cycles of denaturing at 94°C for 15 s, annealing for 30 s, and extension at 72°C for 45 s. Three different annealing temperatures were tested: 54°C, 56°C, and 58°C (Fig. 1). Amplification was completed by an additional cycle at 72°C for 1 min, and products were analyzed by 2% agarose gel electrophoresis under UV exposure. DNA from a healthy dog was used as a PCR negative control. PCR products were cloned using pGEM-T easy vector systems, and three individual clones were selected for sequencing. Additionally, an aliquot of the PCR product was submitted for direct sequencing. Chromatograms were manually evaluated, and consensus sequences were generated for phylogenetic comparisons, conducted in MEGA4 (15).
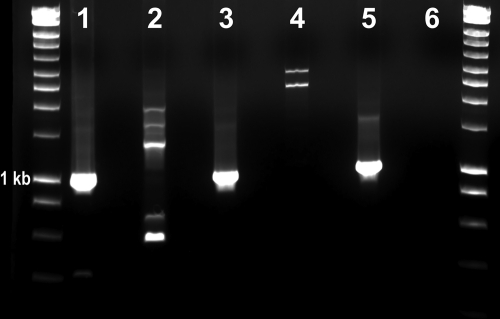
Fig. 1.
Ethidium bromide-stained agarose gel showing DNA amplification using suicide PCR under three different annealing temperatures (lanes 1 and 2, 54°C; lanes 3 and 4, 56°C; lanes 5 and 6, 58°C) for the groEL gene of “Candidatus Neoehrlichia mikurensis.” Lanes 1, 3, and 5, genomic DNA (gDNA) from a German dog with suspected infection; lanes 2, 4, and 6, gDNA from a healthy dog. Molecular marker, 10 kb. The 1-kb ladder is identified.
Successful amplifications were obtained from the preantibiotic sample (Fig. 1) at all three annealing temperatures tested. The 58°C amplification product was used for direct sequencing and cloning. The consensus groEL sequence was 100% homologous to “Candidatus Neoehrlichia mikurensis” (Fig. 2). Amplification and sequencing of other genes were not possible due to insufficient material from the original sample. No amplification product was obtained from the blood sample taken after the 4-week course of doxycycline.
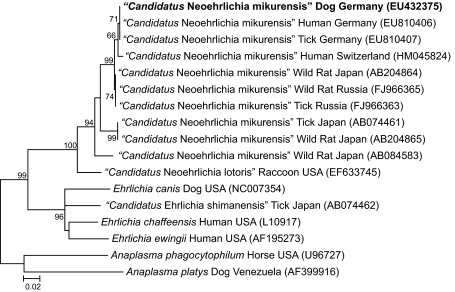
Fig. 2.
Phylogenetic tree based on 968 bp from the GroEL gene sequences of “Candidatus Neoehrlichia mikurensis” EU432375 (obtained from EDTA-blood from our patient, here indicated in boldface) and related organisms by using the maximum-likelihood method based on the Jukes-Cantor model. Each bacterial name is followed by the isolation source and geographic origin, and the GenBank accession number is provided in parentheses. The numbers at the nodes indicate percentages of bootstrap support based on 1,000 replicates. Percentages corresponding to partitions reproduced in fewer than 50% of bootstrap replicates are collapsed. The scale bar indicates 0.02 substitution per nucleotide position. Evolutionary analyses were conducted in MEGA4 (15).
“Candidatus Neoehrlichia mikurensis” infection has not been previously reported in domestic animals. During the last decade, DNA of a new species of intracellular bacteria in the family Anaplasmataceae was sequenced from ticks and rodents from Europe and Asia. In 1999, a new bacterium closely related to Ehrlichia sp. (named the Schotti variant) was detected in eight Ixodes ricinus ticks collected in Netherlands (11). The same organism was detected 2 years later from 21 I. ricinus ticks collected in the Baltic regions of Russia (1). In 2003, an Ehrlichia-like 16S rRNA gene sequence that was 99% similar to the Schotti variant was obtained from Rattus norvegicus in China and named the Ehrlichia sp. Rattus variant (10). In the same year, 16S rRNA gene sequences that were 99.7% similar to the Schotti variant were obtained from 10 I. ricinus ticks removed from asymptomatic human beings in Belluno Province, Italy, and named “Candidatus Ehrlichia walkerii” (3). The first isolate of this organism was obtained in Japan in 2004 from Rattus norvegicus. The morphological and molecular characterization of that isolate, in conjunction with earlier closely related GenBank sequences, supported a novel genetic cluster within the family Anaplasmataceae, and “Candidatus Neoehrlichia mikurensis” was proposed for all current organisms in this group (7). Between 2004 and 2009, this novel bacterium was found in wild rodents in Italy (2), in ticks in Slovakia (13) and the Omsk area of Russia (12), and in ticks and wild rodents in Japan (9). In 2007, the molecular screening of 62 febrile-human samples in Japan did not detect “Candidatus Neoehrlichia mikurensis” DNA (14). However, in 2010, four human cases were reported in Europe: two from Germany (16), one from Sweden (17), and one from Switzerland (5).
Compared with other groEL sequences deposited in GenBank, the dog sequence was 100% similar (968/968 bp) to “Candidatus Neoehrlichia mikurensis” sequences obtained from an Ixodes ricinus tick and a human case in Germany (Fig. 2). Interestingly, the human and canine cases occurred 150 km apart, and both were diagnosed in the second half of 2007 (16). In addition, phylogenetic analysis identified a cluster that included the dog, human, and tick “Candidatus Neoehrlichia mikurensis” groEL sequences from Germany, but these sequences differed from “Candidatus Neoehrlichia mikurensis” sequences obtained from the patient in Switzerland (98% homology) and from wild rats and ticks in Russia and Japan (Fig. 2). On the basis solely of groEL phylogeny, all available GenBank sequences from August 2010 suggest the presence of two geographically distinct clusters of “Candidatus Neoehrlichia mikurensis” distributed in western Europe (Germany and Switzerland) and eastern Asia (Japan and eastern Russia), respectively, as previously suggested (16) (Fig. 2).
Host adaptation of “Candidatus Neoehrlichia mikurensis” may vary between humans and dogs as reported for some other organisms in the Anaplasmataceae family (6). The reported human cases had signs of systemic illness, whereas the dog, despite persistent hematological abnormalities, was asymptomatic. Fever, headache, leukocytosis, and increased C-reactive protein concentrations were common features of this infection among the four human cases. Based upon the limited dog and human case experiences, “Candidatus Neoehrlichia mikurensis” may be associated with coagulation disturbances, a frequent finding in humans and dogs infected with species of the Anaplasmataceae family (4, 6). Despite mild thrombocytopenia (143 × 103 platelets/μl after surgery), profuse subcutaneous hemorrhage occurred postoperatively in the dog, whereas intracerebral and subarachnoid hemorrhage and deep vein thromboses were reported in each of the two German patients, respectively (16), and a cutaneous rash was reported in the Swedish patient (17). Following treatment with doxycycline, the three human patients recovered fully, whereas other antibiotics were generally ineffective (5, 16, 17). In the canine case, it is noteworthy that clinical recovery, with the absence of amplifiable DNA of “Candidatus Neoehrlichia mikurensis” in the bloodstream, was obtained after a second course of doxycycline, administered at a high dose for 4 weeks. Unfortunately, samples were not available to determine if the dog was infected with “Candidatus Neoehrlichia mikurensis” or with another Anaplasmataceae organism at the time of surgery. Consequently, this dog could have been an asymptomatic carrier of “Candidatus Neoehrlichia mikurensis,” and surgery-induced immune alterations may have caused recrudescence of the infection. Alternatively, this dog may have been reinfected within 2 months after the first course of doxycycline, since organisms of the Anaplasmataceae family do not consistently induce protective immunity or block reinfections in dogs (8).
The detection of “Candidatus Neoehrlichia mikurensis” in a dog expands the current list of emerging diseases capable of infecting pets and people. The detection of a bacterial strain in dogs and sick humans supports the possibility of exposure to a common tick vector. Clinical evaluation of additional canine cases will be necessary to define the virulence of “Candidatus Neoehrlichia mikurensis” for dogs.
Nucleotide sequence accession number.
The consensus groEL sequence has been deposited in the GenBank database under accession number EU432375.
Acknowledgments
This study was supported by the Intracellular Pathogens Research Laboratory, North Carolina State University, College of Veterinary Medicine, Raleigh, NC.
Footnotes
Published ahead of print on 2 March 2011.
REFERENCES
- 1. Alekseev A. N., Dubinina H. V., Van De Pol I., Schouls L. M. 2001. Identification of Ehrlichia spp. and Borrelia burgdorferi in Ixodes ticks in the Baltic regions of Russia. J. Clin. Microbiol. 39:2237–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beninati T., Piccolo G., Rizzoli A., Genchi C., Bandi C. 2006. Anaplasmataceae in wild rodents and roe deer from Trento Province (northern Italy). Eur. J. Clin. Microbiol. Infect. Dis. 25:677–678 [DOI] [PubMed] [Google Scholar]
- 3. Brouqui P., Sanogo Y. O., Caruso G., Merola F., Raoult D. 2003. Candidatus Ehrlichia walkerii: a new Ehrlichia detected in Ixodes ricinus tick collected from asymptomatic humans in Northern Italy. Ann. N. Y. Acad. Sci. 990:134–140 [DOI] [PubMed] [Google Scholar]
- 4. Dumler J. S., Madigan J. E., Pusterla N., Bakken J. S. 2007. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin. Infect. Dis. 45(Suppl. 1):S45–S51 [DOI] [PubMed] [Google Scholar]
- 5. Fehr J. S., et al. 2010. Septicemia caused by tick-borne bacterial pathogen Candidatus Neoehrlichia mikurensis. Emerg. Infect. Dis. 16:1127–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harrus S., Waner T. 2011. Diagnosis of canine monocytotropic ehrlichiosis (Ehrlichia canis): an overview. Vet. J. 187:292–296 [DOI] [PubMed] [Google Scholar]
- 7. Kawahara M., et al. 2004. Ultrastructure and phylogenetic analysis of “Candidatus Neoehrlichia mikurensis” in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. Int. J. Syst. Evol. Microbiol. 54:1837–1843 [DOI] [PubMed] [Google Scholar]
- 8. Little S. E. 2010. Ehrlichiosis and anaplasmosis in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 40:1121–1140 [DOI] [PubMed] [Google Scholar]
- 9. Naitou H., et al. 2006. Molecular identification of Ehrlichia species and “Candidatus Neoehrlichia mikurensis” from ticks and wild rodents in Shizuoka and Nagano Prefectures, Japan. Microbiol. Immunol. 50:45–51 [DOI] [PubMed] [Google Scholar]
- 10. Pan H., Liu S., Ma Y., Tong S., Sun Y. 2003. Ehrlichia-like organism gene found in small mammals in the suburban district of Guangzhou of China. Ann. N. Y. Acad. Sci. 990:107–111 [DOI] [PubMed] [Google Scholar]
- 11. Schouls L. M., Van De Pol I., Rijpkema S. G. T., Schot C. S. 1999. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J. Clin. Microbiol. 37:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shpynov S., Fournier P. E., Rudakov N., Tarasevich I., Raoult D. 2006. Detection of members of the genera Rickettsia, Anaplasma, and Ehrlichia in ticks collected in the Asiatic part of Russia. Ann. N. Y. Acad. Sci. 1078:378–383 [DOI] [PubMed] [Google Scholar]
- 13. Spitalská E., Boldis V., Kostanová Z., Kocianová E., Stefanidesová K. 2008. Incidence of various tick-borne microorganisms in rodents and ticks of central Slovakia. Acta Virol. 52:175–179 [PubMed] [Google Scholar]
- 14. Tabara K., et al. 2007. Molecular survey of Babesia microti, Ehrlichia species and Candidatus neoehrlichia mikurensis in wild rodents from Shimane Prefecture, Japan. Microbiol. Immunol. 51:359–367 [DOI] [PubMed] [Google Scholar]
- 15. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 16. von Loewenich F. D., et al. 2010. Detection of “Candidatus Neoehrlichia mikurensis” in two patients with severe febrile illnesses: evidence for a European sequence variant. J. Clin. Microbiol. 48:2630–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Welinder-Olsson C., Kjellin E., Vaht K., Jacobsson S., Wennerås C. 2010. First case of human “Candidatus Neoehrlichia mikurensis” infection in a febrile patient with chronic lymphocytic leukemia. J. Clin. Microbiol. 48:1956–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]