Abstract
Multilocus sequence typing of Borrelia garinii isolates from humans and comparison with rodent and tick isolates were performed. Fifty-nine isolates were divided into two phylogenetic groups, and an association was detected between clinical and rodent isolates, suggesting that, in Japan, human-pathogenic B. garinii comes from rodents via ticks.
TEXT
Lyme disease is a multisystemic disorder caused by infection with the tick-borne spirochetes Borrelia burgdorferi sensu lato (s.l.). B. burgdorferi sensu stricto (s.s.), Borrelia garinii, and Borrelia afzelii, which are the known pathogenic borreliae of humans. B. burgdorferi s.s. is geographically distributed throughout North America and Europe, whereas B. garinii and B. afzelii are distributed throughout Europe and Asia. These Borrelia species are transmitted by Ixodes ricinus in Europe and Ixodes persulcatus in Asia and Russia. (2, 16, 24). In Europe, serological characterization has revealed that B. garinii is composed of several OspA serotypes (27). At present, it is understood that one B. garinii serotype (B. garinii OspA serotype 4) is maintained by rodents (7), although other serotypes of B. garinii are maintained by birds (4, 5, 26). Strains classified into B. garinii OspA serotype 4 were found to be distinguishable from other B. garinii strains by multilocus sequence typing (MLST), which was recently established for Lyme disease borreliae (14, 15). In Japan, B. garinii is known to be the main pathogenic borrelia, and it is transmitted by I. persulcatus (29). However, the natural reservoir host of human pathogenic B. garinii remains unclear since I. persulcatus infests both rodents and birds in Japan (28). To resolve this question, MLST analysis was performed on clinical, tick, and rodent isolates, and phylogenetic relationships among these strains were investigated.
Nineteen B. garinii strains were obtained for MLST analysis from Lyme disease patients with erythema migrans in Japan. Human isolates were cultured from erythema migrans lesions as previously described (22). As for tick and rodent isolates, 40 strains were examined. The sources of these strains are listed in Table 1. Eighteen strains were isolated from I. persulcatus ticks, which were collected from Japan (15 strains) and Russia (3 strains). Twenty-two strains were isolated from rodents. Of these, 10 were from Myodes rufocanus subsp. bedfordiae and 8 from Apodemus speciosus (both sets collected in Japan), and 4 were from A. uralensis (collected in China) (Table 1). The cultivation of borreliae was carried out at 34°C in modified Barbour-Stoenner-Kelly (BSK) medium (using minimal essential medium alpha [BioWest, Germany] as a substitute for CMRL-1066) (1). These strains were stored at −80°C until use. Cultivated bacterial cells (late-log phase) were used in DNA preparation. The genomic DNA of isolated strains was prepared by using a DNA extraction kit (DNeasy blood and tissue kit; Qiagen, Germany) according to the manufacturer's instructions. The PCR assay was performed according to Margos et al. (14, 15). After DNA amplification of eight loci (clpA, ATP-dependent Clp protease subunit A gene; clpX, ATP-dependent Clp protease subunit X gene; nifS, aminotransferase gene; pepX, dipeptidyl aminopeptidase gene; pyrG, CTP synthase; recG, DNA recombinase gene; rplB, 50S ribosomal protein L2 gene; and uvrA, excinuclease ABC subunit A gene), PCR products were purified by using ExoSAP-IT (GE Healthcare UK, Ltd., United Kingdom) and were directly sequenced (ABI Prism 3130xl Genetic Analyzer; Life Technologies Corporation). All sequences were deposited in GenBank (see the table in the supplemental material). In addition, reference sequences of each sequence type (ST) were downloaded from the MLST website (www.mlst.net). After concatenation of the sequences, Bayesian phylogenetic inference was performed (15). The phylogenetic tree was created according to Margos et al. (15), using TreeView software (ver. 1.6.6).
Table 1.
Borrelia garinii strains used in this study
| B. garinii strain (no. of isolates) | Isolation source | Location | Reference |
|---|---|---|---|
| Tick isolates (18) | |||
| HP1, HP3, HT18, HT59, N346, HkIP1, HkIP2 | Ixodes persulcatus | Hokkaido, Japan | 3, 10, this study |
| NP4, NP8, NP76, NP81, NT24, NT25, NT31 | I. persulcatus | Nagano, Japan | 10, 17 |
| FujiP2 | I. persulcatus | Shizuoka, Japan | 11 |
| Ip90 | I. persulcatus | Khabarovsk, Russia | 23 |
| Mp7, Np189 | I. persulcatus | Moscow, Russia | 18 |
| Rodent isolates (22) | |||
| Ear isolates (17) | |||
| HkCR1, HkCR3, HkCR4, HkCR5, HkCR6, HkCR7, HkCR9, HkCR11, HkCR12, | Myodes rufocanus subsp. bedfordiae | Hokkaido, Japan | 9 |
| FsAE1, FsAE2 | Apodemus speciosus | Fukushima, Japan | 8 |
| FiEE11 | A. speciosus | Fukui, Japan | 8 |
| sai8E | A. speciosus | Aomori, Japan | This study |
| ChYAE2 | A. uralensis | Yakeshi, China | 13 |
| CTA1b, CTA4a, CTA5b | A. uralensis | Urumqi, China | 25 |
| Spleen isolate (1) | |||
| ASF | A. speciosus | Hokkaido, Japan | 19 |
| Bladder isolates (4) | |||
| HokkaidoCRB35B | M. rufocanus subsp. bedfordiae | Hokkaido, Japan | This study |
| HokkaidoAS7B | A. speciosus | Hokkaido, Japan | This study |
| sai6B, sai7B | A. speciosus | Aomori, Japan | This study |
| Human skin isolates (19) | |||
| Hiratsuka | Erythema migrans | Niigata, Japan | This study |
| J-14, J-15, J-16, J-17, J-18, J-20T, J-21, J-32, J-33, J-34, J-35, J-37, J-38, J-39, J-40, J-41, J-42 | Erythema migrans | Hokkaido, Japan | Miyamoto et al., unpublished data |
| HH1 | Erythema migrans | Hokkaido, Japan | Sato et al., unpublished data |
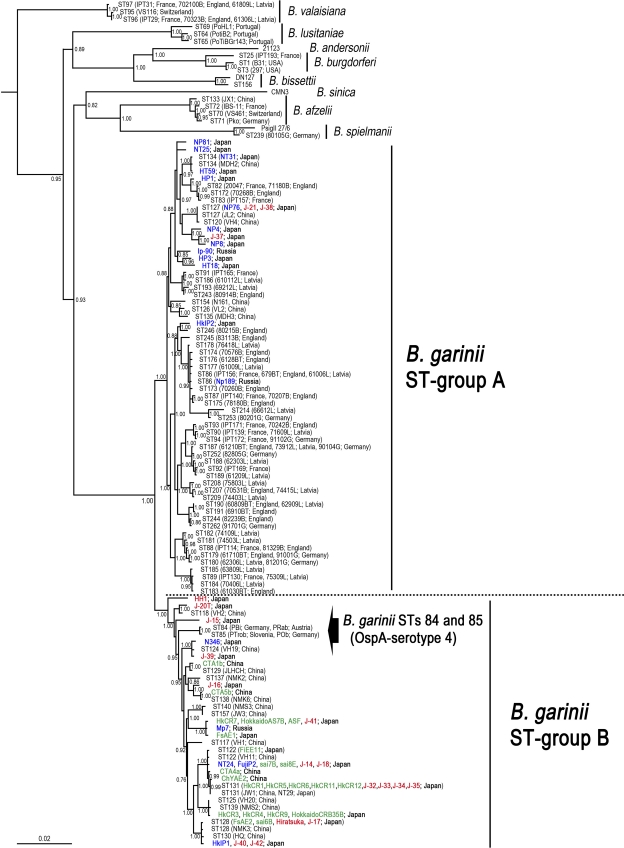
Isolated B. garinii strains were classified into two phylogenetic groups (preliminarily designated B. garinii ST group A and ST group B) by analysis of the concatenated DNA sequences of 8 loci (Fig. 1 and Table 2). The results indicated that B. garinii ST group B contained most of the Japanese clinical isolates (16/19 [84.2%]), all of the rodent isolates from Japan (18/18 [100%]) and China (4/4 [100%]), and 5 isolates from I. persulcatus collected in Japan (4/15 [26.7%]) and Russia (1/3 [33.3%]). Chi-square analysis indicated a confidence level of over 99% that B. garinii ST group B is predominant among Japanese clinical isolates and rodent isolates.
Fig. 1.
Bayesian phylogenetic inference of concatenated housekeeping gene sequences of B. garinii. The phylogenetic tree was constructed based on Bayesian phlylogenetic inference. The posterior probability values of the clades are provided. Bars labeled 0.05 depict 5% divergence. B. garinii human isolates are indicated in red, rodent isolates in green, and isolates from I. persulcatus in blue. The relapsing fever Borrelia spp. (B. duttonii Ly [NC_011229], B. hermsii DAH [NC_010673], B. recurrentis A1 [NC_011244], and B. turicatae 91E135 [NC_008710]) were used as outgroups (data not indicated). The accession numbers of alleles from Borrelia bissettii DN127, Borrelia andersonii 21123, Borrelia sinica CMN3, and Borrelia spielmanii PsigII 27/6 are listed in the table in the supplemental material.
Table 2.
ST groups of Borrelia garinii isolates from Ixodes persulcatus ticks, rodents, and humans
| Isolation source | Country |
B. garinii ST |
|
|---|---|---|---|
| Group A | Group B | ||
| I. persulcatus | Japan | HkIP2, HP1, HP3, HT18, HT59, NP4, NP8, NP76, NP81, NT25, NT31 | HkIP1, N346, NT24, FujiP2 |
| Russia | Ip90, Np189 | Mp7 | |
| Rodents | Japan | None | HkCR1, HkCR3, HkCR4, HkCR5, HkCR6, HkCR7, HkCR9, HkCR11, HkCR12, HokkaidoCRB35B, HokkaidoAS7B, ASF, FsAE1, FsAE2, FiEE11, sai6B, sai7B, sai8E |
| China | None | ChYAE2, CTA1b, CTA4a, CTA5b | |
| Humans | Japan | J-21, J-37, J-38 | Hiratsuka, J-14, J-15, J-16, J-17, J-18, J-20T, J-32, J-33, J-34, J-35, J-39, J-40, J-41, J-42, HH1 |
In this study, all B. garinii isolates from rodents were included in ST group B. In Europe, a recent report designated STs 84 and 85 as “Candidatus Borrelia bavariensis,” and the reservoir host was thought to be rodents (14). Since B. garinii STs 84 and 85 clustered with B. garinii ST group B, we hypothesized that rodents are the main reservoir host of this phylogenetic group. In this study, the STs of 9 clinical isolates (2 of ST128, 4 of ST131, and strains J-14, J-18, and J-41) were found among rodent isolates. Thus, it can be inferred that human-pathogenic B. garinii is maintained by rodents in Japan. B. garinii ST group B was also found among rodent isolates from China. In addition, STs 128 and 131 of B. garinii, which were originally recorded in the MLST database as Chinese isolates, are pathogenic to humans in Japan. These suggest that B. garinii ST group B may also represent a health threat of Lyme disease in China. In contrast, B. garinii ST group A was not isolated from rodents in this study, yet was found to include almost all of the B. garinii isolates in Europe. Given that several reports claim B. garinii is detectable from birds in Asia (10, 20, 21), we suspect that B. garinii ST group A is maintained by birds, as are most of the B. garinii isolates in Europe.
I. persulcatus and I. ricinus ticks are known vectors of pathogenic B. garinii in Asia and Russia and Europe, respectively. In this study, B. garinii ST group A, which is most often isolated in Europe, was infrequent in Japan (Fig. 1). The reason remains unclear, but the inhabitant species of ticks may be associated with this geographical difference. Furthermore, Korenberg et al. recently found that I. pavlovskyi and I. persulcatus ticks differ in their abilities to transmit borrelia (12). This finding may support the notion that the resident tick species contributes to the determination of the endemic species B. garinii.
In this study, it was observed that the B. garinii strains which infect humans in Japan, are often found in rodents, but not nearly as often in ticks. In Europe, it was reported that B. garinii STs 84 and 85 are pathogenic to humans, although they were infrequent among tick isolations (6). These STs are not found in Japan, but they are clustered with most of the Japanese clinical isolates. Therefore, we hypothesized from our data that B. garinii ST group B isolates may be more pathogenic to humans than isolates of B. garinii ST group A.
In conclusion, B. garinii could be divided into two phylogenetic groups by MLST analysis, and one group (B. garinii ST group B) was predominant among clinical and rodent isolates in Japan. These results suggest that rodents are the reservoir host for most human-pathogenic B. garinii isolates in Japan. We also revealed that Japanese clinical isolates may be distinct from most European isolates. This may be due to the different vectors of B. garinii in Asia and Russia versus Europe. Our findings may contribute to the elucidation of B. garinii-caused Lyme disease epidemiology.
Supplementary Material
Acknowledgments
We thank Gabriele Margos (Department of Biology and Biochemistry, University of Bath) for providing information on MLST; Maki Muto and Yumiko Ogasawara (National Institute of Infectious Disease) for technical assistance; and Bettina Wilske, Kenji Miyamoto, Nanao Sato, Haipeng Wang (China Medical University), and Jichun Wang (China Medical University) for providing Borrelia strains.
This study was supported in part by a grant for research on emerging and reemerging infectious diseases from the Japanese Ministry of Health, Labor, and Welfare.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 16 March 2011.
REFERENCES
- 1. Barbour A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521–525 [PMC free article] [PubMed] [Google Scholar]
- 2. Eisen L., Lane R. S. 2002. Vectors of Borrelia burgdorferi sensu lato, p. 91–115 In Gray J. S., Kahl O., Lane R. S., Stanek G. (ed.), Lyme borreliosis biology, epidemiology and control. CABI Publishing, Oxon, United Kingdom [Google Scholar]
- 3. Fukunaga M., Sohnaka M., Takahashi Y., Nakao M., Miyamoto K. 1993. Antigenic and genetic characterization of Borrelia species isolated from Ixodes persulcatus in Hokkaido, Japan. J. Clin. Microbiol. 31:1388–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gern L. 2008. Borrelia burgdorferi sensu lato, the agent of Lyme borreliosis: life in the wilds. Parasite 15:244–247 [DOI] [PubMed] [Google Scholar]
- 5. Hanincová K., et al. 2003. Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl. Environ. Microbiol. 69:2825–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu C. M., Wilske B., Fingerle V., Lobet Y., Gern L. 2001. Transmission of Borrelia garinii OspA serotype 4 to BALB/c mice by Ixodes ricinus ticks collected in the field. J. Clin. Microbiol. 39:1169–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huegli D., Hu C. M., Humair P. F., Wilske B., Gern L. 2002. Apodemus species mice are reservoir hosts of Borrelia garinii OspA serotype 4 in Switzerland. J. Clin. Microbiol. 40:4735–4737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ishiguro F., Takada N. 1996. Lyme Borrelia from Ixodes persulcatus and small rodents from northern to central parts of mainland Japan. Med. Entomol. Zool. 47:183–185 [Google Scholar]
- 9. Ishiguro F., et al. 1996. Reservoir competence of the vole, Clethrionomys rufocanus bedfordiae, for Borrelia garinii or Borrelia afzelii. Microbiol. Immunol. 40:67–69 [DOI] [PubMed] [Google Scholar]
- 10. Ishiguro F., Takada N. T., Masuzawa, Fukui T. 2000. Prevalence of Lyme disease Borrelia spp. in ticks from migratory birds on the Japanese mainland. Appl. Environ. Microbiol. 66:982–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawabata H., Tashibu H., Yamada K., Masuzawa T., Yanagihara Y. 1994. Polymerase chain reaction analysis of Borrelia species isolated in Japan. Microbiol. Immunol. 38:591–598 [DOI] [PubMed] [Google Scholar]
- 12. Korenberg E. I., Nefedova V. V., Romanenko V. N., Gorelova N. B. 2010. The tick Ixodes pavlovskyi as a host of spirochetes pathogenic for humans and its possible role in the epizootiology and epidemiology of borrelioses. Vector Borne Zoonotic Dis. 10:453–458 [DOI] [PubMed] [Google Scholar]
- 13. Li M., et al. 1998. Lyme disease Borrelia species in northeastern China resemble those isolated from far eastern Russia and Japan. Appl. Environ. Microbiol. 64:2705–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Margos G., et al. 2009. A new Borrelia species defined by multilocus sequence analysis of housekeeping genes. Appl. Environ. Microbiol. 75:5410–5416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Margos G., et al. 2008. MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 105:8730–8735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Masuzawa T. 2004. Terrestrial distribution of the Lyme borreliosis agent Borrelia burgdorferi sensu lato in East Asia. Jpn. J. Infect. Dis. 57:229–235 [PubMed] [Google Scholar]
- 17. Masuzawa T., et al. 1996. Comparison of OspA serotypes for Borrelia burgdorferi sensu lato from Japan, Europe and North America. Microbiol. Immunol. 40:539–545 [DOI] [PubMed] [Google Scholar]
- 18. Masuzawa T., et al. 2005. Characterization of Borrelia burgdorferi sensu lato isolated in Moscow province—a sympatric region for Ixodes ricinus and Ixodes persulcatus. Int. J. Med. Microbiol. 294:455–464 [DOI] [PubMed] [Google Scholar]
- 19. Miyamoto K., Nakao M., Sato N., Mori M. 1991. Isolation of Lyme disease spirochetes from an ixodid tick in Hokkaido, Japan. Acta Trop. 49:65–68 [DOI] [PubMed] [Google Scholar]
- 20. Miyamoto K., Sato Y., Okada K., Fukunaga M., Sato F. F. 1997. Competence of a migratory bird, red-bellied thrush (Turdus chrysolaus), as an avian reservoir for the Lyme disease spirochetes in Japan. Acta Trop. 65:43–51 [DOI] [PubMed] [Google Scholar]
- 21. Nakao M., Miyamoto K., Fukunaga M. 1994. Lyme disease spirochetes in Japan: enzootic transmission cycles in birds, rodents, and Ixodes persulcatus ticks. J. Infect. Dis. 170:878–882 [DOI] [PubMed] [Google Scholar]
- 22. Nakao M., Miyamoto K., Kawagishi N., Hashimoto Y., Iizuka H. 1992. Comparison of Borrelia burgdorferi isolated from humans and ixodid ticks in Hokkaido, Japan. Microbiol. Immunol. 36:1189–1193 [DOI] [PubMed] [Google Scholar]
- 23. Noppa L., Burman N., Sadziene A., Barbour A. G., Bergström S. 1995. Expression of the flagellin gene in Borrelia is controlled by an alternative sigma factor. Microbiology 141:85–93 [DOI] [PubMed] [Google Scholar]
- 24. Piesman J., Gern L. 2008. Lyme borreliosis in Europe and North America, p. 220–252 In Bowman A. S., Nuttall P. A. (ed.), Ticks: biology, disease and control. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 25. Takada N., et al. 2001. Lyme disease Borrelia spp. in ticks and rodents from northwestern China. Appl. Environ. Microbiol. 67:5161–5165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taragel'ová V., et al. 2008. Blackbirds and song thrushes constitute a key reservoir of Borrelia garinii, the causative agent of borreliosis in Central Europe. Appl. Environ. Microbiol. 74:1289–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilske B., et al. 1993. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J. Clin. Microbiol. 31:340–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamaguti N., Tipton V. J., Keegan H. L., Toshioka S. 1971. Ticks of Japan, Korea, and the Ryukyu Islands. Brigham Young Univ. Sci. Bull. Biol. Ser. 15:142–148 [Google Scholar]
- 29. Yanagihara Y., Masuzawa T. 1997. Lyme disease (Lyme borreliosis). FEMS Immunol. Med. Microbiol. 18:249–261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.