Abstract
We report the first case of fatal brain infection in an Indian farmer caused by Thielavia subthermophila, a dematiaceous thermophilic fungus in the order Sordariales, and present a review of previous infections from this order. The patient failed amphotericin B therapy combined with surgical excision despite the drug's low MICs in vitro.
CASE REPORT
A 39-year-old male presented in the Emergency Department of Government Medical College Hospital (GMCH), Chandigarh, India, with complaints of multiple episodes of generalized tonic-clonic seizures for the previous 10 days. He experienced, in addition to the seizures, uncontrolled movements of his limbs and rolling of his eyes, incontinence of urine, and production of foam from his mouth. There was a history of fever for 2 days, associated with an attack of seizures. He was a resident of Ambala (Haryana State, northern India) and a farmer by occupation, with a low socioeconomic status. He was first admitted to a local hospital in Ambala and was subsequently transported to the GMCH. In the past, he never had headaches or any other significant complaints. There was no history of any trauma, roadside accidents, near-drowning, or similar predisposing factors.
On examination, the patient was having an altered sensorium and was disoriented, with a Glasgow coma score of E2V2M4. On the basis of generalized tonic-clonic seizures, a presumptive diagnosis of meningioma was made. His chest X ray and electrocardiogram (ECG) were normal. A lumbar puncture was done, and cerebrospinal fluid (CSF) was sent for cytological, biochemical, and microbiological examination. Gram staining, Ziehl-Neelsen (ZN) staining, and fungal smears of CSF were negative, and there was no growth of either bacteria or fungi. The cytological and biochemical examination of CSF was noncontributory. The other laboratory investigations revealed that his hemogram, white blood cells, serum electrolytes, liver function, and glucose concentration were within normal ranges.
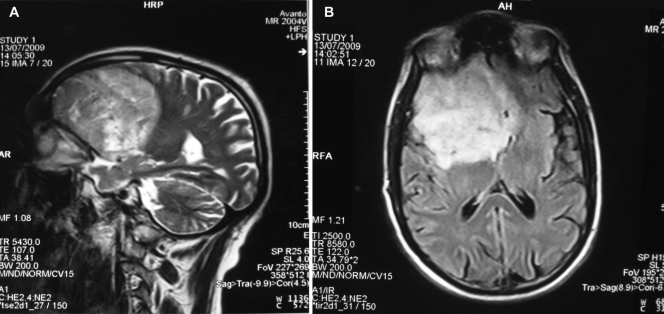
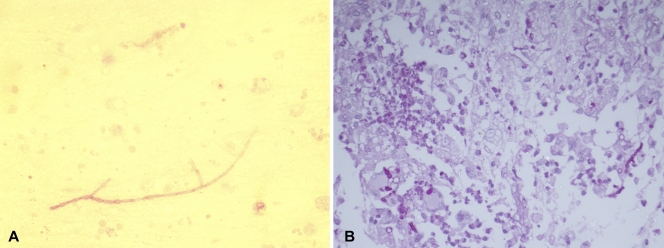
A magnetic resonance image (MRI) of his brain showed a large, supratentorial, intracranial, right-frontotemporal, space-occupying lesion (7.0 by 7.5 by 8.6 cm) and also small components devoid of frank edema alongside the frontal part of the falx cerebri, suggestive of meningioma in the right sphenoidal wing (Fig. 1A and B). Therefore, right-frontotemporal craniotomy was done for excision of the intracranial mass, which intraoperatively showed a white, cheesy, and gelatinous substance, suggestive of infective pathology rather than of meningioma. The excised intracranial mass was sent for histopathological and microbiological examination. Direct microscopy revealed neither Mycobacterium nor other bacteria with Ziehl-Neelsen (ZN) staining or Gram staining, respectively, but KOH preparations showed septate, branched fungal hyphae (Fig. 2A). Histopathological examination with periodic acid-Schiff (PAS) staining showed fungal granulomas in the brain parenchyma centered around blood vessels. Granulomas were made up of epithelioid cells with giant cells and collections of neutrophils. These granulomas and giant cells contained septate fungal hyphae, and blood vessels showed evidence of vasculitis. There was also angioinvasion by the fungal hyphae; however, necrosis was not seen (Fig. 2B). Fungal culture was done with Sabouraud's dextrose agar (SDA; HiMedia, Mumbai, India) with and without antibiotics and incubated at both 37°C and 22°C. The bacterial and mycobacterial cultures remained sterile. On the second day of incubation, SDA plates showed mycelial growth at both 37°C and 22°C. Lactophenol cotton blue (LCB) mounts of the black fungal growth showed sterile, dematiaceous hyphae which did not allow morphological identification.
Fig. 1.
Coronal (A) and axial (B) MRI images of the brain demonstrating a large, supratentorial, intracranial, right-frontotemporal, space-occupying lesion and also small, left-frontal components devoid of frank edema on both sides of the falx cerebri.
Fig. 2.
(A) Septate hyphae observed in a KOH wet mount; (B) photomicrograph of brain parenchyma showing granuloma and septate hyphae (PAS staining; magnification, ×400).
The patient was treated with intravenous amphotericin B deoxycholate (1 mg/kg of body weight/day), but his condition deteriorated rapidly and he developed respiratory distress, for which a tracheostomy was necessary. Despite antifungal therapy, the patient died 2 weeks later due to cardiac arrest and respiratory failure.
Mycology.
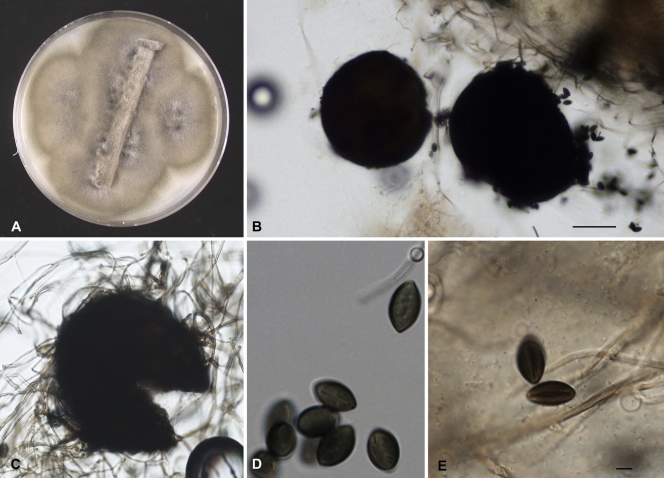
The fungal culture was deposited in the reference collection of CBS-KNAW, Utrecht, Netherlands, under accession number CBS 125981. Stock cultures were maintained on slants of 2% malt extract agar (MEA; Difco, Leeuwarden, Netherlands) and oatmeal agar (OA) at 24°C (14). Colonies showed rapid growth and were flat and velvety to floccose, with an olivaceous-black reverse on OA (Fig. 3A). Smears from old cultures were prepared in lactic acid and in sterile water and examined with a Nikon Eclipse 80i microscope equipped with a Nikon digital-sight DS-Fi1 camera. Septate, branching, dark olivaceous hyphae were observed. Supplementary cultures were prepared on MEA, potato carrot agar (PCA; Difco), potato dextrose agar (PDA), and oatmeal agar (OA) with or without lupine stems and incubated at 25, 35, 42, 45, and 50°C for a period of 3 weeks under alternate near-UV light to suppress the growth of aerial hyphae and induce adequate ascoma formation (14). After 2 weeks of incubation, scattered black ascomata were observed on all media tested (Fig. 3B). Walls of the ascomata consisted of textura epidermoidea (i.e., of jigsaw-shaped cells [14]) coated with dark hyphae. The brown, fusiform ascospores were single celled (10 to 12 by 7.5 to 8.5 μm) and had a characteristic subapical germ pore measuring 1 to 1.5 μm (Fig. 3C to E). Thermotolerance tests showed that the isolate grew rapidly at both 35°C and higher temperatures of 42°C, 45°C, and 50°C. Subsequently, the fungus was phenotypically identified as a Thielavia species. Sequencing was used to further identify it to the species level.
Fig. 3.
Thielavia subthermophila (CBS 125981). (A) A culture on oatmeal agar at 42°C after 2 weeks in darkness with lupine stem grew rapidly and was flat and velvety or floccose, with an olivaceous-black reverse. (B and C) Scattered black ascomata developing within the hyphae. (D and E) The brown, fusiform ascospores are single celled (10 to 12 by 7.5 to 8.5 μm) and have a characteristic subapical germ pore measuring 1 to 1.5 μm. Scale bars, 10 μm.
For molecular analyses, the fungus was grown on 2% MEA plates, and DNA was extracted using an UltraClean microbial DNA isolation kit (MO BIO, Carlsbad, CA) according to the manufacturer's instructions. PCR amplification and sequencing were carried out according to the method of Badali et al. (7). Briefly, the universal fungal primer pairs V9G (5′-TTACGTCCCTGCCCTTTGTA-3′)/LS266 (5′-GCATTCCCAAACAACTCGACTC-3′) and LROR/LR7 were used for amplification of internal transcribed spacer (ITS) ribosomal DNA (rDNA) and 28S rRNA (nucLSU), respectively. PCRs were performed on a GeneAmp PCR system 9700 (Applied Biosystems, Foster City, CA) in 50-μl volumes containing 25 ng template DNA, 5 μl reaction buffer (0.1 M Tris-HCl, pH 8.0, 0.5 M KCl, 15 mM MgCl2, 0.1% gelatin, 1% Triton X-100), 0.2 mM each deoxynucleoside triphosphate (dNTP), and 2.0 U Taq DNA polymerase (ITK Diagnostics, Leiden, Netherlands). Amplification of ITS and nucLSU was performed with cycles of 2 min at 94°C for primary denaturation, followed by 35 cycles at 94°C (45 s), 52°C (30 s), and 72°C (120 s), with a final 7-min extension step at 72°C. Amplicons were purified using GFX PCR DNA and a gel band purification kit (GE Healthcare, Ltd., Buckinghamshire, United Kingdom). Sequencing was performed as follows: 95°C for 1 min, followed by 30 cycles consisting of 95°C for 10 s, 50°C for 5 s, and 60°C for 2 min. Reaction mixtures were purified with Sephadex G-50 fine (GE Healthcare Bio-Sciences AB, Uppsala, Sweden), and sequencing was done on an ABI 3730xl automatic sequencer (Applied Biosystems, Foster City, CA). Sequence data obtained in this study were adjusted using Lasergene SeqMan software (DNAStar, Inc., Madison, WI). Sequences were compared with entries in GenBank and by using local BLAST searching in a molecular database maintained at the CBS and validated by ex-type strains. Isolate CBS 125981 was identified as Thielavia subthermophila by 98.98% and 100% identities in its ITS rDNA and nucLSU regions, respectively, with GenBank submissions, which included accession number AJ271575.1 from the recent revision of Thielavia by Stchigel et al. (28), and by 99.6% identity with CBS 509.74, the ex-type strain of T. subthermophila. This led to the final diagnosis of the infection as a cerebral phaeohyphomycosis due to Thielavia subthermophila.
In vitro antifungal susceptibility testing was performed using the M38-A2 reference method of the Clinical and Laboratory Standards Institute (CLSI) by using a broth microdilution format (13, 16). Briefly, ascospore suspensions were prepared from >3-week-old cultures on OA with lupine stems (14) at 37°C by gently scraping the surfaces of mature colonies with a sterile moistened cotton swab. If large aggregates existed, they were allowed to settle for several minutes, the homogenous suspension of asci and ascospores was then transferred to sterile tubes, and the asci and ascospores in the supernatants were counted with a hemocytometer. Broth dilutions were done in RPMI 1640 medium with l-glutamine, without bicarbonate, and buffered with 0.165 M morpholinepropanesulfonic acid (MOPS). Paecilomyces variotii (ATCC 22319), Candida parapsilosis (ATCC 22019), and Candida krusei (ATCC 6258) were used as quality control organisms (13). The isolate showed the following antifungal susceptibility profile: amphotericin B, 0.03 μg/ml; fluconazole, 8 μg/ml; itraconazole, 0.016 μg/ml; voriconazole, 0.016 μg/ml; posaconazole, 0.016 μg/ml; and isavuconazole, 0.016 μg/ml. The minimum effective concentrations of caspofungin and micafungin were 4 and 0.25 μg/ml, respectively.
Discussion.
During recent decades, the diversity of fungal agents causing systemic disease has increased dramatically, especially in immunocompromised hosts, patients using broad-spectrum antibiotics, or patients with severe underlying diseases or undergoing solid organ transplantation. Melanized (dematiaceous) fungi are particularly significant because they infect not only debilitated hosts but also apparently healthy individuals, giving infections which range from mild cutaneous infections to fatal brain disease (12, 15, 19, 20, 22, 29). Primary cerebral phaeohyphomycosis is a rare disorder characterized by black necrotic tissue and the production of pus, frequently occurring in humans without known predisposing factors or immunodeficiency (19, 22, 26). The infection is recognized as a disease associated with high mortality and ultimately a poor prognosis despite the application of surgery and antifungal therapy (12, 22). If untreated, the infection may lead to death within weeks, months, or, occasionally, years. The majority of etiological agents belong to a single order of known environmental fungi, the Chaetothyriales (e.g., Cladophialophora, Exophiala, Rhinocladiella, and Fonsecaea). Occasionally species from other orders are involved, such as Sordariales (Chaetomium), Pleosporales (Bipolaris, Exserohilum), Xylariales (Nodulisporium), and Botryosphaeriales (Neoscytalidium) (22).
Thielavia is a common genus of environmental ascomycetes belonging to the family Chaetomiaceae in the order Sordariales. The genus is characterized by spherical, nonostiolate ascomata with a thin peridium (ascoma wall), producing one-celled, darkly pigmented ascospores (14, 25, 31, 32). Taxonomy and phylogeny of Thielavia have been the subject of some confusion, because optimal markers for species distinction have not yet been established (28). A morphotaxonomic revision by von Arx et al. (31, 32) described 14 species, but developments in molecular techniques revolutionized species concepts. Thielavia subthermophila (not to be confused with Thielavia thermophila Fergus & Sinden) was introduced by Mouchacca (24) for a species from desert soil which showed good growth at 45°C. The species is further characterized by the presence of pigmented mycelium and brownish-black, hairy ascomata. Thielavia gigaspora (25), Thielavia arenaria, Thielavia hyrcania, and Thielavia microspora (31) are distinguished by the criteria of ascospore size and position of germ pores (28). Thielavia species have rarely been proven to be involved in human infections. Bourbeau et al. (11) described a disseminated infection due to Myceliophthora thermophila, the anamorph of Thielavia heterothallica, and Theoulakis et al. (29) reported a keratitis due to T. subthermophila in a 10-year-old girl. Our patient is the first reported case of severe systemic infection due to T. subthermophila. Identification by a molecular approach like sequencing of ITS rDNA and comparison to sequences in GenBank is the best and most simple approach for the difficult identification of these sporulating fungi. This species is an addition to the list of potential agents of primary brain abscesses in apparently healthy individuals, which includes its black-yeast-like relatives Cladophialophora bantiana, Exophiala dermatitidis, Fonsecaea monophora, and Rhinocladiella mackenziei (7–9, 27). Primary or secondary brain infections are distinguished by their modes of infection, either by supposed hematogenous spread from an unrecognized pulmonary focus or through direct extension from an adjacent focus, e.g., in paranasal sinuses or after a penetrating trauma to the head (12, 19, 21, 27).
Thielavia is closely related to Chaetomium (Sordariales, Chaetomiaceae), a large genus of saprobic ascomycetes that are widespread in soil, in plant debris, and on wood. Both species have been recovered during air surveys in jute fields in India (30), suggesting that the probable source for our patient, a farmer, was airborne. Chaetomium globosum, Chaetomium atrobrunneum, Chaetomium funicola, and Chaetomium strumarium have occasionally been encountered in a wide variety of human infections, such as onychomycosis, sinusitis, pneumonia, and cerebral abscess. Fatal cerebral infection was repeatedly reported in intravenous drug users, with a high mortality despite the administration of antifungal combination therapy (1). The disorder may be underdiagnosed, because many ascomycetes in the Sordariales do not produce anamorphs and may appear as sterile mycelium. In addition, the correct identification of species described 20 years ago can be doubted, as these isolates have not been reexamined with molecular methods. We have the impression that members of the Sordariales target relatively frequently the cerebrum (22). The optimal temperature for most Chaetomium species lies between 25 and 35°C (10, 23, 28), while those that have been reported to cause invasive infection grow very well above 35°C to 45°C. The invasive infections due to Thielavia and Chaetomium species reported to date are summarized in Table 1.
Table 1.
Overview of reported cases of systemic infections by members of the Sordarialesa
| Agent | Age (yr) | Sex | Host status | Site of infection | Therapy | Outcome | Reference |
|---|---|---|---|---|---|---|---|
| T. subthermophila | 39 | M | Healthy | Brain | AmB | Died | This study |
| 10 | F | Healthy | Cornea | Ciprofloxacin, tobramycin, AmB, cefazolin | Cured | 28 | |
| C. strumarium | 20 | M | IVDA | Brain | Ceftriaxone, penicillin, acyclovir | Died | 1 |
| 25 | M | IVDA | Brain | Amoxicillin, acyclovir, AmB, rifampin, isoniazid | Died | 1 | |
| 28 | M | IVDA | Brain | Oxacillin, cefotaxime, metronidazole | Died | 1 | |
| C. atrobrunneum | 31 | M | Multiple myeloma, allogeneic BMT | Brain, lung | AmB, ITC | Died | 17 |
| 32 | M | Renal transplant | Brain | Unknown | Died | 5 | |
| 12 | M | AML | CSF, brain | AmB, itraconazole, L-AmB | Died | 4 | |
| <1 | M | Anemia, pancytopenia | Lung | Piperacillin-tazobactam, gentamicin, AmB, L-AmB, ITC | Died | 4 | |
| C. globosum | 19 | F | Lymphoma/autologous BMT | Lung pleura | Imipenem, vancomycin, amikacin, AmB | Died | 21 |
| 24 | M | ALL | Lung | AmB | Died | 18 | |
| C. perlucidum | 78 | F | Asthma, chronic bronchiectasis | Lung | RML lobectomy | Cured | 10 |
| 47 | F | Leukemia, umbilical cord blood transplant | Multiple organs | L-AmB | Died | 10 | |
| Chaetomium sp. | 19 | M | AML | Lung | L-AmB | Died | 33 |
| 73 | F | None | Left maxillary sinus | Infundibulectomy | Cured | 6 |
M, male; F, female; BMT, bone marrow transplant recipient; RML, right middle lobe; IVDA, intravenous drug abuser; AML, acute myelogenous leukemia patient; ALL, acute lymphocytic leukemia patient; AmB, amphotericin B; ITC, itraconazole; L-AmB, liposomal amphotericin B.
Cases of brain infection due to C. strumarium have occurred in intravenous drug abusers and in patients with hematologic malignancies and solid organ transplantation (1). The outcome was generally poor. Al-Aidaroos et al. (4) reported fatal invasive infections in two immunocompromised pediatric patients due to C. atrobrunneum. The present study adds another thermophilic member of the Sordariales (T. subthermophila) to the list of potential agents of phaeohyphomycotic brain infections.
The keratitis due to T. subthermophila reported by Theoulakis et al. (29) was successfully treated with topical amphotericin B and oral voriconazole. Treatment of cerebral phaeohyphomycosis generally includes surgical debridement, combined with antifungal therapy and/or immune enhancement. Although the etiologic agent in our case had a low in vitro MIC of amphotericin B, the patient died despite amphotericin B therapy. Murine studies showed no benefit from amphotericin B in cerebral phaeohyphomycosis due to poor penetration into the central nervous system (2), which may explain, in part, the treatment failure. Currently, there is no accepted standard therapy for brain infections by melanized fungi, but in vitro data (7, 8, 9) and one clinical study (3) suggest that posaconazole may be a potential choice.
Nucleotide sequence accession numbers.
The ITS rDNA and nucLSU sequences from isolate CBS 125981 determined in this study have been deposited in GenBank under accession numbers HM448441 and HM448442, respectively.
Acknowledgments
This study was supported by a grant (no. 13081) to H. Badali from the Ministry of Health and Medical Education of the Islamic Republic of Iran and the School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran. J. F. Meis received grants form Astellas, Merck, Basilea, and Schering-Plough. He has been a consultant to Astellas, Basilea, and Merck and received speaker's fees from Merck, Pfizer, Schering-Plough, and Janssen Pharmaceutica. All other authors report no conflicts of interest.
We are most grateful to Walter Gams for suggestions with respect to phenotypic identification.
Footnotes
Published ahead of print on 16 March 2011.
REFERENCES
- 1. Abbott S. P., et al. 1995. Fatal cerebral mycoses caused by the ascomycete Chaetomium strumarium. J. Clin. Microbiol. 33:2692–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al-Abdely H. M., et al. 2000. SCH 56592, amphotericin B, or itraconazole therapy of experimental murine cerebral phaeohyphomycosis due to Ramichloridium obovoideum (“Ramichloridium mackenziei”). Antimicrob. Agents Chemother. 44:1159–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al-Abdely H. M., et al. 2005. Successful therapy of cerebral phaeohyphomycosis due to Ramichloridium mackenziei with the new triazole posaconazole. Med. Mycol. 43:91–95 [DOI] [PubMed] [Google Scholar]
- 4. Al-Aidaroos A., et al. 2007. Invasive Chaetomium infection in two immunocompromised paediatric patients. Pediatr. Infect. Dis. J. 26:456–458 [DOI] [PubMed] [Google Scholar]
- 5. Anandi V., et al. 1989. Cerebral phaeohyphomycosis caused by Chaetomium globosum in a renal transplant recipient. J. Clin. Microbiol. 27:2226–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aru A., Munk-Nielsen L., Federspiel B. H. 1997. The soil fungus Chaetomium in the human paranasal sinuses. Eur. Arch. Otorhinolaryngol. 254:350–352 [DOI] [PubMed] [Google Scholar]
- 7. Badali H., De Hoog G. S., Curfs-Breuker I., Klaassen C. H. W., Meis J. F. 2010. Use of amplified fragment length polymorphism to identify 42 Cladophialophora strains related to cerebral phaeohyphomycosis with in vitro antifungal susceptibility. J. Clin. Microbiol. 48:2350–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Badali H., De Hoog G. S., Curfs-Breuker I., Meis J. F. 2010. In vitro activities of antifungal drugs against Rhinocladiella mackenziei, an agent of fatal brain infection. J. Antimicrob. Chemother. 65:175–177 [DOI] [PubMed] [Google Scholar]
- 9. Badali H., et al. 2010. First autochthonous case of Rhinocladiella mackenziei cerebral abscess outside the Middle East. J. Clin. Microbiol. 48:646–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barron M. A., et al. 2003. Invasive mycotic infections caused by Chaetomium perlucidum, a new agent of cerebral phaeohyphomycosis. J. Clin. Microbiol. 41:5302–5307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bourbeau P., McGough D. A., Fraser H., Shah N., Rinaldi M. G. 1992. Fatal disseminated infection caused by Myceliophthora thermophila, a new agent of mycosis: case history and laboratory characteristics. J. Clin. Microbiol. 30:3019–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chakrabarti A. 2007. Epidemiology of central nervous system mycoses. Neurol. India 55:191–197 [DOI] [PubMed] [Google Scholar]
- 13. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard, 2nd ed Document M38-A2 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 14. De Hoog G. S., Guarro J., Gené J., Figueras M. J. 2000. Atlas of clinical fungi, 2nd ed Centraalbureau voor Schimmelcultures, Utrecht, Netherlands [Google Scholar]
- 15. Guarro J. 1998. Comments on recent human infections caused by ascomycetes. Med. Mycol. 36:349–350 [PubMed] [Google Scholar]
- 16. Guarro J., Soler L., Rinaldi M. G. 1995. Pathogenicity and antifungal susceptibility of Chaetomium species. Eur. J. Clin. Microbiol. Infect. Dis. 14:613–618 [DOI] [PubMed] [Google Scholar]
- 17. Guppy K. H., Thomas C., Thomas K., Anderson D. 1998. Cerebral fungal infections in the immunocompromised host: a literature review and a new pathogen Chaetomium atrobrunneum: case report. Neurosurgery 43:1463–1469 [DOI] [PubMed] [Google Scholar]
- 18. Hoppin E. C., McCoy E. L., Rinaldi M. G. 1983. Opportunistic mycotic infection caused by Chaetomium in a patient with acute leukemia. Cancer 52:555–556 [DOI] [PubMed] [Google Scholar]
- 19. Horré R., De Hoog G. S. 1999. Primary cerebral infections by melanized fungi: a review. Stud. Mycol. 43:176–193 [Google Scholar]
- 20. Kantarcioglu A. S., De Hoog G. S. 2004. Infections of the central nervous system by melanized fungi: a review of cases presented between 1999 and 2004. Mycoses 47:4–13 [DOI] [PubMed] [Google Scholar]
- 21. Lesire V., et al. 1999. Possible role of Chaetomium globosum in infection after autologous bone marrow transplantation. Intensive Care Med. 25:124–125 [DOI] [PubMed] [Google Scholar]
- 22. Li D. M., De Hoog G. S. 2009. Cerebral phaeohyphomycosis—a cure at what lengths? Lancet Infect. Dis. 9:376–383 [DOI] [PubMed] [Google Scholar]
- 23. Malloch D., Cain R. F. 1973. The genus Thielavia. Mycologia 65:1055–1077 [Google Scholar]
- 24. Mouchacca J. 1973. Les Thielavia des sols arides: espèces nouvelles et analyse générique. Bull. Trim. Soc. Fr. Mycol. 89:295–311 [Google Scholar]
- 25. Moustafa A.-W. F., Abdel-Azeem A. M. 2008. Thielavia gigaspora, a new thermotolerant ascomycete from Egypt. Microbiol. Res. 163:441–444 [DOI] [PubMed] [Google Scholar]
- 26. Revankar S. G., Sutton D. A., Rinaldi M. G. 2004. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin. Infect. Dis. 38:206–216 [DOI] [PubMed] [Google Scholar]
- 27. Revankar S. G., Sutton D. A. 2010. Melanized fungi in human disease. Clin. Microbiol. Rev. 23:884–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stchigel A. M., Figueras L., Cano J., Guarro J. 2002. New species of Thielavia, with a molecular study of representative species of the genus. Mycol. Res. 106:975–983 [Google Scholar]
- 29. Theoulakis P., Goldblum D., Zimmerli S., Muehlethaler K., Frueh B. E. 2009. Keratitis resulting from Thielavia subthermophila Mouchacca. Cornea 28:1067–1069 [DOI] [PubMed] [Google Scholar]
- 30. Uddin N. 2005. Estimation of aeromycoflora in jute fields. Aerobiologia 21:75–80 [Google Scholar]
- 31. von Arx J. A. 1975. On Thielavia and some similar genera of ascomycetes. Stud. Mycol. 8:1–32 [Google Scholar]
- 32. von Arx J. A., Guarro J., Figueras M. J. 1988. Sordariaceous ascomycetes without ascospore ejaculation. Beih. Nova Hedwigia 94:1–104 [Google Scholar]
- 33. Yeghen T., et al. 1996. Chaetomium pneumonia in a patient with acute myeloid leukaemia. J. Clin. Pathol. 49:184–186 [DOI] [PMC free article] [PubMed] [Google Scholar]