Abstract
We report the first documented case of a posttraumatic fungal keratitis caused by Neosartorya udagawae. The patient was empirically treated with fluconazole until a corneal scraping grew an Aspergillus fumigatus-like fungus, and itraconazole therapy was then established. A sequence-based approach assigned the isolate to the species. Five months after completion of antifungal therapy, endophthalmitis occurred and orbital exenteration was necessary.
CASE REPORT
A 39-year-old man, a logger who had immigrated to Italy for some weeks, presented on 19 August 2009 with a history of pain and defective vision in his left eye of 9 days' duration following a nonpenetrating eye injury from a tree branch. He had been treated elsewhere earlier, and his medications consisted of ofloxacin, netilmicin, and cyclopentolate hydrochloride eye drops, with subsequent addition of oral ciprofloxacin. On 14 August 2009, the patient was referred to the Ophthalmology Ambulatory of the Campo di Marte Hospital in Lucca, where intensive antimicrobial therapy was started. Fortified gentamicin, vancomycin, and amphotericin B eye drops were given, along with cyclopentolate hydrochloride eye drops and oral ciprofloxacin. Five days later, the patient's symptoms worsened and he was hospitalized.
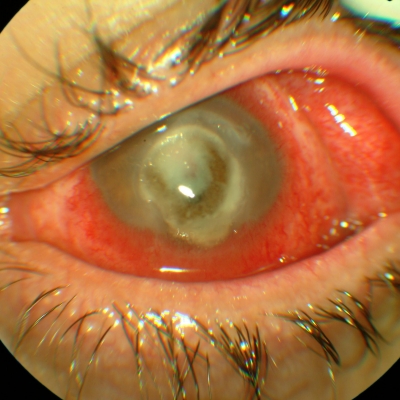
On examination, visual acuity in the left eye was reduced to motu manus. Slit-lamp evaluation revealed a large, central corneal ulcer, 4 by 6 mm in size, involving the anterior 1/3 of the stroma. An area of infiltration (6.2 to 7.5 mm) was noted at the peripheral edges of the ulcer (Fig. 1). The infiltrate involved all the layers of the stroma, and there was a dense endothelial plaque. A hypopyon of 1 mm was present, whereas thinning and melting of the cornea with high risk of perforation was noted. The patient was asked to continue the same topical therapy, but intravenous therapy consisting of levofloxacin, teicoplanin, and vancomycin was also administered.
Fig. 1.
Corneal picture of the patient at presentation showing a large ulcer with infiltrate. Vision was reduced to motu manus.
Corneal scrapings were taken from the base and edges of the ulcer using a sterile no. 15 surgical blade, whereas fluid from the anterior chamber was aspirated using a sterile syringe and needle. All the specimens were evaluated microscopically using 10% KOH and Gram staining and plated on 5% sheep blood agar, chocolate agar, and Sabouraud dextrose agar (SDA) slant. Plates were incubated in 5% CO2 at 37°C for 3 days, whereas SDA slants were incubated under aerobic conditions at 30°C for 14 days. Corneal scrapings from the ulcerative lesion and material from the anterior camera did not reveal any organism in smear studies or microbial growth in cultures. Based on clinical impression, oral ciprofloxacin was substituted with intravenous meropenem and oral fluconazole (400 mg/day) was added to the medical regimen. A contact lens was then placed on the ocular surface, and the patient was given oral diclofenamide to lower intraocular pressure and methylprednisolone acetate to suppress the development of intraocular inflammation. Concomitantly, repeat corneal scrapings were obtained. While the smears were once again negative, the laboratory culture results were returned 4 days later (on 1 September 2009) with the presence of a fungus which was initially identified as a member of Aspergillus section Fumigati. As growth of the same organism was detected on all inoculated media in the absence of fungal elements in smears, a diagnosis of mycotic keratitis was made. This was in accordance with definite criteria used to assess the significance of a fungus isolated from culture (23). Based on these findings, treatment with fortified eye drops and oral itraconazole (100 mg twice a day) was initiated, whereas methylprednisolone acetate was de-escalating. On 5 September 2009, the patient slightly improved and was discharged on oral itraconazole at the indicated dosage for the duration of 1 month, supplemented with amphotericin B eye drops and amoxicillin/clavulanate. Upon the antifungal susceptibility testing results (see below), the patient was also advised to give fortified itraconazole eye drops.
The patient was lost to follow-up until 5 months later, when he presented with a heavily compromised clinical situation because endophthalmitis had developed. On 15 March 2010, a thorough discussion of all treatment options and possible outcomes led clinicians to exenterate the patient's eye.
Mycological study and diagnosis.
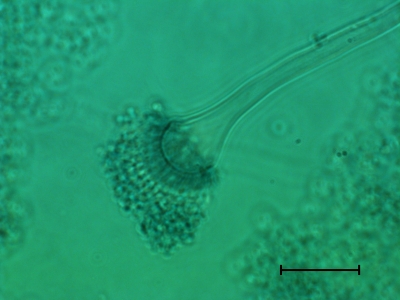
For morphological studies (8), the isolate was subcultured on Czapek Dox yeast extract and malt extract agar plates that were incubated at 30°C for 5 days. Under microscopic analysis, the isolate showed conidial heads similar to those of Aspergillus fumigatus (Fig. 2). Fungal colony surface was cottony with abundant white aerial hyphae, with conidia being sparsely produced, as opposed to velvety colonies of A. fumigatus, which are covered with a lawn of bluish green conidia. Growth assays at temperatures ranging from 10 to 45°C for 5 days were carried out. The isolate grew minimally at 10°C and did not grow at a temperature higher than 42°C. On the other hand, the reference strain A. fumigatus ATCC 1028 failed to grow at 10°C but grew well at ≥45°C. We also compared the growth of both the isolates at 37°C. While the colonies of the ATCC 1028 strain grew rapidly, the case isolate grew slowly for the first 2 days, but after 4 days the colonies reached a size similar to those of the ATCC 1028 strain.
Fig. 2.
Light microscopy of the lactophenol cotton-blue stained culture of Neosartorya udagawae from the case patient, showing conidial heads. Scale bar: 100 μm.
For molecular studies, the isolate was subcultured on SDA at 37°C for 5 days. DNA from fungal cultures was extracted using the DNeasy plant DNA extraction minikit (Qiagen, Milan, Italy) according to the manufacturer's instructions. PCR amplification was performed using fungus-specific primers for the internal transcribed spacer 1 and 2 regions flanking 5.8S ribosomal DNA (rDNA) (ITS1-5.8S-ITS2) (8) and partial portions of the β-tubulin and calmodulin genes (2, 3). PCR products were sequenced as previously described (20), and species identification was performed by searching databases using the BLAST sequence analysis tool (http://www.ncbi.nlm.nih.gov/BLAST/). The isolate was assigned to Neosartorya udagawae based on the complete identity with the corresponding sequences of the N. udagawae type strain CBS 114217.
Antifungal susceptibility testing.
Antifungal susceptibility testing was performed by the reference broth microdilution method according to the Clinical and Laboratory Standards Institute document M38-A2 (5). MIC values for the isolate at 48 h were reported for amphotericin B, fluconazole, itraconazole, voriconazole, and posaconazole. Minimum effective concentrations (MECs) at 48 h were reported for caspofungin, micafungin, and anidulafungin. According to previous studies (19, 25), the isolate was found to be resistant not only to fluconazole (MIC, >256 μg/ml) as expected, but also to voriconazole (8 μg/ml), whereas MICs of other antifungal agents demonstrated full susceptibility of the isolate to amphotericin B (0.125 μg/ml), itraconazole (0.25 μg/ml), and posaconazole (0.25 μg/ml). MECs of caspofungin, micafungin, and anidulafungin were 0.5, 0.25, and 0.016 μg/ml, respectively. A comparable antifungal susceptibility profile was seen for the A. fumigatus reference strain, with the exception of voriconazole and posaconazole, for which MICs were 0.5 μg/ml and 0.06 μg/ml, respectively.
Discussion.
Although less common than bacterial keratitis, fungal keratitis is more devastating, with deep infection often difficult to cure by antifungal medication (1, 27). Aspergillus flavus, A. fumigatus, Aspergillus terreus, and Aspergillus niger (14) and, less frequently, Aspergillus tamari (12), Aspergillus nomius (16), Aspergillus tubingensis (13), and Aspergillus brasiliensis (15) have been isolated from keratomycosis cases. In contrast, N. udagawae, an ascomycete with an A. fumigatus-like anamorph (21), originally identified from Brazilian soil (11) and only recently implicated in cases of invasive aspergillosis (25), has not yet been reported from mycotic keratitis.
Aspergillus lentulus and Neosartorya species (N. fischeri, N. pseudofischeri, N. spinosa, N. hiratsukae, and N. udagawae) fall within section Fumigati of the genus Aspergillus (3). Identification of species within a section, e.g., discrimination of A. fumigatus from N. udagawae, may be important given that different species have variable susceptibilities to multiple antifungal drugs (3). Most isolates of A. fumigatus are considered to be susceptible to amphotericin B, extended-spectrum triazoles, and caspofungin (26), whereas Fumigati-mimetic molds, including N. udagawae, tend to demonstrate in vitro resistance to various agents, potentially contributing to refractory disease (25).
Morphology-based identification methods for Aspergillus species are often inadequate, as members of the section Fumigati have overlapping morphological features (4). A polyphasic identification approach was therefore developed that included phenotypic (i.e., macro- and micromorphology, growth temperature regimens, and extrolite patterns) and genotypic characters (i.e., comparative sequence-based analysis) (3). Together with other researchers (4, 25), we demonstrated that DNA sequence analysis of partial β-tubulin and calmodulin genes allowed us to assign our isolate to N. udagawae.
Although members of the genus Neosartorya present ubiquitously, they have only rarely been reported to be pathogens, perhaps because Aspergillus species with known sexual stages (teleomorphs) tend to produce fewer asexual spores than their nonteleomorphic relatives (9). However, invasive or deep Neosartorya infections involving N. fischeri, N. pseudofischeri, and N. hiratsukae have been described in patients with various underlying diseases, and mortality rates ranged from 33 to 67% (25). Notably, one of these cases involved a patient with no history of trauma to his infected left eye, in whom keratitis progressed to endophthalmitis despite treatment with ketoconazole, thus requiring eye evisceration (6). Furthermore, a recent report on several cases of invasive aspergillosis caused by N. udagawae showed that the clinical course for these cases was distinct from that of typical disease due to A. fumigatus (25). The difference in the progression of the disease caused by the two species may be consistent with distinctive growth behavior and susceptibility to the host defenses in vitro. With respect to A. fumigatus, conidia of N. udagawae are more susceptible to neutrophils/hydrogen peroxide, suggesting a reduced virulence for this species (21).
According to our report, the finding of repeat negative cultures of corneal scrapes in very deep infections is not surprising (28). Paradoxically, in cases of contact lens-related microbial keratitis in which the corneal scraping is culture negative, contact lens culture may sometimes be a clue to the possible organism involved (7). This may also explain the superior results obtained by some authors when culturing corneal biopsy specimens compared to those obtained from culturing corneal scrapings (23). Nevertheless, it is plausible that the use of an antibiotic-steroid combination in the initial management of our patient before diagnosis had exacerbated the N. udagawae infection in the absence of antifungal treatment, as described elsewhere (18). Therefore, the use of topical steroids should be discouraged once the cause of infection is known.
Low sensitivity and time retardation of corneal cultures imply a delay in both diagnosis and institution of an appropriate antifungal therapy. We took several days to obtain a positive culture result from our patient's specimens. However, in the presence of a high suspicion of fungal infection, we started empirical antifungal therapy with oral fluconazole that was continued until culture reports became available. Although revealing itself to be inadequate, the choice of using fluconazole was suggested by several pieces of evidence in the literature (23). One of them is that older-generation azoles, with the exception of fluconazole, achieve only limited concentrations in the eye; another piece of evidence is the generally lower frequency, at least in temperate climates, of Aspergillus infections sustained by ocular trauma with respect to those due to Fusarium spp. and Curvularia spp., the last two infections being successfully treated with fluconazole (23). In our case, fluconazole was changed to itraconazole only when the fungus was conventionally identified as an Aspergillus section Fumigati species on the basis of morphological and microscopic characteristics. Subsequent susceptibility testing results confirmed the appropriateness of the therapeutic treatment, also in view of the in vitro voriconazole resistance observed with our isolate. Although in retrospect itraconazole proved to be a better choice than voriconazole, it should be noted that voriconazole is becoming the drug of choice for all fungal keratitis and endophthalmitis (10). First, the new-generation triazole has an excellent broad spectrum of antifungal activity and is active against species (except for N. udagawae) that are known to be resistant to the other antifungal agents commonly used in fungal keratitis (1). Second, there are good data to suggest that voriconazole achieves therapeutic concentrations in both the aqueous and vitreous humor following oral administration (10), as well as that topically administered 1% voriconazole solution results in aqueous concentrations exceeding the MIC at which 90% of isolates are inhibited for Aspergillus, Fusarium, and Candida species (24).
Fungal infection continues to be an important cause of ocular morbidity. An early diagnosis and accurate identification of the fungal etiological agent may improve the poor clinical outcome of keratomycosis (2). In this context, in vivo confocal microscopy has recently emerged as a highly sensitive tool for rapid diagnosis in severe infectious keratitis (22). In addition, a new molecular-based method consisting of DNA-stabilizing filter paper for specimen collection together with direct PCR could be a promising alternative for rapid detection of fungal keratitis (17).
Footnotes
Published ahead of print on 30 March 2011.
REFERENCES
- 1. Al-Badriyeh D., Neoh C. F., Stewart K., Kong D. C. 2010. Clinical utility of voriconazole eye drops in ophthalmic fungal keratitis. Clin. Ophthalmol. 4:391–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balajee S. A., et al. 2009. Sequence-based identification of Aspergillus, Fusarium, and Mucorales species in the clinical mycology laboratory: where are we and where should we go from here? J. Clin. Microbiol. 47:877–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balajee S. A., et al. 2007. Aspergillus species identification in the clinical setting. Stud. Mycol. 59:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balajee S. A., Nickle D., Varga J., Marr K. A. 2006. Molecular studies reveal frequent misidentification of Aspergillus fumigatus by morphotyping. Eukaryot. Cell 5:1705–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clinical Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, second edition. CLSI document M38-A2. CLSI, Wayne, PA [Google Scholar]
- 6. Coriglione G., et al. 1990. Neosartorya fischeri var. fischeri (Wehmer) Malloch and Cain 1972 (anamorph: Aspergillus fischerianus Samson and Grams 1985) as a cause of mycotic keratitis. Eur. J. Epidemiol. 6:382–385 [DOI] [PubMed] [Google Scholar]
- 7. Das S., Sheorey H., Taylor H. R., Vajpayee R. B. 2007. Association between cultures of contact lens and corneal scraping in contact lens related microbial keratitis. Arch. Ophthalmol. 125:1182–1185 [DOI] [PubMed] [Google Scholar]
- 8. de Hoog G. S., Guarro J., Gené J., Figueras M. J. 2000. Atlas of clinical fungi. Centraalbureau voor Schimmelcultures, Utrecht, Netherlands [Google Scholar]
- 9. Geiser D. M. 2009. Sexual structures in Aspergillus: morphology, importance and genomics. Med. Mycol. 47:S21–S26 [DOI] [PubMed] [Google Scholar]
- 10. Hariprasad S. M., Mieler W. F., Lin T. K., Sponsel W. E., Graybill J. R. 2008. Voriconazole in the treatment of fungal eye infections: a review of current literature. Br. J. Ophthalmol. 92:871–878 [DOI] [PubMed] [Google Scholar]
- 11. Horie Y., Miyaji M., Nishimura K., Franco M. F., Coelho K. I. 1995. Two new species of Neosartorya from Brazilian soil. Mycoscience 36:159–165 [Google Scholar]
- 12. Kredics L., et al. 2007. Case of keratitis caused by Aspergillus tamarii. J. Clin. Microbiol. 45:3464–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kredics L., et al. 2009. Infectious keratitis caused by Aspergillus tubingensis. Cornea 28:951–954 [DOI] [PubMed] [Google Scholar]
- 14. Manikandan P., et al. 2008. Aspergillus species in human keratomycosis, p. 293–328 In Varga J., Samson R. (ed.), Aspergillus in the genomic era. Wageningen Academic Publishers, Wageningen, Netherlands [Google Scholar]
- 15. Manikandan P., et al. 2010. Keratitis caused by the recently described new species Aspergillus brasiliensis: two case reports. J. Med. Case Rep. 4:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manikandan P., et al. 2009. Mycotic keratitis due to Aspergillus nomius. J. Clin. Microbiol. 47:3382–3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Menassa N., et al. 2010. Rapid detection of fungal keratitis with DNA-stabilizing FTA filter paper. Invest. Ophthalmol. Vis. Sci. 51:1905–1910 [DOI] [PubMed] [Google Scholar]
- 18. Peponis V., Herz J. B., Kaufman H. E. 2004. The role of corticosteroids in fungal keratitis: a different view. Br. J. Ophthalmol. 88:1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pfaller M., et al. 2011. Use of epidemiological cutoff values to examine 9-year trends in susceptibility of Aspergillus species to the triazoles. J. Clin. Microbiol. 49:586–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanguinetti M., et al. 2007. Evaluation of VITEK 2 and RapID yeast plus systems for yeast species identification: experience at a large clinical microbiology laboratory. J. Clin. Microbiol. 45:1343–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sugui J. A., et al. 2010. Neosartorya udagawae (Aspergillus udagawae), an emerging agent of aspergillosis: how different is it from Aspergillus fumigatus? J. Clin. Microbiol. 48:220–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takezawa Y., et al. 2010. Effectiveness of in vivo confocal microscopy in detecting filamentous fungi during clinical course of fungal keratitis. Cornea 29:1346–1352 [DOI] [PubMed] [Google Scholar]
- 23. Thomas P. A. 2003. Current perspectives on ophthalmic mycoses. Clin. Microbiol. Rev. 16:730–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vemulakonda G. A., et al. 2008. Aqueous and vitreous concentrations following topical administration of 1% voriconazole in humans. Arch. Ophthalmol. 126:18–22 [DOI] [PubMed] [Google Scholar]
- 25. Vinh D. C., et al. 2009. Invasive aspergillosis due to Neosartorya udagawae. Clin. Infect. Dis. 49:102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walsh T. J., et al. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327–360 [DOI] [PubMed] [Google Scholar]
- 27. Xie L., Dong X., Shi W. 2001. Treatment of fungal keratitis by penetrating keratoplasty. Br. J. Ophthalmol. 85:1070–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yildiz E. H., et al. 2010. Update on fungal keratitis from 1999 to 2008. Cornea 29:1406–1411 [DOI] [PubMed] [Google Scholar]