Abstract
The prevalence of class D extended-spectrum oxacillinases (ES-OXAs) in ceftazidime-resistant strains of Pseudomonas aeruginosa is often underestimated by double-disk synergy tests (DDST) using clavulanate. A DDST with a customized distance between a disk of ceftazidime or cefepime and inhibitors (clavulanate and imipenem) detected 14 out of 15 different ES-OXAs.
TEXT
The development of enzymatic resistance to β-lactams in the opportunistic pathogen Pseudomonas aeruginosa results from mutational overproduction of the intrinsic cephalosporinase AmpC, from acquisition of transferable genes coding for a variety of secondary β-lactamases, or both (20). A growing number of Ambler class A extended-spectrum β-lactamases (ESBLs) such as TEM, SHV, PER, GES, VEB, BEL, KPC, and CTX-M, class B carbapenemases (metallo-β-lactamases [MBLs], such as IMP, VIM, SPM, and GIM), and 17 class D extended-spectrum oxacillinases (ES-OXAs) have been found in clinical strains of P. aeruginosa from various geographical origins (reviewed in references 10, 17, and 30 to 32). Inhibition of ESBL and MBL activities by clavulanate and cation chelators (EDTA, thiol compounds, and dipicolinic acid), respectively, has allowed the development of useful phenotypic screening assays to detect most of these enzymes in clinical strains (2, 22).
ES-OXA production confers a high-level resistance to antipseudomonal cephalosporins (10). Epidemiological studies usually neglect ES-OXAs. However, an unexpected proportion (33%) of ES-OXAs among β-lactamases with extended-spectrum produced has recently been found in clinical P. aeruginosa (15). In addition, new ES-OXAs have been found recently in several European countries (17, 30), as has as the spread of multidrug-resistant clones of P. aeruginosa producing ES-OXAs (5, 13a, 19). Most of the 17 ES-OXA genes identified so far in P. aeruginosa are located on plasmid-borne integrons, allowing their diffusion and spread (30). Altogether, these data suggest the emergence of ES-OXAs among clinical isolates of P. aeruginosa and support the need for a targeted screening test. A regular double-disk synergy test (DDST) using clavulanate usually failed to detect the ES-OXAs (with the exception of the clavulanate-inhibited OXA-18). ES-OXAs are characterized by an important genetic diversity: they derive from OXA-10 (OXA-11, -14, -16, -17, -142, -145, and -147), OXA-2 (OXA-15, -32, -144, and -161), OXA-1 (OXA-31), or OXA-13 (OXA-19, -28, and -183) or are more distant from these groups (OXA-18 and -45) (30). In addition, narrow-spectrum oxacillinase-encoding genes (e.g., blaOXA-10) which are frequently expressed by clinical isolates (14, 15) differ from blaES-OXA only by point mutations. This renders a PCR-based approach difficult for ES-OXA screening.
Although the inhibitory activity of clavulanate on ES-OXAs is poor, it has been repeatedly documented (5, 8, 12, 17, 24). Likewise, imipenem inhibits the activity of ES-OXAs derived from blaOXA-10, as observed with members of the BEL, GES, and VEB groups (24). Jiang et al. have shown that reducing the distance between disks enhanced the performance of the DDST (16). Based on these data, we designed a strain-tailored DDST for the detection of P. aeruginosa producing ES-OXAs with a customized distance between the disks of substrates (ceftazidime and cefepime) and inhibitors (clavulanate and imipenem).
Strain-tailored double-disk synergy test.
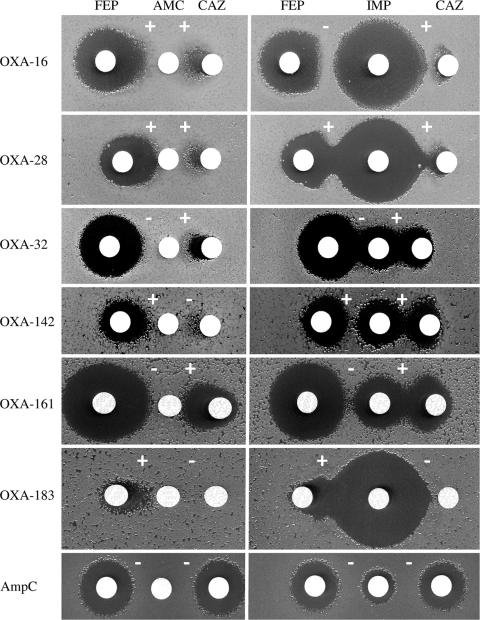
A routine disk susceptibility test was first performed to determine inhibition zones around disks containing separate compounds. Mueller-Hinton (MH) agar plates (Bio-Rad, Ivry-sur-Seine, France) were inoculated with a 1/100 dilution of a 0.1 McFarland suspension, and disks (Bio-Rad) containing clavulanate (10 μg in amoxicillin-clavulanate disk), ceftazidime (30 μg), cefepime (30 μg), and imipenem (10 μg) were tested (25). The day after and under identical conditions, the synergy between disks of substrates (ceftazidime and cefepime) and inhibitors (clavulanate and imipenem) was tested in duplicate. Distances between the disks were adapted to each strain based on inhibition zone diameters around disks containing each compound tested separately. Optimal detection of ES-OXAs was yielded, with a distance of 5 ± 1 mm between the edges of the two inhibition zones around the disks of considered agents (Fig. 1). A collection of clinical P. aeruginosa producing ES-OXAs (OXA-11, -14 to -19, -28, -32, -142, -144, -145, -147, -161, and -183) was assayed. We controlled the ability of that test to detect ESBL-producing (PER-1, VEB-1, GES-1, and BEL-1) and MBL-producing (VIM-1) isolates. In addition, P. aeruginosa isolates from our laboratory collection were tested (Table 1). The presence of the bla genes encoding ES-OXAs, ESBLs, and MBLs was assessed by sequencing experiments (see their origin in Table 1). As a negative control, we tested a reference strain (PAOΔdacB) and a collection of 12 clinical isolates overproducing the chromosomally encoded cephalosporinase AmpC (3, 23). After 18 h of incubation, the presence of an enlarged zone (≥2 mm) or a synergy zone between any disks of antimicrobial agents and a disk of inhibitor was considered a positive result.
Fig. 1.
Double-disk synergy test with P. aeruginosa isolates producing the extended-spectrum oxacillinases OXA-16 and OXA-142 (OXA-10 derived), OXA-28 and OXA-183 (OXA-13 derived), or OXA-32 and OXA-161 (OXA-2 derived) or overproducing the cephalosporinase AmpC (AmpC). Distances between the disks were adapted to each strain, based on the inhibition zone diameter around disks containing each compound tested separately. For instance, if no inhibition zone was noticed around clavulanate- and ceftazidime-containing disks, the distance between their two disks is 5 ± 1 mm. Abbreviations: FEP, cefepime (30 μg); AMC, amoxicillin-clavulanate (20/10 μg); CAZ, ceftazidime (30 μg); IMP, imipenem (10 μg). Interpretative results are given (see Table 1).
Table 1.
Results of strain-tailored DDST applied to a collection of P. aeruginosa strains producing ES-OXAs, ESBLs, or MBLs or overproducing the AmpC cephalosporinasea
| Enzyme produced | Reference strain |
No. of additional clinical strains tested | Synergy between disks containingb: |
||||
|---|---|---|---|---|---|---|---|
| Reference or accession no. | CAZ MIC (μg/ml) | CLA-FEP | CLA-CAZ | IMP-FEP | IMP-CAZ | ||
| OXA-11 | 13 | 512 | 0 | + | + | + | + |
| OXA-14 | 7 | 512 | 1c | − | − | + | + |
| OXA-15 | 8 | 128 | 0 | + | + | − | + |
| OXA-16 | 9 | ≥128 | 0 | + | + | − | + |
| OXA-17 | 6 | 128 | 0 | + | + | + | + |
| OXA-18 | 26 | 128 | 0 | + | + | + | + |
| OXA-19 | 24 | 512 | 48d | + | + | + | + |
| OXA-28 | 29 | 256 | 13e | + | + | + | + |
| OXA-32 | 28 | 128 | 0 | − | + | − | + |
| OXA-142 | EU358785 | 128 | 0 | + | − | + | + |
| OXA-144 | 18 | >64 | 0 | − | − | − | − |
| OXA-145 | FJ790516 | 128 | 0 | + | − | + | + |
| OXA-147 | 12 | 256 | 0 | + | − | + | − |
| OXA-161 | 17 | 128 | 0 | − | + | − | + |
| OXA-183 | HQ111474 | 128 | 0 | + | − | + | − |
| PER-1 | 21 | 512 | 3f | + | + | + | + |
| GES-1 | 11 | 32 | 0 | + | + | + | + |
| VEB-1 | 1 | >512 | 1f | + | + | + | + |
| BEL-1 | 27 | 32 | 0 | + | + | − | + |
| VIM-1 | 1 | >128 | 0 | − | − | − | − |
| AmpC | 23 | 32 | 12g | − | − | − | − |
Strain-tailored DDST detects ES-OXAs (Fig. 1).
Positive DDSTs were obtained with 13/15 and 14/15 reference strains producing different ES-OXAs by using clavulanate and imipenem, respectively. Only OXA-144 failed to be detected by both inhibitors (Table 1). Synergies were noticed with the 4 reference strains producing ESBLs but not with the MBL-producing strain. The reference strain overproducing AmpC was found to be negative with our DDST. All the additional clinical isolates taken from our collection behaved in the same way as the reference strains (Table 1).
The strain-tailored DDST thus appears to be a reproducible and cost-effective screening test for both ES-OXA- and ESBL-producing isolates in the medical laboratory. Since AmpC-overproducing mutants are very prevalent among β-lactam-resistant strains of P. aeruginosa, the DDST should optimize the use of molecular methods (i.e., PCR and DNA sequencing) by restricting the number of strains requiring complementary analyses. Production of ES-OXAs or ESBLs in P. aeruginosa typically leads to a high level of resistance to ceftazidime (10, 32). We therefore suggest testing of resistant isolates (as defined by the CLSI [25]) with a MIC of ≥32 μg/ml or with an inhibition zone of ≤15 mm around the ceftazidime-containing disk. It could be perfectly possible that rare ESBL- or ES-OXA-producing strains yield a MIC of <32 μg/ml, but a lower threshold could render this screening test hardly realistic and less cost-effective in the clinical laboratory. The test described here is now used routinely in our reference laboratory. It has allowed the detection of known ES-OXAs in clinical strains (4, 5, 15) (Table 1) and the discovery of new variants (OXA-145, OXA-147, and OXA-183) (12, 13a). However, it needs to be evaluated on a larger scale, but we believe that it may significantly improve the detection of ES-OXAs. In conjunction with an MBL-targeted screening test, it may help expedite implementation of control measures for preventing the spread of multidrug-resistant strains harboring emerging resistance mechanisms.
Acknowledgments
The National Reference Center for Antibiotic Resistance in Besançon is funded by the French Ministry of Health via the Institut de Veille Sanitaire. D.H. was supported by a grant from the European Community (7th PCRD; PIOF-GA-2009-235009).
We thank Laurent Poirel and Patrice Nordmann (Faculté de Médecine Paris-Sud, Kremlin-Bicêtre) for the gift of the isolates producing OXA-28, OXA-32, GES-1, VEB-1, BEL-1, and VIM-1 and Antonio Oliver (Instituto Universitario de Investigación en Ciencias de la Salud, Palma de Mallorca, Spain) for the isolates producing OXA-144 and OXA-161 and for the strain PAO1ΔdacB. Stuart Shapiro (Basilea Pharmaceutica, Basel, Switzerland) kindly provided isolates producing OXA-11, -14, -15, -16, and -17.
Footnotes
Published ahead of print on 30 March 2011.
REFERENCES
- 1. Aubert D., Girlich D., Naas T., Nagarajan S., Nordmann P. 2004. Functional and structural characterization of the genetic environment of an extended-spectrum β-lactamase blaVEB gene from a Pseudomonas aeruginosa isolate obtained in India. Antimicrob. Agents Chemother. 48:3284–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bradford P. A. 2001. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cavallo J. D., Hocquet D., Plésiat P., Fabre R., Roussel-Delvallez M. 2007. Susceptibility of Pseudomonas aeruginosa to antimicrobials: a 2004 French multicentre hospital study. J. Antimicrob. Chemother. 59:1021–1024 [DOI] [PubMed] [Google Scholar]
- 4. Cholley P., et al. 2010. Molecular epidemiology of multidrug-resistant Pseudomonas aeruginosa in a French university hospital. J. Hosp. Infect. 76:316–319 [DOI] [PubMed] [Google Scholar]
- 5. Cholley P., et al. 2010. Hospital outbreak of Pseudomonas aeruginosa producing extended-spectrum oxacillinase OXA-19. J. Med. Microbiol. 59:866–869 [DOI] [PubMed] [Google Scholar]
- 6. Danel F., Hall L. M., Duke B., Gur D., Livermore D. M. 1999. OXA-17, a further extended-spectrum variant of OXA-10 β-lactamase, isolated from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:1362–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Danel F., Hall L. M., Gur D., Livermore D. M. 1995. OXA-14, another extended-spectrum variant of OXA-10 (PSE-2) β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 39:1881–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Danel F., Hall L. M., Gur D., Livermore D. M. 1997. OXA-15, an extended-spectrum variant of OXA-2 β-lactamase, isolated from a Pseudomonas aeruginosa strain. Antimicrob. Agents Chemother. 41:785–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Danel F., Hall L. M., Gur D., Livermore D. M. 1998. OXA-16, a further extended-spectrum variant of OXA-10 β-lactamase, from two Pseudomonas aeruginosa isolates. Antimicrob. Agents Chemother. 42:3117–3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Danel F., Page M. G. P., Livermore D. M. 2007. Class D β-lactamases, p. 163–194 In Bonomo R. A., Tolmasky M. E. (ed.), Enzyme-mediated resistance to antibiotics. ASM Press, Washington, DC [Google Scholar]
- 11. Dubois V., et al. 2002. Molecular characterization of a novel class 1 integron containing bla(GES-1) and a fused product of aac3-Ib/aac6′-Ib′ gene cassettes in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 46:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fournier D., Hocquet D., Dehecq B., Cholley P., Plésiat P. 2010. Detection of a new extended-spectrum oxacillinase in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 65:364–365 [DOI] [PubMed] [Google Scholar]
- 13. Hall L. M., Livermore D. M., Gur D., Akova M., Akalin H. E. 1993. OXA-11, an extended-spectrum variant of OXA-10 (PSE-2) β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 37:1637–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a. Hocquet D., et al. Ceftazidime-hydrolysing β-lactamase OXA-145 with impared hydrolysis of penicillins in Pseudomonas aeruginosa. J. Antimicrob. Chemother., in press [DOI] [PubMed] [Google Scholar]
- 14. Hocquet D., Nordmann P., El Garch F., Cabanne L., Plésiat P. 2006. Involvement of the MexXY-OprM efflux system in emergence of cefepime resistance in clinical strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:1347–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hocquet D., et al. 2010. Nationwide investigation of extended-spectrum β-lactamases, metallo-β-lactamases and extended-spectrum oxacillinases produced by ceftazidime-resistant Pseudomonas aeruginosa in France. Antimicrob. Agents Chemother. 54:3512–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang X., et al. 2006. Detection of extended-spectrum β-lactamases in clinical isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:2990–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Juan C., et al. 2009. Detection of the novel extended-spectrum β-lactamase OXA-161 from a plasmid-located integron in Pseudomonas aeruginosa clinical isolates from Spain. Antimicrob. Agents Chemother. 53:5288–5290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Juan C., Zamorano L., Perez J. L., Ge Y., Oliver A. 2010. Activity of a new antipseudomonal cephalosporin, CXA-101 (FR264205), against carbapenem-resistant and multidrug-resistant Pseudomonas aeruginosa clinical strains. Antimicrob. Agents Chemother. 54:846–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kalai Blagui S., et al. 2007. Nosocomial outbreak of OXA-18-producing Pseudomonas aeruginosa in Tunisia. Clin. Microbiol. Infect. 13:794–800 [DOI] [PubMed] [Google Scholar]
- 20. Livermore D. M. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis. 34:634–640 [DOI] [PubMed] [Google Scholar]
- 21. Llanes C., Neuwirth C., El Garch F., Hocquet D., Plésiat P. 2006. Genetic analysis of a multiresistant strain of Pseudomonas aeruginosa producing PER-1 β-lactamase. Clin. Microbiol. Infect. 12:270–278 [DOI] [PubMed] [Google Scholar]
- 22. Miriagou V., et al. 2010. Acquired carbapenemases in Gram-negative bacterial pathogens: detection and surveillance issues. Clin. Microbiol. Infect. 16:112–122 [DOI] [PubMed] [Google Scholar]
- 23. Moya B., et al. 2009. β-Lactam resistance response triggered by inactivation of a nonessential penicillin-binding protein. PLoS Pathog. 5:e1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mugnier P., Casin I., Bouthors A. T., Collatz E. 1998. Novel OXA-10-derived extended-spectrum β-lactamases selected in vivo or in vitro. Antimicrob. Agents Chemother. 42:3113–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. NCCLS/CLSI 2009. Method for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 8th ed., M7-A7 NCCLS/CLSI, Wayne, PA [Google Scholar]
- 26. Philippon L., Naas T., Bouthors A., Barakett V., Nordmann P. 1997. OXA-18, a class D clavulanic acid-inhibited extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 41:2188–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poirel L., Brinas L., Verlinde A., Ide L., Nordmann P. 2005. BEL-1, a novel clavulanic acid-inhibited extended-spectrum β-lactamase, and the class 1 integron In120 in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:3743–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Poirel L., et al. 2002. Integron-located oxa-32 gene cassette encoding an extended-spectrum variant of OXA-2 β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 46:566–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poirel L., Girlich D., Naas T., Nordmann P. 2001. OXA-28, an extended-spectrum variant of OXA-10 β-lactamase from Pseudomonas aeruginosa and its plasmid- and integron-located gene. Antimicrob. Agents Chemother. 45:447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poirel L., Naas T., Nordmann P. 2010. Diversity, epidemiology, and genetics of class D β-lactamases. Antimicrob. Agents Chemother. 54:24–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rossolini G. M., Docquier J. D. 2007. Class B β-lactamases, p. 115–144 In Bonomo R. A., Tolmasky M. E. (ed.), Enzyme-mediated resistance to antibiotics. ASM Press, Washington, DC [Google Scholar]
- 32. Weldhagen G. F., Poirel L., Nordmann P. 2003. Ambler class A extended-spectrum β-lactamases in Pseudomonas aeruginosa: novel developments and clinical impact. Antimicrob. Agents Chemother. 47:2385–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]