Abstract
Respiratory tract colonization by molds in patients with cystic fibrosis (CF) were analyzed, with particular focus on the frequency, genotype, and underlying mechanism of azole resistance among Aspergillus fumigatus isolates. Clinical and demographic data were also analyzed. A total of 3,336 respiratory samples from 287 CF patients were collected during two 6-month periods in 2007 and 2009. Azole resistance was detected using an itraconazole screening agar (4 mg/liter) and the EUCAST method. cyp51A gene sequencing and microsatellite genotyping were performed for isolates from patients harboring azole-resistant A. fumigatus. Aspergillus spp. were present in 145 patients (51%), of whom 63 (22%) were persistently colonized. Twelve patients (4%) harbored other molds. Persistently colonized patients were older, provided more samples, and more often had a chronic bacterial infection. Six of 133 patients (4.5%) harbored azole-nonsusceptible or -resistant A. fumigatus isolates, and five of those six patients had isolates with Cyp51A alterations (M220K, tandem repeat [TR]/L98H, TR/L98H-S297T-F495I, M220I-V101F, and Y431C). All six patients were previously exposed to azoles. Genotyping revealed (i) microevolution for A. fumigatus isolates received consecutively over the 2-year period, (ii) susceptible and resistant isolates (not involving TR/L98H isolates) with identical or very closely related genotypes (two patients), and (iii) two related susceptible isolates and a third unrelated resistant isolate with a unique genotype and the TR/L98H resistance combination (one patient). Aspergilli were frequently found in Danish CF patients, with 4.5% of the A. fumigatus isolates being azole nonsusceptible or resistant. Genotyping suggested selection of resistance in the patient as well as resistance being achieved in the environment.
INTRODUCTION
Molds are frequently recovered from cultures of respiratory samples from patients with cystic fibrosis (CF). Aspergillus fumigatus is the predominant species, with reported prevalence rates from 6% to nearly 60% (32). Aspergillus terreus has been reported as the second most common Aspergillus species (42). Other fungi, such as Scedosporium spp., Exophiala dermatitidis, Acrophialophora fusispora, and recently Geosmithia argillacea, have also been detected (6, 13, 14, 25). However, reported rates are influenced by both laboratory- and patient-related factors, e.g., type of culture media used, incubation time, age of patients, applied definitions of transient or persistent mold colonization, and sampling frequencies (8, 32).
Aspergillus may cause several pulmonary manifestations in CF patients. Allergic bronchopulmonary aspergillosis (ABPA) is recognized as a severe complication and characterized by an accelerated decline in lung function. ABPA in CF affects mainly older children and adults, with a prevalence rate of 6 to 25% (17, 49). Aspergillus bronchitis is observed in CF patients not fulfilling the criteria for ABPA but with positive respiratory tract cultures, and clinical improvement occurs with appropriate antifungal treatment (28, 46). Invasive aspergillosis in the absence of lung transplantation is rare among CF patients (33). Finally, Aspergillus may colonize the lungs without causing apparent clinical disease, and the effects on lung function are not clear (2, 22). Genotyping studies of A. fumigatus isolates indicate that patients may be simultaneous or sequentially colonized with several different strains but eventually a single strain becomes dominant (15, 20, 51).
Itraconazole is often used for the treatment of chronic, noninvasive forms of aspergillosis (28) and has proven effective and steroid sparing in ABPA cases (31). Voriconazole is recommended as first-line therapy for invasive aspergillosis (24), and posaconazole is recommended for prophylaxis in severely immunocompromised patients (16). Resistance of A. fumigatus to itraconazole is well recognized and was first reported in 1997 for three clinical isolates obtained in the late 1980s (19). The main mechanism of azole resistance currently reported is alterations to the drug target encoded by the cyp51A gene. There have been reports describing azole resistance in azole-exposed patients (27, 30, 48) and also in azole-naïve patients, as well as in the environment in the Netherlands (11, 47, 48) and Denmark (36). However, a recent study found no azole-resistant isolates in CF patients (3).
At our CF center, with a tradition of close monitoring of patients and aggressive treatment of infections, all three azoles are frequently used. Thus, we explored the prevalence and dynamics of molds in respiratory tract samples from a cohort of approximately 300 CF patients, and we studied particularly the frequency, genotype, and underlying mechanism of azole resistance among the detected A. fumigatus isolates. Clinical and demographic data for patients with and without Aspergillus spp. in their respiratory samples were also analyzed.
(This work was presented in part at the 1st Meeting of the ISHAM Working Group on Fungal Respiratory Infections in Cystic Fibrosis, 7 to 8 June 2009, Angers, France, and at the 4th Trends in Medical Mycology Meeting, 18 to 21 October 2009, Athens, Greece.)
MATERIALS AND METHODS
Patients and samples.
At the CF center at Copenhagen University Hospital Rigshospitalet, patients have been followed with monthly visits in the outpatient clinic for clinical status and pulmonary function and microbiological investigations of lower respiratory tract secretions. The diagnosis of CF was based on abnormal sweat electrolytes, characteristic clinical features, or mutations in the gene encoding the CFTR protein. Clinical and demographic information was accessed by using the Copenhagen CF center database. ABPA was defined according to consensus criteria (49). Patients were treated with azoles (weeks of therapy were recorded) according to the following guidelines. Aspergillus colonization was treated if patients were symptomatic and no response was achieved on antibacterial therapy. The first-line treatment was itraconazole (10 mg/kg of body weight/day), followed by voriconazole (200 to 400 mg twice a day; the dose was adjusted if the weight of the patient was below 40 kg) or posaconazole (400 mg twice a day). ABPA was treated with an azole in combination with systemic steroid therapy. The duration of therapy was based on response but was generally 3 to 6 months. Therapeutic drug monitoring (TDM) was performed using a bioassay at the Statens Serum Institut although not systematically.
Respiratory samples were included prospectively during two sample periods: sample period I was from 1 July to 31 December 2007 (1,715 samples from 266 patients), and sample period II was from 1 July to 31 December 2009 (1,620 samples from 274 patients). Two hundred fifty-three patients (88.2%) out of 287 patients provided samples in both periods. Three patients died during sample periods, and three died between sample periods; four of the six were lung transplant recipients. The respiratory sample types were sputum, endolaryngeal suction, tracheal suction, and bronchoalveolar lavage (BAL) samples.
Cases were classified as (i) “mold negative” if no molds were detected in any of the respiratory samples (n = 130), (ii) “new Aspergillus cases” if patients were negative for Aspergillus or no samples were provided in 2007 and Aspergillus was present in 2009 (n = 44), (iii) “persistent Aspergillus colonization” if Aspergillus species were detected in both sample periods (n = 63), (iv) “Aspergillus colonization cleared” if patients were Aspergillus positive in 2007 but samples were negative or no samples were provided in 2009 (n = 38), or (v) “other molds” if other molds than Aspergillus spp. were detected in one or both sampling periods (n = 12).
Plating and identification.
Primary culture was performed using Sabouraud glucose (pH 4) agar and 5 bacteriological agars (SSI Diagnostika, Hillerød, Denmark), incubation at 37°C, and daily examination for up to 5 days. Species identification and susceptibility testing of the mold isolate(s) were done at the national reference laboratory according to morphological criteria (18). A. fumigatus complex isolates with reduced susceptibility to one or several azoles were further incubated at 48°C to separate A. fumigatus sensu stricto from cryptic A. fumigatus complex species. Aspergillus isolates that could not be identified to the species level were sequenced as described below.
Susceptibility testing and screening for itraconazole resistance in Aspergillus.
Unique Aspergillus isolates (isolated >6 months apart) underwent EUCAST susceptibility testing (44) as part of the clinical routine in 2007. Subsequent isolates were stored at −80°C and screened for azole resistance in parallel with isolates from 2009 by using an itraconazole-containing agar (4 mg/liter) (SSI Diagnostika) (47). Isolates which grew on the itraconazole agar were further examined according to the EUCAST method (44). Stock solutions (5,000 mg/liter in dimethyl sulfoxide [Sigma-Aldrich, Brøndby, Denmark]) were as follows (with manufacturers listed parenthetically): itraconazole (Sigma-Aldrich), voriconazole (Pfizer, Ballerup, Denmark), and posaconazole (Schering-Plough, Glostrup, Denmark). Final drug concentration ranges were 0.03 to 4 mg/liter. MICs were determined visually as a no-growth endpoint at 48 h of incubation time. Four A. fumigatus isolates with prominent but not absolute growth inhibition in the highest itraconazole concentration were further analyzed by using an extended range up to 16 mg/liter (and these are referred to as nonsusceptible). Quality control strains Candida krusei ATCC 6258 and C. parapsilosis ATCC 22019 were included but read after 24 h of incubation (50). In addition, resistant isolates were tested for susceptibility to amphotericin B and caspofungin by using Etest strips (AB Biodisk, Solna, Sweden) on RPMI 1640–2% glucose agar (SSI Diagnostika). For caspofungin, aberrant growth in the inhibition zone was ignored.
The following interpretative breakpoints were used for A. fumigatus and azoles. For itraconazole and voriconazole, the susceptible breakpoint was ≤1 mg/liter, the intermediate breakpoint was 2 mg/liter, and the resistant breakpoint was ≥4 mg/liter. For posaconazole, the susceptible breakpoint was ≤0.5 mg/liter, the intermediate breakpoint was 0.5 mg/liter, and the resistant breakpoint was ≥1 mg/liter (41, 43, 52).
Molecular identification and detection of resistance mechanisms.
DNA was extracted from fungal cultures as previously described (10). Three isolates of Aspergillus thermomutatus were identified by sequencing of the β-tubulin gene (5). An isolate of Aspergillus ustus, two isolates of Aspergillus nidulans, and two isolates of Penicillium sp. were identified by using universal primers ITS1 and ITS4 (23). The promoter and full coding region of the cyp51A gene were amplified by PCR using the primers 0F (5′-TCATATGTTGCTCAGCGG-3′) and 4R (5′-CCTATTCCGATCACACCAAA-3′) and, in addition, the primers 1R (5′-CATTGAGCAAGATTGCCG-3′), 2F (5′-CGGCAATCTTGCTCAATG-3′), 2R (5′-GGTGAATCGCGCAGATAGT-3′), 3F (5′-ACTATCTGCGCGATTCACC-3′), 3R (5′-GTCAAGATCCTTGTACTGGAGC-3′), and 4F (5′-CTCCAGTACAAGGATCTTGAC-3′) for the sequencing of the PCR product (34). The sequences were compared to the sequence of an azole-susceptible wild-type A. fumigatus isolate (GenBank accession no. AF338659).
Genotyping.
Genotyping was performed with a panel of nine short tandem repeats (TRs) as described previously (21). Microevolution was defined as small changes in repeat numbers (e.g., addition of one or two repeat units in a polymorphic locus), indicating a very high degree of relatedness between isolates. Construction of a phylogenetic tree was performed by using four microsatellite markers (3A, 3C, 4A, and 4B) used to allow inclusion of data from the University of Manchester for reference. Distance matrices between the microsatellite types were generated using the SplitsTree multilocus sequence typing (MLST) analysis tool on the PubMLST website (http://pubmlst.org/perl/mlstanalyse/mlstanalyse.pl?site=pubmlst&page=splitstree&referer=pubmlst.org), saved as files in NEXUS format, and imported into SplitsTree v.4.11.3 (www.splitstree.org). A nonrooted tree was generated using the neighbor-joining algorithm.
Data analysis.
The Mann-Whitney test was used for comparison of two groups of continuous data without normal distribution. Fisher's exact test was used to compare two groups of categorical data. P values less than 0.05 were considered statistically significant.
RESULTS
Cohort characteristics.
A total of 3,336 respiratory samples from 287 CF patients were included. Aspergillus spp. were present in 145 patients (51%), of which 63 patients (22%) were persistently colonized, whereas 12 patients (4%) harbored other molds (Table 1). Persistently colonized patients and patients who cleared their Aspergillus colonization were older, provided more respiratory samples, and were more often chronically colonized with bacteria than those who were negative for molds (Table 1). A higher proportion of patients with persistent Aspergillus colonization had ABPA than those with no molds (14% versus 5%; P = 0.041). Fewer persistently colonized patients had diabetes than mold-negative patients (14% versus 28%; P = 0.032). Patients who cleared their Aspergillus colonization were more often recipients of lung transplantation than mold-negative patients (29% versus 8%; P = 0.0014). Finally, females were numerically (but not significantly) more often persistently colonized with Aspergillus (Table 1). Neither lung function nor body mass index (BMI) z-scores differed significantly between the groups. Patients with other molds present had the highest proportion of ABPA, provided the most respiratory samples, and had the poorest lung function.
Table 1.
Cohort characteristics by mold colonization statusp
| Parametera | Patients with no molds (n = 130) | New Aspergillus cases (n = 44)b | Patients with persistent Aspergillus colonization (n = 63)c | Patients who cleared Aspergillus colonization (n = 38)d | Patients with other molds (n = 12) |
|---|---|---|---|---|---|
| Median yr of age (range) | 17.5 (0–51) | 18.5 (0–49) | 23.0 (5–57)g | 25.0 (2–47)h | 25.0 (8–36) |
| No. of females/males (% females) | 66/64 (51) | 22/22 (50) | 38/25 (60) | 20/18 (53) | 7/5 (58) |
| No. (%) of patients with CF genotype | |||||
| ΔF508/ΔF508 | 80e (62) | 31 (70) | 45 (71) | 30f (79) | 9 (75) |
| ΔF508/other | 41 (32) | 11 (25) | 18 (29) | 5 (13) | 3 (25) |
| Other/other | 5 (4) | 2 (5) | 0 | 0 | 0 |
| No. (%) of patients with CF-related diabetes | 37 (28) | 13 (30) | 9 (14)i | 10 (26) | 4 (33) |
| No. (%) of LTX | 10 (8) | 5 (11) | 4 (6) | 11 (29)j | 0 |
| No. (%) of patients with ABPA | 6 (5) | 1 (2) | 9 (14)k | 5 (13) | 3 (25) |
| Median FEV1% predicted (range) (n) | |||||
| 2007 | 80.9 (20.0–118.2) (101) | 79.3 (36.8–129.7) (30) | 75.4 (29.8–122.5) (60) | 74.6 (22.0–112.9) (32) | 70.9 (40.1–116.1) (12) |
| 2009 | 81.0 (17.0–115.8) (108) | 85.4 (33.1–135.8) (36) | 75.1 (28.8–115.0) (60) | 78.1 (26.8–125.5) (27) | 72.1 (37.5–116.5) (12) |
| Median BMI z-score (range) (n) | |||||
| 2007 | −0.2 (−3.5 to 2.2) (113) | −0.3 (−3.8 to 1.7) (38) | −0.4 (−2.5 to 2.7) (60) | −0.4 (−5.9 to 0.7) (33) | 0.3 (−1.3 to 1.2) (12) |
| 2009 | −0.4 (−4.0 to 2.4) (120) | −0.3 (−2.7 to 0.9) (40) | −0.4 (−2.7 to 2.6) (60) | −0.3 (−3.1 to 1.1) (27) | 0.3 (−1.7 to 1.6) (12) |
| Respiratory samples | |||||
| Total no. | 1,317 | 490 | 874 | 458 | 197 |
| Median no./patient (range) | 10 (1–29) | 12 (2–22) | 12 (4–28)l | 12 (3–20)m | 17 (8–28) |
| No. (%) of patients with chronic bacterial infection | |||||
| Pseudomonas aeruginosa | 37 (28) | 14 (32) | 25 (40) | 16 (42) | 6 (50) |
| Burkholderia spp. | 11 (8) | 2 (5) | 3 (5) | 2 (5) | 0 |
| Achromobacter spp. | 10 (8) | 4 (9) | 6 (10) | 6 (16) | 0 |
| Stenotrophomonas maltophilia | 3 (2) | 0 | 3 (5) | 3 (8) | 1 (8) |
| Other Gram-negative bacteria | 2 (2) | 2 (5) | 1 (2) | 1 (3) | 1 (8) |
| Mycobacteria | 0 | 1 (2) | 1 (2) | 0 | 0 |
| Nocardia spp. | 0 | 0 | 2 (3) | 0 | 0 |
| Total | 63 (48) | 23 (52) | 41 (65)n | 28 (74)o | 8 (67) |
LTX, lung transplant recipients; ABPA, allergic bronchopulmonary aspergillosis; FEV1% predicted, forced expiratory volume in 1 s (FEV1) divided by forced vital capacity (as a percentage) divided by the average population FEV1 for a similar patient.
34/44 were colonized with A. fumigatus.
57/63 were persistently colonized with A. fumigatus, 4/63 were colonized with A. fumigatus in 2007 and A. niger 2009, 1/63 was persistently colonized with A. terreus, and 1/63 was colonized with A. fumigatus in 2007 and other Aspergillus species in 2009. In total, 62/63 were colonized with A. fumigatus.
37/38 were colonized with A. fumigatus.
Insufficient data for four patients.
Insufficient data for three patients.
P = 0.015 (relative to the mold-negative value).
P = 0.017 (relative to the mold-negative value).
P = 0.032 (relative to the mold-negative value).
P = 0.0014 (relative to the mold-negative value).
P = 0.041 (relative to the mold-negative value).
P < 0.0001 (relative to the mold-negative value).
P = 0.023 (relative to the mold-negative value).
P = 0.032 (relative to the mold-negative value).
P = 0.009 (relative to the mold-negative value).
Continuous and categorical data of groups (the rightmost four columns) were compared to the group of patients with no molds by using the Mann-Whitney test and Fisher's exact test, respectively.
Detection of Aspergillus spp. and other molds.
Totals of 1,715 and 1,621 samples from 266 and 274 patients were analyzed in 2007 and 2009, respectively. Aspergillus was the most frequently recovered genus, with a constant detection rate among patients in the two periods (38.0% in 2007 versus 38.3% in 2009). In both periods, A. fumigatus was the most common species (37.2% and 33.2% of the cases), followed by Aspergillus flavus (4.1% and 4.4% of the cases) and A. terreus (1.9% and 2.6% of the cases). More patients harbored Aspergillus niger in 2009 than in 2007 (4.7% versus 0.8%). Finally, one patient harbored another Aspergillus species in 2007 (A. nidulans), and four patients harbored other species in 2009 (A. nidulans, A. versicolor, A. thermomutatus, and A. ustus). Among other molds, Penicillium spp. and Paecilomyces spp. were isolated more frequently from patients in 2009 (5.5% and 2.6% versus 0.8% and 0.8% in 2007, respectively). Other molds were detected in 1 to 3 patients per sampling period and included Scedosporium apiospermum, Fusarium spp., Acremonium spp., Trichoderma spp., Scopulariopsis spp., other hyaline hyphomycetes, Cladosporium spp., other dematiaceous fungi, Rhizopus microsporus, and Lichtheimia corymbifera. Thus, overall, 41% and 44.5% of the patients harbored at least one mold in the 2007 and 2009 study periods, respectively.
Azole susceptibility for Aspergillus isolates.
Overall, itraconazole MICs of ≥2 mg/liter were found in Aspergillus isolates from 8/145 (5.5%) patients, including 6/133 (4.5%) patients with A. fumigatus (Tables 2 and 3), 1/9 patients with A. terreus (itraconazole MIC of 4 mg/liter and voriconazole MIC of 2 to 4 mg/liter), and 1/1 with three A. thermomutatus isolates for which the itraconazole, voriconazole, and posaconazole MICs were >4 mg/liter, ≥4 mg/liter, and 0.125 to 0.25 mg/liter, respectively. One patient harbored four A. fumigatus isolates for which growth was notably reduced in wells with an itraconazole concentration of ≥2 mg/liter. However, with a stereo microscope and a stringent no-growth endpoint, the MICs were >16 mg/liter and are thus indicated as 2/>16 mg/liter in Table 3.
Table 2.
Characteristics of patients with azole-resistant A. fumigatus isolates
| Patient no. | Age (yr)/sexd | No. resistant A. fumigatus isolates, mo. and yr of first/last isolation | Total no. of A. fumigatus isolates/total no. of respiratory samples | Resistance mechanism | Aspergillus colonization pattern | Chronic bacterial infection | Wks of exposure to anti-Aspergillus azoles |
Serum azole concn (mg/liter)e | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 2001–2003 | 2004–2006 | 2007–2009 | ||||||||
| R1a | 25/F | 1, Nov. 2007 | 1/10 | M220K | Cleared | Achromobacter xylosoxidans | 156 | 104 | 19 | ND |
| R2 | 19/M | 1, Oct. 2007 | 1/15 | TR/L98H-S297T-F495I | Cleared | Stenotrophomonas maltophilia | 0 | 103 | 52 | ND |
| R3b | 30/F | 4, Sept. 2007 | 7/18 | Y431C | Cleared | Pandoraea apista | 0 | 26 | 21 | 0.1 POS |
| R4 | 29/M | 9, July 2009/Dec. 2009 | 13/24 | M220I-V101F | Persistent | Pseudomonas aeruginosa | 72 | 94 | 138 | <0.1 ITC, 0.4–1.8 POS |
| R5c | 29/F | 7, Aug. 2007/Nov. 2009 | 15/16 | TR/L98H-S297T-F495I, TR/L98H | Persistent | A. xylosoxidans | 0 | 6 | 121 | <0.1-2.1 ITC, 0.9–1.2 VRC |
| R6 | 17/M | 2, Aug. 2009/Oct. 2009 | 17/22 | Not found | Persistent | No chronic infection | 0 | 58 | 117 | <0.1–0.3 VRC |
| Total wks of overall exposure (avg no. of wks per patient) | ||||||||||
| Azole-resistant-A. fumigatus-positive patients (n = 6) | 228 (38) | 391 (65) | 468 (78) | |||||||
| Aspergillus positive (excluding azole-resistant patients) (n = 139) | 1,514 (11) | 2,691 (19) | 3,999 (29) | |||||||
| No molds (n = 130) | 1,216 (9) | 1,882 (14) | 2,034 (16) | |||||||
An azole-resistant M220K isolate was also recovered in January 2007.
This patient underwent lung transplantation 1 December 2007; three additional isolates were lost and not available for susceptibility testing.
An azole-resistant TR/L98H-S297T-F495I isolate was also recovered in May 2009.
F, female; M, male.
ND, not determined; ITC, itraconazole; VRC, voriconazole; POS, posaconazole.
Table 3.
MICs, Cyp51A amino acid alterations, and genotypes by patients with azole-resistant, azole-susceptible, and environmental A. fumigatus isolates
| Isolate | Sample date (day.mo.yr) | ITC agar resulte | MIC (mg/liter)f |
Cyp51A alteration | Genotyped |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EUCAST |
Etest |
||||||||||||||||
| ITC | VRC | POS | AMB | CAS | 2A | 2B | 2C | 3A | 3B | 3C | 4A | 4B | 4C | ||||
| R1-07-1_Ra | 04.01.2007 | + | >4 | 4 | >4 | 1 | 0.064 | M220K | 23 | 23 | 15 | 35 | 10 | 20 | 13 | 9 | 5 |
| R1-07-2_R | 22.11.2007 | + | >4 | 2 | >4 | 0.5 | ND | M220K | 23 | 23 | 15 | 35 | 10 | 20 | 13 | 9 | 5 |
| R2-07-1_R | 22.10.2007 | + | >4 | 0.5 | 0.5 | 0.25 | ND | TR/L98H-S297T-F495I | 14 | 20 | 8 | 40 | 9 | 11 | 8 | 10 | 20 |
| R3-07-1_nS | 07.08.2007 | v | 2/>16g | 2 | 0.25 | 0.5 | 0.016 | Y431C | 19 | 26 | 9 | 31 | 11 | 36 | 20 | 9 | 8 |
| R3-07-2_nS | 21.08.2007 | v | >2/>16g | 2 | 0.125 | 0.25 | 0.016 | Y431C | 19 | 26 | 9 | 31 | 11 | 36 | 20 | 9 | 8 |
| R3-07-3_nS | 27.08.2007 | v | >2/>16g | 2 | 0.125 | 0.25 | 0.016 | Y431C | 19 | 26 | 9 | 31 | 11 | 36 | 20 | 9 | 8 |
| R3-07-4_nS | 14.09.2007 | v | >2/>16g | 1 | 0.125 | 0.5 | 0.032 | Y431C | 19 | 26 | 9 | 31 | 11 | 36 | 20 | 9 | 8 |
| R4-07-1_S | 17.07.2007 | − | 1 | 1 | 0.25 | 0.5 | 0.032 | Not found | 18 | 23 | 16 | 35 | 13 | 18 | 15 | 9 | 10 |
| R4-07-2_S | 05.12.2007 | − | 0.5 | 1 | 0.5 | 0.5 | 0.016 | Not found | 18 | 23 | 16 | 35 | 13 | 18 | 15 | 9 | 10 |
| R4-09-1_R | 09.07.2009 | + | >4 | 1 | 4 | 0.5 | 0.016 | M220I-V101F | 18 | 23 | 16 | 35 | 13 | 18 | 15 | 9 | 10 |
| R4-09-2_R | 16.07.2009 | + | >4 | 1 | 4 | 0.25 | 0.032 | M220I-V101F | 18 | 23 | 16 | 35 | 13 | 18 | 15 | 12 | 10 |
| R4-09-3_Rb | 21.07.2009 | + | >4 | 2 | 4 | 0.5 | 0.016 | M220I-V101F | 18 | 23 | 16 | 35 | 13 | 18 | 15 | 9 | 10 |
| R4-09-4_R | 10.08.2009 | + | >4 | 1 | 4 | 0.25 | 0.032 | M220I-V101F | 18 | 23 | 16 | 35 | 13 | 18 | 15 | 9 | 10 |
| R4-09-5_R | 07.09.2009 | + | >4 | 1 | 4 | 1 | 0.032 | M220I-V101F | 18 | 23 | 16 | 35 | 13 | 18 | 15 | 12 | 10 |
| R4-09-6_R | 25.09.2009 | + | >4 | 4 | 0.5 | 0.5 | 0.125 | Not found | 18 | 23 | 16 | 35 | 13 | 18 | 15 | 9 | 10 |
| R4-09-7_R | 23.10.2009 | + | >4 | 4 | 4 | 0.5 | 0.032 | M220I-V101F | 18 | 23 | 16 | 35 | 13 | 18 | 15 | 12 | 10 |
| R4-09-8_R | 24.11.2009 | + | >4 | 2 | 1 | 1 | 0.094 | M220I-V101F | 18 | 23 | 16 | 35 | 13 | 18 | 15 | 9 | 10 |
| R4-09-9_S | 26.11.2009 | − | 0.5 | 0.5 | 0.25 | 1 | 0.016 | Not found | 18 | 23 | 16 | 35 | 13 | 18 | 15 | 9 | 10 |
| R4-09-10_R | 14.12.2009 | + | >4 | 2 | 4 | 0.5 | 0.016 | M220I-V101F | 18 | 23 | 16 | 35 | 13 | 18 | 15 | 9 | 10 |
| R5-07-1_R | 01.08.2007 | + | >4 | 1 | 0.5 | 0.5 | 0.016 | TR/L98H-S297T-F495I | 14 | 20 | 8 | 40 | 9 | 11 | 8 | 10 | 20 |
| R5-07-2_R | 16.11.2007 | + | >4 | 1 | 1 | 0.5 | 0.032 | TR/L98H-S297T-F495I | 14 | 20 | 8 | 40 | 9 | 11 | 8 | 10 | 20 |
| R5-07-3_R | 20.11.2007 | + | >4 | 1 | 0.5 | 0.5 | 0.016 | TR/L98H-S297T-F495I | 14 | 20 | 8 | 40 | 9 | 11 | 8 | 10 | 20 |
| R5-07-4_R | 06.12.2007 | + | >4 | 1 | 1 | 0.5 | 0.016 | TR/L98H-S297T-F495I | 14 | 20 | 8 | 40 | 9 | 11 | 8 | 10 | 20 |
| R5-09-1_Rc | 12.05.2009 | + | >4 | 4 | 2 | 0.5 | 0.032 | TR/L98H-S297T-F495I | 14 | 20 | 8 | 40 | 9 | 11 | 8 | 10 | 20 |
| R5-09-2_S | 28.07.2009 | − | 0.25 | 0.25 | 0.06 | 0.5 | 0.064 | Not found | 23 | 24 | 15 | 34 | 12 | 19 | 13 | 9 | 5 |
| R5-09-3_R | 31.08.2009 | + | >4 | 2 | 1 | 1 | 0.032 | TR/L98H-S297T-F495I | 14 | 20 | 8 | 40 | 9 | 11 | 8 | 10 | 20 |
| R5-09-4_S | 13.11.2009 | − | 0.25 | 0.25 | 0.06 | 0.5 | 0.064 | Not found | 13 | 20 | 9 | 34 | 9 | 10 | 10 | 10 | 19 |
| R5-09-5a_R | 24.11.2009 | + | >4 | 2 | 1 | 0.5 | 0.032 | TR/L98H-S297T-F495I | 14 | 20 | 8 | 40 | 9 | 11 | 8 | 10 | 20 |
| R5-09-5b_R | 24.11.2009 | + | >4 | 4 | 1 | 0.5 | 0.016 | TR/L98H | 23 | 10 | 9 | 10 | 10 | 6 | 8 | 10 | 20 |
| R6-07-1_S | 01.08.2007 | − | NAh | NA | NA | NA | NA | NA | 25 | 20 | 12 | 34 | 9 | 10 | 2 | 10 | 17 |
| R6-07-2_S | 27.08.2007 | − | NA | NA | NA | NA | NA | Not found | 23 | 23 | 15 | 36 | 11 | 20 | 13 | 9 | 5 |
| R6-07-3_S | 11.10.2007 | − | NA | NA | NA | NA | NA | NA | 23 | 23 | 15 | 36 | 11 | 20 | 13 | 9 | 5 |
| R6-09-1_Sc | 09.03.2009 | − | 0.5 | 1 | 0.25 | 0.5 | 0.125 | Not found | 23 | 23 | 15 | 37 | 11 | 20 | 13 | 9 | 5 |
| R6-09-2_S | 14.07.2009 | − | NA | NA | NA | NA | NA | NA | 23 | 23 | 15 | 36 | 11 | 20 | 13 | 9 | 5 |
| R6-09-3_R | 10.08.2009 | + | >4 | 2 | 0.5 | 2 | 0.064 | Not found | 18 | 19 | 8 | 37 | 15 | 20 | 9 | 9 | 5 |
| R6-09-4_S | 23.08.2009 | − | NA | NA | NA | NA | NA | NA | 18 | 19 | 8 | 37 | 15 | 20 | 9 | 9 | 5 |
| R6-09-5_S | 27.08.2009 | − | NA | NA | NA | NA | NA | NA | 23 | 23 | 15 | 36 | 11 | 20 | 13 | 9 | 5 |
| R6-09-6_S | 21.09.2009 | − | NA | NA | NA | NA | NA | NA | 23 | 23 | 15 | 36 | 11 | 20 | 13 | 9 | 5 |
| R6-09-7_S | 06.10.2009 | − | NA | NA | NA | NA | NA | NA | 14 | 19 | 8 | 36 | 18 | 7 | 8 | 27 | 5 |
| R6-09-8_R | 13.10.2009 | + | >4 | 2 | 0.25 | 2 | 0.064 | Not found | 24 | 23 | 15 | 36 | 11 | 20 | 13 | 9 | 5 |
| R6-09-9_S | 18.11.2009 | − | NA | NA | NA | NA | NA | NA | 23 | 23 | 15 | 36 | 11 | 20 | 13 | 9 | 5 |
| R6-09-10_S | 07.12.2009 | − | NA | NA | NA | NA | NA | NA | 23 | 23 | 15 | 36 | 11 | 20 | 13 | 9 | 5 |
| R6-09-11_S | 28.12.2009 | − | NA | NA | NA | NA | NA | NA | 23 | 23 | 15 | 36 | 11 | 20 | 13 | 9 | 5 |
| S1-07-1_S | 07.08.2007 | − | 0.125 | 0.25 | 0.06 | 0.5 | 0.125 | NA | 18 | 17 | 15 | 35 | 21 | 24 | 16 | 9 | 8 |
| S1-07-2_S | 25.10.2007 | − | 1 | 0.25 | 0.125 | 0.5 | ND | NA | 18 | 23 | 19 | 32 | 13 | 27 | 17 | 9 | 5 |
| S1-07-3_S | 26.10.2007 | − | 0.5 | 1 | 0.25 | 0.5 | 0.094 | Not found | 18 | 17 | 15 | 36 | 21 | 24 | 16 | 9 | 8 |
| S1-07-4_S | 10.12.2007 | − | 0.25 | 0.125 | 0.06 | 0.5 | ND | NA | 18 | 17 | 15 | 35 | 21 | 24 | 16 | 9 | 8 |
| S1-09-1_S | 03.08.2009 | − | 0.125 | 0.5 | 0.125 | 0.5 | 0.032 | NA | 18 | 17 | 15 | 35 | 21 | 24 | 16 | 9 | 8 |
| S1-09-2_S | 07.09.2009 | − | NA | NA | NA | NA | NA | NA | 18 | 23 | 19 | 33 | 13 | 27 | 17 | 9 | 5 |
| S1-09-3_S | 26.10.2009 | − | NA | NA | NA | NA | NA | NA | 18 | 23 | 19 | 32 | 13 | 27 | 17 | 9 | 5 |
| S1-09-4_S | 04.12.2009 | − | NA | NA | NA | NA | NA | NA | 18 | 17 | 15 | 35 | 21 | 24 | 16 | 9 | 8 |
| S1-09-5_Sb | 07.12.2009 | − | NA | NA | NA | NA | NA | NA | 18 | 17 | 15 | 35 | 21 | 24 | 16 | 9 | 8 |
| S2-09-1_S | 23.11.2009 | − | 0.25 | 1 | 0.06 | 1 | 0.064 | Not found | 25 | 20 | 8 | 10 | 10 | 21 | 9 | 10 | 5 |
| T11_R | 03.07.2009 | + | >4 | 4 | 2 | NA | NA | TR/L98H | 14 | 21 | 12 | 31 | 9 | 38 | 8 | 10 | 5 |
| T18_R | 03.07.2009 | + | >4 | 4 | 0.5 | NA | NA | TR/L98H | 10 | 20 | 8 | 32 | 9 | 7 | 8 | 9 | 19 |
| T22_R | 03.07.2009 | + | >4 | 4 | 0.25 | NA | NA | TR/L98H | 14 | 24 | 14 | 39 | 10 | 7 | 8 | 11 | 5 |
| R13_Rb | 14.07.2009 | + | >4 | 4 | 1 | NA | NA | TR/L98H | 20 | 10 | 12 | 84 | 10 | 13 | 8 | 9 | 11 |
Isolates obtained prior to sample periods.
Possible contamination by an isolate with another genotype. R4-09-3 also gave 12 repeats at marker 4B, S1-09-5 gave 23, 13, and 27 repeats at markers 2B, 3B, and 3C, respectively, and R13 gave 0 repeats at marker 3A.
Isolates obtained between sample periods.
Number of tandem repeats at the given microsatellite number.
ITC, itraconazole; +, growth of A. fumigatus; −, no growth; v, variable growth.
VRC, voriconazole; POS, posaconazole; CAS, caspofungin; AMB, amphotericin B; ND, not determined.
By using a stringent no-growth endpoint, the MIC was >16 mg/liter; however, growth was notably reduced in wells containing ≥2 mg/liter itraconazole. Thus, the MIC is indicated as 2/>16 mg/liter.
NA, not analyzed.
Mutations in the cyp51A gene of A. fumigatus.
Seven different amino acid substitutions in the Cyp51A target enzyme were detected (Tables 2 and 3). For three patients (R1 to R3), only nonsusceptible or resistant isolates were found, while for three patients (R4 to R6), susceptible as well as resistant isolates were recovered. A replacement of leucine with histidine at codon 98 (L98H) in combination with a 34-bp tandem repeat in the promoter region of the cyp51A gene (TR/L98H) was detected in 1/1 and 8/10 isolates from patients R2 and R5, respectively. Furthermore, in eight of these nine isolates, this was combined with two additional substitutions (S297T and F495I). Noticeably, in one sample from patient R5, two distinct azole-resistant isolates were recovered; one isolate had the TR/L98H-S297T-F495I substitutions, and the second had only the TR/L98H substitution. In two patients, substitutions were detected at the amino acid hot spot position 220 (M220K for patient R1 and M220I for patient R4), and for patient R4, an additional substitution (V101F) was also found. From patient R3, we found four itraconazole-nonsusceptible isolates with a Y431C substitution. No Cyp51A substitutions were found in three azole-resistant isolates from patients R4 and R6. One of the Cyp51A substitutions was associated with multiazole resistance (M220K), while the isolates with M220I and TR/L98H substitutions were consistently resistant to itraconazole and posaconazole but variably susceptible to voriconazole.
Exposure to anti-Aspergillus azoles.
All six patients with azole-resistant A. fumigatus isolates had been exposed to mold-active azoles for a total of 46 to 278 weeks prior to the detection of the resistant isolate and overall for more weeks than patients with no mold or susceptible Aspergillus isolates (Table 2). Patients R1 and R4 with the hot spot mutation M220 received azole therapy for prolonged periods, 260 and 278 weeks, respectively. The remaining patients with azole-resistant isolates were treated with azoles for shorter periods, 30 to 175 weeks, and not at all in the years 2001 to 2003. TDM was performed on a total of 20 serum samples from four of the six patients since 2007, showing drug concentrations below the recommended level from each of the patients.
Genotyping.
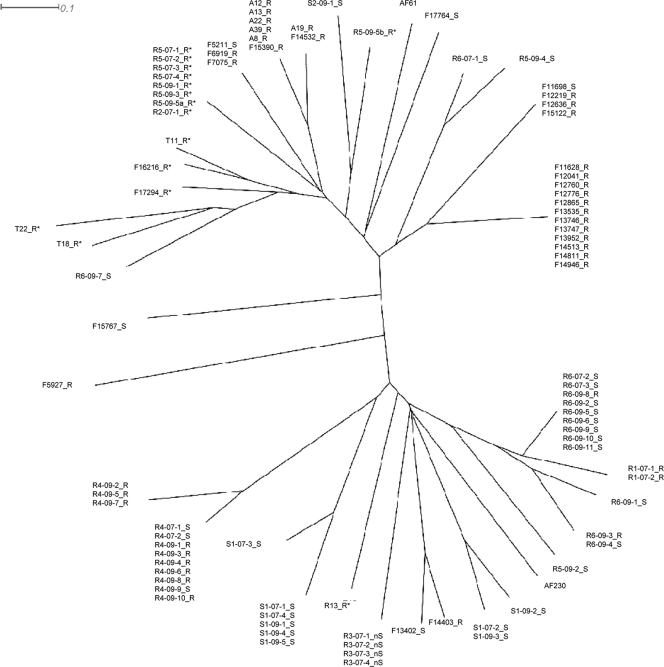
Microsatellite typing was used to analyze the relatedness of isolates obtained from patients R1 to R6, S1, and S2 and four environmental azole-resistant isolates harboring the TR/L98H substitution (T11, T18, T22, and R13; Table 3) (36). For three patients (R1, R3, and R4) identical or nearly identical genotypes were found within each individual, despite the fact that both azole-susceptible and azole-resistant isolates were recovered from patient R4. Each of three other patients (R5, R6, and S1) harbored multiple isolates which had two or more unrelated genotypes. The azole-resistant isolates from patient R5 were unrelated to the susceptible isolates, whereas the genotypes of resistant and susceptible isolates from patient R6 were identical or nearly identical. A unique azole-resistant isolate from patient R2 had a genotype identical to those of the seven isolates from patient R5. Figure 1 shows a phylogenetic tree based on four microsatellite markers of the genetic relatedness of the isolates in comparison with isolates from Manchester for reference. The resistant clinical isolates (both with and without Cyp51A substitutions) and environmental isolates are distributed among susceptible isolates, and both Danish and Manchester isolates are distributed among each other.
Fig. 1.
Phylogenetic tree of A. fumigatus isolates from Danish CF patients with azole-resistant or -nonsusceptible isolates (R1 to R3), with both azole-resistant and -susceptible isolates (R4 to R6), and with a susceptible isolate(s) (S1 and S2) in relation to azole-resistant environmental isolates from Denmark (T11, T18, T22, and R13) and to isolates from patients from a geographically separated environment in Manchester, United Kingdom (F, A, and AF). _S, _nS, and _R indicate azole-susceptible, -nonsusceptible, and -resistant isolates, respectively; * indicates TR/L98H isolates. The scale bar indicates the number of nucleotide substitutions per site.
DISCUSSION
This study has shown that molds could be recovered from more than 40% of our CF patients, with more than 20% being persistently colonized with Aspergillus spp. A. fumigatus was the predominant species and involved in the majority of cases of persistent colonization. We observed that among the patients with A. fumigatus, 4.5% harbored nonsusceptible or azole-resistant isolates, and all of these patients were previously exposed to azoles. This frequency is in the high end of the range from earlier reports (26). Furthermore, we showed that the isolates with the TR/L98H resistance mechanism which we recently detected in environmental samples in Denmark already existed in the CF patients (36). Finally, we observed that patients persistently colonized with Aspergillus spp. were older, provided more samples, and experienced more ABPA, and the proportion of these patients with chronic bacterial infections was greater than the proportion of mold-negative patients with these infections.
The detection frequency for molds varies considerably between centers. Most studies have focused on A. fumigatus and reported detection rates ranging from 5.9 to 57% (4, 7, 12, 39, 40, 45). We included both children and adults and showed an A. fumigatus detection rate of more than 33% (both sample periods), which falls in the middle of this wide range. We found that the detection rates for most mold species were stable over the two periods, but species such as A. niger, Penicillium spp., and Paecilomyces spp. were isolated from more patients in the second period. Seasonal variation was not the cause of this, as both sample periods were the last 6 months of the year.
We found that A. flavus and A. terreus were consistently the second and third most frequently detected in both sample periods, respectively. Other centers have reported that S. apiospermum and A. terreus are the second and third most frequently observed filamentous fungi (42). However, the routine media and incubation time used in our study are not optimal for species like S. apiospermum and E. dermatitidis, and thus, the prevalence rates of these are probably underestimated (9, 38).
A number of studies have investigated the clinical significance of molds in the airways of CF patients not fulfilling the criteria for ABPA, and most have been limited to A. fumigatus (29, 37). Amin et al. (2) recently reported more pulmonary exacerbations requiring hospitalization in persistently colonized CF patients, whereas de Vrankrijker et al. (22) did not find a negative impact on lung function. In our study, patients persistently colonized with Aspergillus had more pronounced obstructive lung disease, but the association was not statistically significant. Moreover, we found that persistently colonized patients were older, and the proportion of females in this group was higher, supporting similar findings reported by others (35). Traditionally, diabetes has been considered a predisposing condition for fungal infections, but surprisingly, a recent French study showed that diabetes was less common in patients with Aspergillus than in those without it (40). We confirm this observation among the persistently colonized patients, and thus, the linkage between Aspergillus and a lower incidence of diabetes should be further explored.
Azole resistance in A. fumigatus from CF patients has been only sporadically detected (27, 30, 48). In, to our knowledge, the most comprehensive published report, the authors studied 159 isolates from 11 patients defined as chronically colonized but found no azole-resistant isolates (3). We analyzed 413 A. fumigatus isolates from 133 patients and found nonsusceptible or azole-resistant isolates in the samples from six patients (4.5%), a rate that may underestimate the true rate of voriconazole- and posaconazole-resistant isolates, as we used for the majority of isolates an itraconazole screening agar (1, 11, 54). Seven different substitutions in Cyp51A were found. Six of the substitutions have been reported previously (27, 47), but to our knowledge, the V101F substitution is novel. This was found exclusively in combination with a confirmed resistance mechanism (M220), so it is unclear whether V101F is associated with susceptibility. Furthermore, we found the Y431C amino acid substitution in four isolates with no other target gene mutations. The isolates showed variable growth on the itraconazole agar and prominent but not absolute growth inhibition visible using a stereo microscope in the wells with 2 to 16 mg/liter itraconazole in the EUCAST plate. The Y431C substitution has previously been found in a multiazole-resistant isolate in a cohort from Manchester (27). Though the present findings suggest a potential role for this alteration in resistance development, other factors (such as the presence of efflux pumps or overexpression of a target gene) may be required to result in the multiazole resistance phenotype observed in Manchester.
An interesting aspect of this study is the variety of different cyp51A mutations found, which parallels what has been described in Manchester (United Kingdom) from a center which manages patients with long-term azole therapy (27). In contrast, in the Netherlands, more than 90% of azole-resistant isolates shared the same TR/L98H resistance mechanism (48), which has also been demonstrated in azole-resistant environmental isolates (53, 54). We detected the TR/L98H resistance mechanism in nine isolates from two patients. In addition, eight of these isolates also had two extra substitutions in the Cyp51A protein (S297T and F495I). Though any potential role for these substitutions in resistance has yet to be confirmed, they have also been found in clinical and environmental samples from the Netherlands, but only in combination with the TR/L98H alteration (47).
It has previously been suggested that patients can acquire azole-resistant isolates by two means: selection in the individual patient during treatment or de novo inhalation of resistant isolates present in the environment. Our data support both theories. First, there was evidence of evolution within the lung, by identification of susceptible and resistant isolates of the identical or closely related genotypes, suggesting selection of resistance in vivo. Second, two patients had the same isolates, both with TR/L98-S297-F495 alterations, suggesting acquisition of a single strain which was resistant prior to colonization. Third, none of the susceptible isolates shared a genotype with the TR/L98H isolates from the same patients, while patients with isolates with other resistance mechanisms simultaneously harbored susceptible isolates with identical genotypes. Finally, there was a trend toward patients with the TR/L98H phenotype having less azole exposure than the patients with M220 substitutions.
Previously, it was shown that CF patients may harbor several genotypes of A. fumigatus in their lungs both at the same time and in serial samples (3, 20). Our analysis of both azole-susceptible and -resistant isolates from eight patients showed that several genotypes could be detected in a single patient as well as in a single sample. Isolates with identical or nearly identical genotypes were detected in patients R1, R3, and R4 (both azole-susceptible and -resistant isolates). From patient R2, we had a single TR/L98H-S297T-F495I isolate with a genotype identical to that of all the isolates with the same resistance mechanism from patient R5. We were not able to identify the source of this coincidence and cannot exclude the possibility of mislabeling of a respiratory sample. However, transmission from patient R5 to R2 is also, at least in theory, a possibility, albeit unlikely. Alternatively, both patients could have independently acquired this resistant isolate from the environment.
In conclusion, the emergence of azole resistance in the A. fumigatus isolates of CF patients is of concern. First, patients may later develop Aspergillus bronchitis or severe complications, such as ABPA. Second, in patients undergoing lung transplantation, an azole-resistant invasive infection may develop. Due to the fact that azoles are the only available oral group of drugs for the treatment of these conditions, clinical management of azole-resistant infections is challenging. More longitudinal and prospective studies on the clinical significance of molds in the CF respiratory tract are needed, especially in order to define which patients will benefit from the treatment of fungal colonization outside the ABPA setting.
ACKNOWLEDGMENTS
We thank laboratory technicians at the Department of Clinical Microbiology, Rigshospitalet, and the Mycology Unit, Statens Serum Institut, for excellent assistance in sample handling. We also thank Mie Hørbo and Christine Rønne Hansen for assistance in data extraction.
K.L.M. is member of the ISHAM Working Group on Fungal Respiratory Infections in Cystic Fibrosis and has received travel grants from Pfizer, Schering-Plough (now MSD), and MSD. R.H.J. has received a travel grant from MSD. E.M. was supported by the Ministerio de Ciencia e Innovacion (MICINN; grant number SAF2008-04143) and has been paid for a talk on behalf of Gilead. S.J.H. has received support grants from Gilead, Pfizer, and the Fungal Research Trust and travel grants from Astellas and Schering-Plough and has been paid for talks on behalf of Pfizer and Astellas. M.C.A. has been paid for talks on behalf of Astellas, Merck, Pfizer, Schering-Plough, Spepharm, Gilead, and Swedish Orphan, acted as a consultant for Merck, Astellas, Spepharm, and Pfizer, received research grants from Astellas, Pfizer, Roche, Schering-Plough, and Merck and received travel grants from Astellas, Merck, Pfizer, Schering-Plough, and Swedish Orphan. H.K.J., M.S., T.P., and H.L. have no conflicts to report.
Footnotes
Published ahead of print on 20 April 2011.
REFERENCES
- 1. Alcazar-Fuoli L., Mellado E., Alastruey-Izquierdo A., Cuenca-Estrella M., Rodriguez-Tudela J. L. 2008. Aspergillus section Fumigati: antifungal susceptibility patterns and sequence-based identification. Antimicrob. Agents Chemother. 52:1244–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amin R., Dupuis A., Aaron S. D., Ratjen F. 2010. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest 137:171–176 [DOI] [PubMed] [Google Scholar]
- 3. Amorim A., Guedes-Vaz L., Araujo R. 2010. Susceptibility to five antifungals of Aspergillus fumigatus strains isolated from chronically colonised cystic fibrosis patients receiving azole therapy. Int. J. Antimicrob. Agents 35:396–399 [DOI] [PubMed] [Google Scholar]
- 4. Bakare N., Rickerts V., Bargon J., Just-Nubling G. 2003. Prevalence of Aspergillus fumigatus and other fungal species in the sputum of adult patients with cystic fibrosis. Mycoses 46:19–23 [DOI] [PubMed] [Google Scholar]
- 5. Balajee S. A., Gribskov J. L., Hanley E., Nickle D., Marr K. A. 2005. Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryot. Cell 4:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barton R. C., et al. 2010. Isolation of the fungus Geosmithia argillacea in the sputum of people with cystic fibrosis. J. Clin. Microbiol. 48:2615–2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bauernfeind A., et al. 1987. Qualitative and quantitative microbiological analysis of sputa of 102 patients with cystic fibrosis. Infection 15:270–277 [DOI] [PubMed] [Google Scholar]
- 8. Borman A. M., et al. 2010. Lack of standardization in the procedures for mycological examination of sputum samples from CF patients: a possible cause for variations in the prevalence of filamentous fungi. Med. Mycol. 48:S88–S97 [DOI] [PubMed] [Google Scholar]
- 9. Bouchara J. P., et al. 2009. Development of an oligonucleotide array for direct detection of fungi in sputum samples from patients with cystic fibrosis. J. Clin. Microbiol. 47:142–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brillowska-Dabrowska A., Saunte D. M., Arendrup M. C. 2007. Five-hour diagnosis of dermatophyte nail infections with specific detection of Trichophyton rubrum. J. Clin. Microbiol. 45:1200–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bueid A., et al. 2010. Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J. Antimicrob. Chemother. 65:2116–2118 [DOI] [PubMed] [Google Scholar]
- 12. Burns J. L., et al. 1998. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin. Infect. Dis. 27:158–163 [DOI] [PubMed] [Google Scholar]
- 13. Cimon B., et al. 2000. Clinical significance of Scedosporium apiospermum in patients with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 19:53–56 [DOI] [PubMed] [Google Scholar]
- 14. Cimon B., et al. 2005. Airway colonization by Acrophialophora fusispora in patients with cystic fibrosis. J. Clin. Microbiol. 43:1484–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cimon B., et al. 2001. Molecular epidemiology of airway colonisation by Aspergillus fumigatus in cystic fibrosis patients. J. Med. Microbiol. 50:367–374 [DOI] [PubMed] [Google Scholar]
- 16. Cornely O. A., et al. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356:348–359 [DOI] [PubMed] [Google Scholar]
- 17. de Almeida M. B., Bussamra M. H., Rodrigues J. C. 2006. Allergic bronchopulmonary aspergillosis in paediatric cystic fibrosis patients. Paediatr. Respir. Rev. 7:67–72 [DOI] [PubMed] [Google Scholar]
- 18. de Hoog G. S., Guarro J., Gene J., Figueras M. J. 2001. Atlas of clinical fungi. ASM Press, Washington, DC [Google Scholar]
- 19. Denning D. W., et al. 1997. Itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1364–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Valk H. A., et al. 2009. Molecular typing and colonization patterns of Aspergillus fumigatus in patients with cystic fibrosis. J. Cyst. Fibros. 8:110–114 [DOI] [PubMed] [Google Scholar]
- 21. de Valk H. A., et al. 2005. Use of a novel panel of nine short tandem repeats for exact and high-resolution fingerprinting of Aspergillus fumigatus isolates. J. Clin. Microbiol. 43:4112–4120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Vrankrijker A. M., et al. Aspergillus fumigatus colonisation in cystic fibrosis: implications for lung function? Clin. Microbiol. Infect. 2010 doi: 10.1111/j.1469-0691.2010.03429.x. [Epub ahead of print.] doi: 10.1111/j.1469-0691.2010.03429.x. [DOI] [PubMed] [Google Scholar]
- 23. Henry T., Iwen P. C., Hinrichs S. H. 2000. Identification of Aspergillus species using internal transcribed spacer regions 1 and 2. J. Clin. Microbiol. 38:1510–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herbrecht R., et al. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408–415 [DOI] [PubMed] [Google Scholar]
- 25. Horre R., et al. 2004. Isolation of fungi, especially Exophiala dermatitidis, in patients suffering from cystic fibrosis. A prospective study. Respiration 71:360–366 [DOI] [PubMed] [Google Scholar]
- 26. Howard S. J., Arendrup M. C. 2011. Acquired antifungal drug resistance in Aspergillus fumigatus: epidemiology and detection. Med. Mycol. 49:S90–S95 [DOI] [PubMed] [Google Scholar]
- 27. Howard S. J., et al. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 15:1068–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Howard S. J., Pasqualotto A. C., Denning D. W. 2010. Azole resistance in allergic bronchopulmonary aspergillosis and Aspergillus bronchitis. Clin. Microbiol. Infect. 16:683–688 [DOI] [PubMed] [Google Scholar]
- 29. Kraemer R., Delosea N., Ballinari P., Gallati S., Crameri R. 2006. Effect of allergic bronchopulmonary aspergillosis on lung function in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 174:1211–1220 [DOI] [PubMed] [Google Scholar]
- 30. Lagrou K., et al. 2008. Triazole resistance among clinical Aspergillus fumigatus isolates, abstr. P-33. 3rd Adv. Aspergillosis Meet., 16 to 19 January 2008, Miami, FL [Google Scholar]
- 31. Leon E. E., Craig T. J. 1999. Antifungals in the treatment of allergic bronchopulmonary aspergillosis. Ann. Allergy Asthma Immunol. 82:511–516 [DOI] [PubMed] [Google Scholar]
- 32. Lipuma J. J. 2010. The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 23:299–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Massam J., et al. 2011. Invasive aspergillosis in cystic fibrosis: a fatal case in an adolescent and review of the literature. Pediatr. Infect. Dis. J. 30:178–180 [DOI] [PubMed] [Google Scholar]
- 34. Mellado E., Diaz-Guerra T. M., Cuenca-Estrella M., Rodriguez-Tudela J. L. 2001. Identification of two different 14-alpha sterol demethylase-related genes (cyp51A and cyp51B) in Aspergillus fumigatus and other Aspergillus species. J. Clin. Microbiol. 39:2431–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Milla C. E., Wielinski C. L., Regelmann W. E. 1996. Clinical significance of the recovery of Aspergillus species from the respiratory secretions of cystic fibrosis patients. Pediatr. Pulmonol. 21:6–10 [DOI] [PubMed] [Google Scholar]
- 36. Mortensen K. L., et al. 2010. Environmental study of azole-resistant Aspergillus fumigatus and other aspergilli in Austria, Denmark, and Spain. Antimicrob. Agents Chemother. 54:4545–4549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mroueh S., Spock A. 1994. Allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. Chest 105:32–36 [DOI] [PubMed] [Google Scholar]
- 38. Nagano Y., et al. 2010. Comparison of techniques to examine the diversity of fungi in adult patients with cystic fibrosis. Med. Mycol. 48:166–176 [DOI] [PubMed] [Google Scholar]
- 39. Nelson L. A., Callerame M. L., Schwartz R. H. 1979. Aspergillosis and atopy in cystic fibrosis. Am. Rev. Respir. Dis. 120:863–873 [DOI] [PubMed] [Google Scholar]
- 40. Paugam A., et al. 2010. Characteristics and consequences of airway colonization by filamentous fungi in 201 adult patients with cystic fibrosis in France. Med. Mycol. 48:S32–S36 [DOI] [PubMed] [Google Scholar]
- 41. Pfaller M. A., et al. 2008. In vitro survey of triazole cross-resistance among more than 700 clinical isolates of Aspergillus species. J. Clin. Microbiol. 46:2568–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pihet M., et al. 2009. Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis—a review. Med. Mycol. 47:387–397 [DOI] [PubMed] [Google Scholar]
- 43. Rodriguez-Tudela J. L., et al. 2008. Epidemiological cutoffs and cross-resistance to azole drugs in Aspergillus fumigatus. Antimicrob. Agents Chemother. 52:2468–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rodriguez-Tudela J. L., et al. 2008. EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 14:982–984 [DOI] [PubMed] [Google Scholar]
- 45. Schønheyder H., Jensen T., Hoiby N., Andersen P., Koch C. 1985. Frequency of Aspergillus fumigatus isolates and antibodies to Aspergillus antigens in cystic fibrosis. Acta Pathol. Microbiol. Immunol. Scand. B. 93:105–112 [DOI] [PubMed] [Google Scholar]
- 46. Shoseyov D., Brownlee K. G., Conway S. P., Kerem E. 2006. Aspergillus bronchitis in cystic fibrosis. Chest 130:222–226 [DOI] [PubMed] [Google Scholar]
- 47. Snelders E., et al. 2009. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl. Environ. Microbiol. 75:4053–4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Snelders E., et al. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 5:e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stevens D. A., et al. 2003. Allergic bronchopulmonary aspergillosis in cystic fibrosis—state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin. Infect. Dis. 37(Suppl. 3):S225–S264 [DOI] [PubMed] [Google Scholar]
- 50. Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST) 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 14:398–405 [DOI] [PubMed] [Google Scholar]
- 51. Vanhee L. M., Symoens F., Bouchara J. P., Nelis H. J., Coenye T. 2008. High-resolution genotyping of Aspergillus fumigatus isolates recovered from chronically colonised patients with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 27:1005–1007 [DOI] [PubMed] [Google Scholar]
- 52. Verweij P. E., Howard S. J., Melchers W. J., Denning D. W. 2009. Azole-resistance in Aspergillus: proposed nomenclature and breakpoints. Drug Resist. Updat. 12:141–147 [DOI] [PubMed] [Google Scholar]
- 53. Verweij P. E., Snelders E., Kema G. H., Mellado E., Melchers W. J. 2009. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect. Dis. 9:789–795 [DOI] [PubMed] [Google Scholar]
- 54. Verweij P. E., et al. 2010. A new resistance machanism from environmental origin conferring voriconazole resistance has emerged in clinical Aspergillus fumigatus isolates in the Netherlands. 50th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-624 [Google Scholar]