Abstract
A case of Acremonium kiliense peritonitis is described. Diagnosis was established by repeated isolation of the fungus from peritoneal dialysate and by its identification on the basis of morphological characteristics and sequencing of internal transcribed spacer (ITS) regions of ribosomal DNA (rDNA). This report and available literature suggest that A. kiliense may have a greater clinical significance than hitherto recognized.
CASE REPORT
A 75-year-old Jordanian man with a long history of diabetes mellitus and hypertension developed end-stage kidney disease requiring continuous ambulatory peritoneal dialysis (CAPD). His course of CAPD was largely uneventful except for a single episode of bacterial peritonitis, for which he was hospitalized and treated with parenteral antibiotics, with an excellent response. In January 2010, he became febrile, with clinical evidence of peritonitis, and was admitted to the hospital. The peritoneal fluid was turbid, with a white blood cell (WBC) count of 2.1 × 109/liter. On initial microscopic examination, the presence of fungal elements in the peritoneal dialysate was missed. The peritoneal fluid sample was inoculated into aerobic Bactec blood culture bottles, which yielded a growth after 59 h of incubation. The Gram-stained smear from blood culture bottles showed branched hyphal elements. On Sabouraud dextrose agar (SDA; Oxoid Ltd., Basingstoke, England), the specimen yielded slimy colonies with a pinkish appearance (Fig. 1). Microscopic examination of the primary culture (isolate Kw441-2010) showed hyaline hyphae, with scanty sporulation. A provisional identification of Acremonium/Fusarium species was made, and the growth was subcultured on Sabouraud dextrose agar and oatmeal agar (OMA; oatmeal [30 g], agar [20 g], distilled water [1 liter]) for further identification and antifungal susceptibility testing. Subsequent cultures of the peritoneal fluid yielded the same fungus on three occasions. A serum sample was obtained for the detection of galactomannan (Platelia Aspergillus enzyme immunoassay [EIA] kit; Bio-Rad, Marnes-la-Coquette, France) and (1-3)-β-d-glucan (Fungitell; Associates of Cape Cod); the latter test was positive (253 pg/ml). An Etest performed on RPMI 1640 medium supplemented with 2% glucose revealed that the isolate was resistant to amphotericin B and caspofungin but susceptible to voriconazole and posaconazole, with MIC values of >32 μg/ml, >32 μg/ml, 0.064 μg/ml, and 0.75 μg/ml, respectively. The patient was started on voriconazole, with a loading dose of 400 mg, followed by a maintenance dose of 200 mg, given every 12 h via the oral route. Although the patient showed clinical improvement after 2 weeks of voriconazole therapy, the peritoneal dialysate remained turbid (WBC counts, 2.0 × 109/liter). Abdominal ultrasound examination did not reveal any evidence of intraperitoneal adhesions or organ invasion. Since the response to treatment was not adequate, the Tenckhoff catheter was removed and the patient was temporarily switched to hemodialysis. After 1 week of additional voriconazole therapy, the peritoneal dialysate became clear, and microscopic examination and culture were negative. The patient was discharged with advice to continue oral voriconazole (200 mg twice daily) for 1 month with regular follow-up in the CAPD unit. He remained symptom free for about 3 weeks but was readmitted with symptoms of severe septicemia due to Staphylococcus aureus and died of septic shock despite treatment.
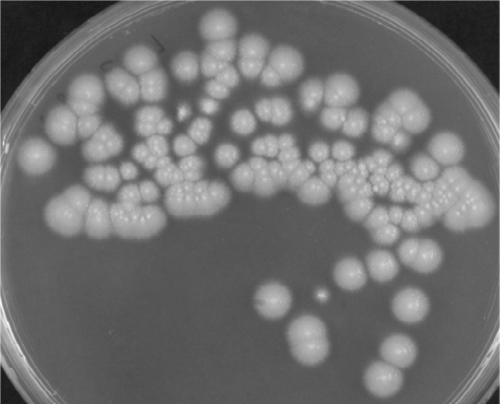
Fig. 1.
Colonies of A. kiliense on SDA grown from the sediment of peritoneal dialysate.
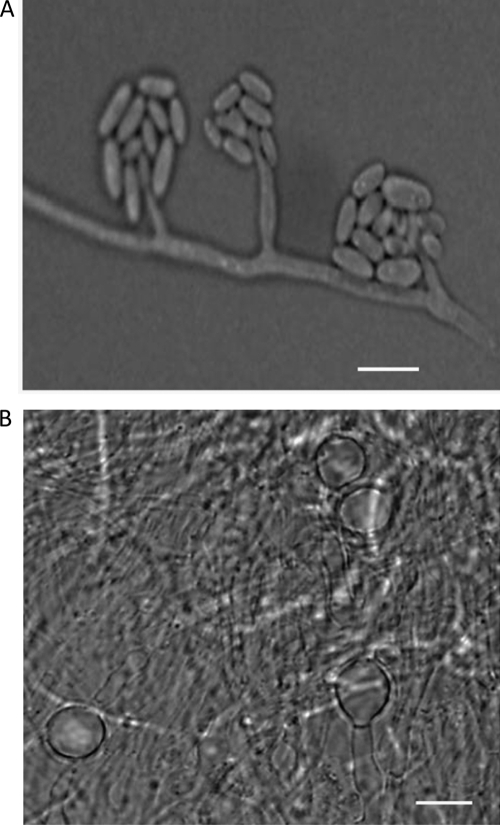
Colonies of the isolate on SDA at 30°C were initially white and glabrous but became pinkish on further incubation. On microscopic examination, the growth showed mostly fasciculate mycelium, which gave rise to erect, slender phialides (18 to 54 by 1.6 to 3 μm) (Fig. 2), forming hyaline, thin-walled, slightly curved, cylindrical-to-ellipsoidal conidia (3 to 5 by 1.2 to 2.4 μm) at the tip, occurring mostly in groups (Fig. 2). On OMA medium, after 10 days of incubation at 24°C, the isolate formed adelophialides (Fig. 3A) and unicellular terminal and intercalary thick-walled chlamydospores (Fig. 3B). These phenotypic characteristics identified the isolate as Acremonium kiliense (30).
Fig. 2.
Slide culture of A. kiliense on SDA showing aculeate phialides arising from a fasciculate aerial mycelium bearing cylindrical-to-ellipsoidal conidia in a lactophenol cotton blue mount. Bar = 5 μm.
Fig. 3.
Adelophialides (A) and chlamydospores (B) of A. kiliense formed on oatmeal agar medium after 10 days of incubation at 24°C. Bar = 5 μm.
The DNA from the isolate was prepared as described in detail previously (1). The entire internal transcribed spacer (ITS) region (containing ITS-1, 5.8S rRNA, and ITS-2) of the ribosomal DNA (rDNA) was amplified by PCR by using panfungal primers ITS1 and ITS4 as described previously (3). The amplicons were purified by using a PCR product purification kit (Qiagen, Hilden, Germany), and both strands were sequenced by using ITS1, ITS4, ITS1FS, ITS2, ITS3, or ITS4RS as sequencing primers as described in detail previously (13). For determining the sequence-specific identity of our isolate, pairwise comparisons were made by using ClustalW. The entire ITS region sequence (490 nucleotides) of our isolate exhibited 100% identity with the corresponding sequence from the type strain (MUCL 9724T) of A. kiliense. Based on previous observations that strains belonging to same species exhibit >99% nucleotide identity in the ITS-1 and ITS-2 regions of their rDNA, the molecular identity of our isolate was determined as A. kiliense (30, 31).
In vitro susceptibility was determined by the Etest (AB Biodisk, Solna, Sweden) on RPMI 1640 medium supplemented with 2% glucose and buffered with morpholinepropanesulfonic acid. The test was performed according to the manufacturer's instructions. Growth from a 7-day-old culture was uniformly suspended in 1 ml of normal saline. The clumps were allowed to settle, and the supernatant was used as an inoculum. The plates were inoculated by dipping a sterile swab into the growth suspension and streaking it uniformly across the surface of the agar. The plates were dried at ambient temperature for 15 min before Etest strips were applied. The plates were incubated at 35°C and read at 48 h. The Etest MICs were read at the point where dense colonial growth intersected the strip (24). The isolate was considered susceptible to voriconazole (0.064 μg/ml) and posaconazole (0.75 μg/ml) and resistant to amphotericin B (>32 μg/ml), fluconazole (>256 μg/ml), 5-fluorocytosine (>32 μg/ml), and caspofungin (>32 μg/ml).
Discussion.
The last 2 decades have witnessed a steady increase in the spectrum of hyaline fungi incriminated as opportunistic pathogens in immunocompromised patients (5, 14, 28). Many soil saprobes and plant pathogens with no obvious pathogenic potential have now emerged as etiologic agents under a variety of clinical conditions, thus posing new diagnostic and therapeutic challenges (38). Although Aspergillus and Fusarium are two major pathogenic filamentous genera, the role of Acremonium spp. is also being increasingly recognized in both localized and systemic infections (5, 7, 28). In immunocompetent individuals, keratitis, endophthalmitis, mycetoma, onychomycosis, or cutaneous infections are the most familiar forms of localized infections (5, 38). On the other hand, in severely immunocompromised patients, Acremonium species may cause disseminated infections involving multiple organs following fungemia (9, 10, 38). In one autopsy-proven study of invasive mold infections in cancer patients, 10% of the cases were caused by Acremonium spp. (14).
The present case of peritonitis highlights the clinical importance of A. kiliense in human infections. The diagnosis was established by repeated isolation of the fungus from peritoneal dialysate in culture and its identification by typical microscopic characteristics and sequencing of the ITS regions of rDNA. So far, nine cases of Acremonium peritonitis (including the present case) have been reported and are summarized in Table 1 (12, 18, 21, 22, 25, 34). Three of these cases were caused by A. kiliense and two by Acremonium strictum; in the remaining four cases, the Acremonium species were not identified (Table 1). Patients of all age groups (7 months to 75 years) were affected. Four patients were cured following antifungal therapy and/or removal of their Tenckhoff catheter and three died, and for two patients, the outcome was not known. Of the three patients who did not survive, two apparently died of bacterial sepsis; the other patient probably had parenchymal invasion, and the total dose of amphotericin B (105 mg) administered was probably not sufficient for a favorable outcome (18). For the remaining two patients (22), who were treated with itraconazole (400 mg/day), the cultures became negative for the period of follow-up; however, the final outcome was not reported. The source of Acremonium infection in patients on peritoneal dialysis is difficult to ascertain; however, considering the manipulations involved in the procedure, environmental contamination is highly possible. Generally, the management of Acremonium peritonitis includes catheter removal and systemic antifungal therapy. Although amphotericin B has been used with favorable outcomes in some early reports, voriconazole or posaconazole may be better alternatives, particularly in patients with renal insufficiency. Presently, the clinical experience with the latter drugs in the treatment of Acremonium infections is limited (23, 36).
Table 1.
Summary of cases of peritonitis caused by Acremonium spp.a
| Reference | Country | Year | Age/sex | Underlying condition(s) | Species | Treatment | Patient outcome |
|---|---|---|---|---|---|---|---|
| Landay et al. (18) | USA | 1982 | 68 yr/M | CAPD, ERD | Acremonium sp. and Klebsiella sp. | Amphotericin B | Died |
| Lopes et al. (21) | Brazil | 1995 | 8 yr/M | Chronic renal failure | A. kiliense | Ketoconazole, catheter removal | Cured |
| 49 yr/M | DM, hypertension, cirrhosis, chronic renal failure | A. kiliense | Amphotericin B, catheter removal | Cured | |||
| Nedret Koc et al. (25) | Turkey | 1998 | 22 yr/M | ERD | A. strictum | Amphotericin B i.p., i.v. | Cured |
| Manzano-Gayosso et al. (22) | Mexico | 2003 | 58 yr/M | Renal failure | Acremonium sp. | Itraconazole | Improved |
| 60 yr/F | Renal failure | Acremonium sp. | Itraconazole | Improved | |||
| Kendirli et al. (12) | Turkey | 2008 | 7 mo | Hyponatremia, Down syndrome, congenital heart diseases | Acremonium sp. | Fluconazole, amphotericin B, catheter removal | Died due to VAP, sepsis |
| Sener et al. (34) | Turkey | 2008 | 47 yr/F | CAPD, ERD | A. strictum | Fluconazole, catheter removal | Cured |
| This study | Kuwait | 2010 | 75 yr/M | ERD | A. kiliense | Voriconazole, catheter removal | Improved, died of Staphylococcus septicemia |
Abbreviations: M, male; F, female; CAPD, continuous ambulatory peritoneal dialysis; DM, diabetes mellitus; ERD, end-stage renal disease; i.p., intraperitoneally; i.v., intravenously; VAP, ventilator-associated pneumonia.
In a review of published cases of Acremonium infections other than peritonitis since 1981 for which etiologic species were identified to the species level, 18 of them were reportedly caused by A. kiliense (Table 2). Five of these cases were reported from the United States (4, 8), four each were reported from Brazil (15, 16, 20, 29) and India (6, 11, 37), two each were reported from Argentina (2, 26) and France (17, 19), and one was reported from Hungary (35). Only three of these cases occurred in immunocompromised patients, and the fungus was isolated from heart and brain tissue (15), blood (17), and lung tissue (29). Of these patients, one died (15) and two survived the infection (17, 29). In the remaining 15 patients, who were apparently immunocompetent, the infections were localized and were treated with antifungal agents with or without surgical debridement of the infected tissue (Table 2).
Table 2.
Summary of cases caused by Acremonium kiliense infectiona
| Reference | Country | Yr | Age/Sex | Underlying condition | Site(s) of disease or type of infection | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Lacaz et al. (15) | Brazil | 1981 | 47 yr/M | Prosthetic heart valve | Heart, brain | Surgery, AB (local) + FC | Died |
| Brabender et al. 4 | USA | 1985 | 35 yr/M | Head trauma | Head (osteomyelitis) | Surgical debridement (craniectomy), AB, KE | Cured |
| Lacroix et al. (17) | France | 1988 | 32 yr/F | Myeloma with large tumor burden | Septicemia, blood, catheter, feces? | AB, FL, NYS | Cured |
| Simon et al. (35) | Hungary | 1991 | 11 yr/M | Esophagus stenosis | Mycotic esophagitis | IT, NTM, NYS, surgery | Cured |
| Venugopal and Venugopal (37) | India | 1995 | 24 yr/M | Trauma | Right foot | KE | Cured |
| 36 yr/M | Not indicated | Back and perineum | KE, debridement | Cured | |||
| Lopez et al. (20) | Brazil | 1995 | 4 yr/M | None | Scalp | EC, systemic griseofulvin | Cured |
| Fridkin et al. (8)b | USA | 1996 | 73 yr/F | Cataract extraction | Endophthalmitis | FL topically, orally, AB intravitreously | Cured |
| 57 yr/M | Cataract extraction (postoperative) | Eyes | FL topically and orally, AB intravitreously | Cured | |||
| 88 yr/F | Cataract extraction | Eyes | FL topically and orally, AB intravitreously | Cured | |||
| 81 yr/F | Cataract extraction | Eyes | AB intravitreously, FL orally | Not cured | |||
| Le Guen et al. (19) | France | 1997 | 73 yr/M | Corneal ulcers | Keratomycosis | KE, AB by ocular instillation | Cured |
| Lacaz et al. (16) | Brazil | 1999 | ?/M | NA | Podalic mycetoma | IT | Cured |
| Gupta et al. (11) | India | 2003 | NA | Cataract surgery | Endophthalmitis | Surgery, AB | Cured |
| Pastorino et al. (29) | Brazil | 2005 | 25 days/M | CGD | Pneumonia | IT (6 wk) | Cured |
| Negroni et al. (26) | Argentina | 2006 | NA | NA | Mycetoma | KE, IT | Cured |
| Albrecht et al. (2) | Argentina | 2007 | 18 yr/M | Trauma | Onychomycosis | 8% ciclopirox nail solution, IT orally | Cured |
| Das et al. (6) | India | 2010 | 46 yr/M | Type 2 diabetes | Nodular swelling in soles, toenail | FL, TER | Cured |
Abbreviations: AB, amphotericin B; EC, econazole; FC, 5-fluorocytosine; FL, fluconazole; IT, itraconazole; KE, ketoconazole; NA, not available; NTM, natamycin; NYS, nystatin; s.c., subcutaneously; TER, terbinafine; CGD, chronic granulomatous disease.
The management of these cases was apparently described by Weissgold et al. (39).
Considering the difficulties in identifying clinical Acremonium spp., it is probable that the etiologic species described in some of the case reports may have been misidentified. In fact, A. strictum, which has been described as the etiologic agent in most of the case reports (5, 9), could be one such species; its etiologic role in human infections appears to be uncertain (10). Since there are close morphological similarities between A. strictum and A. kiliense, it is possible that these cases were actually caused by the latter species or by some other species of the genus. In this context, attention may be drawn to two recent case reports (9, 27) where sequenced isolates identified as A. strictum in fact belonged to the Acremonium sclerotigenum-Acremonium egyptiacum group (30). In an attempt to differentiate A. kiliense from A. strictum, Perdomo et al. (30) observed that isolates of A. kiliense form unicellular chlamydospores and adelophialides (reduced forms of phialides without a basal septum) in the vegetative or substrate hyphae (not in aerial hyphae) when grown on oatmeal agar at 24°C for about 2 weeks. These morphological structures were also observed in our isolate, which confirmed its identity as A. kiliense.
In order to unequivocally establish the molecular identity of our isolate, we retrieved the ITS region sequences from type or reference strains of well-known Acremonium spp. of clinical relevance which have recently become available in the DNA sequence database (30). These included A. kiliense (CBS 122.29/ATCC 34716/MUCL 9724T, GenBank accession no. AJ 621775), Acremonium zeae (CBS 800.69T, accession no. FN691451), A. strictum (CBS 346.70/ATCC34717T, accession no. FN691453), Acremonium implicatum (MUCL 4112, accession no. FN706553), Acremonium glaucum (CBS 796.69T, accession no. FN691451), A. sclerotigenum (CBS 270.86 and CBS 281.80, accession no. FN706551 and FN706549, respectively), A. egyptiacum (CBS 114785T, accession no. FN706550), and Acremonium persicinum (CBS 310.59T, accession no. FN706554). As mentioned above, the entire ITS region sequence (490 nucleotides) of our isolate exhibited 100% identity with the corresponding sequence from A. kiliense MUCL 9724T. However, it differed at 15 or more nucleotide positions from the ITS region sequences from type or reference strains of the other Acremonium spp. mentioned above. With the availability of ITS region sequences of type or reference strains in GenBank and with an improved understanding of the morphological characteristics of individual Acremonium spp., it should be possible to identify clinical isolates to the species level with greater accuracy.
Due to the difficulties previously described for the phenotypic identification of Acremonium spp., most clinical laboratories identify isolates only to the genus level. Thus, the relative etiologic role of an individual Acremonium sp. under different clinical conditions remains under-documented. Moreover, morphologically similar fungal isolates belonging to other genera may be misidentified as Acremonium spp. (30). To clarify taxonomic uncertainties about the identification of Acremonium spp. and to assess their relative etiologic significance in human infections, Perdomo et al. (30) reexamined 75 phenotypically identified clinical isolates by studying morphological characteristics and comparing their observations with 29 type/reference strains available in the Centraalbureau voor Schimmelcultures (CBS-KNAW, Netherlands) and the Mycotheque de l'Université Catholique de Louvain (MUC; Belgium). These investigators also sequenced ITS regions of rDNAs of these clinical isolates and compared them with type/reference strain sequences. This comprehensive study provided new insight into the etiologic spectrum of Acremonium spp. associated with human infections. Contrary to the generally held view, it was A. kiliense (30%), and not A. strictum, Acremonium recifei, or Acremonium potronii, which was the predominant species, followed by A. sclerotigenum-A. egyptiacum (22%), A. implicatum (14%), A. persicinum (14%), and Acremonium atrogriseum (8%) among the 50 clinical isolates that were identified by morphological and molecular methods. Of the 15 A. kiliense isolates, four came from eye, three from blood, three from respiratory tract, and one each from cerebrospinal fluid (CSF), vertebral disc, sinus, foot mass, and scalp specimens, but none came from nails. Another interesting finding of the study was the identification of several species that were not previously incriminated in human infections. It remains to be investigated if clinical isolates from other tropical and temperate geographic regions exhibit similar etiologic spectra or if they vary according to ecological or environmental conditions. It is also possible that individual Acremonium species prefer to cause a particular type of infection, as reflected by the apparent association between A. recifei and mycetoma (5).
Presently, there is a paucity of data on the antifungal susceptibilities of Acremonium spp. The reduced susceptibility of our strain to amphotericin B, 5-fluorocytosine, fluconazole, and caspofungin is consistent with findings of previously published studies (10, 30). Most of the information on antifungal susceptibility has originated from single isolates described in case reports where the etiologic agent has been identified (or misidentified) as A. strictum. In some other studies, only small numbers of Acremonium species isolates (without species-level identification) have been tested, yielding inconsistent results, particularly with reference to amphotericin B (7, 32, 33). Guarro et al. (10) presented in vitro antifungal susceptibility data for 5 reference strains of A. kiliense against amphotericin B, miconazole, itraconazole, fluconazole, ketoconazole, and 5-fluorocytosine. There were wide interstrain variations in MICs and minimum fungicidal concentrations. Most of these reference strains exhibited reduced susceptibilities to the drugs tested. More recently, Perdomo et al. (30) tested 50 well-characterized isolates of Acremonium spp., including 15 strains of A. kiliense, by the CLSI broth dilution method described in document M38-A2 (4a). All the strains of A. kiliense showed reduced susceptibility to amphotericin B (MIC90, 16 μg/ml), itraconazole (MIC90, >16 μg/ml), posaconazole (MIC90, >16 μg/ml), voriconazole (MIC90, 4 μg/ml), terbinafine (MIC90, 2 μg/ml), natamycin (MIC90, 8 μg/ml), micafungin (MIC90, >16 μg/ml), anidulafungin (MIC90, >16 μg/ml), and caspofungin (MIC90, 16 μg/ml). Similar susceptibility profiles were obtained with respect to other Acremonium spp. Although our isolate was also resistant to amphotericin B and caspofungin, it was susceptible to voriconazole and posaconazole. The differing susceptibilities of our isolate to the last two drugs may be due to either strain variation or the susceptibility method used. With the availability of authentic clinical isolates of Acremonium spp., attention should now be focused on developing experimental models to elucidate comparative levels of virulence of Acremonium spp. and to evaluate in vivo efficacies of currently available antifungal drugs in systemic and localized infections caused by them.
In conclusion, Acremonium spp. are capable of causing a variety of clinical conditions in immunocompromised as well as immunocompetent individuals. In cases where causative agents have been unambiguously identified, A. kiliense appears to be the predominant species associated with invasive infections. Further studies using a larger number of clinical isolates from different geographic regions are needed to elucidate the etiologic significance of individual Acremonium spp. Because of the difficulties in accurately identifying Acremonium spp. in routine clinical microbiology laboratories, a need for greater application of molecular methods is imperative.
Nucleotide sequence accession numbers.
The A. kiliense strain has been deposited in the CBS Fungal Biodiversity Center, Utrecht, Netherlands, under accession no. CBS129075. The ITS region sequence of our isolate has been deposited in the EMBL database under accession no. FR694874.
Acknowledgments
We thank Salwa Al-Hajri for technical support.
This study was supported in part by KURA grant MI01/09.
Footnotes
Published ahead of print on 30 March 2011.
REFERENCES
- 1. Ahmad S., Khan Z. U., Theyyathel A. M. 2007. Value of Aspergillus terreus-specific DNA, (1-3)-β-d-glucan and galactomannan detection in serum and bronchoalveolar lavage specimens of experimentally infected mice. Diagn. Microbiol. Infect. Dis. 59:165–171 [DOI] [PubMed] [Google Scholar]
- 2. Albrecht M. C., Fraenza L. B., Ramonda G. T. 2007. Rev. Argent. Dermatol. 88:40–44 [Google Scholar]
- 3. Al-Sweih N., Ahmad S., Khan Z. U., Khan S., Chandy R. 2005. Prevalence of Candida dubliniensis among germ tube-positive Candida isolates in a maternity hospital in Kuwait. Mycoses 48:347–351 [DOI] [PubMed] [Google Scholar]
- 4. Brabender W., Ketcherside J., Hodges G. R., Rengachary S., Barnes W. G. 1985. Acremonium kiliense osteomyelitis of the calvarium. Neurosurgery 16:554–556 [PubMed] [Google Scholar]
- 4a. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard—Second Edition. Document M38-A2 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Das S., Saha R., Dar S. A., Ramachandran V. G. 2010. Acremonium species: a review of the etiologic agents of emerging hyalohyphomycosis. Mycopathologia 170:361–375 [DOI] [PubMed] [Google Scholar]
- 6. Das S., Saha R., Bhattacharya S. N., Mishra K., Dar S. A. 2010. Hyalohyphomycosis: an unusual presentation and review of literature. Indian J. Med. Spec. 1:123–129 [Google Scholar]
- 7. Fincher R. M., et al. 1991. Infection due to the fungus Acremonium (Cephalosporium). Medicine (Baltimore, MD) 70:398–409 [DOI] [PubMed] [Google Scholar]
- 8. Fridkin S. K., et al. 1996. Acremonium kiliense endophthalmitis that occurred after cataract extraction in an ambulatory surgical center and was traced to an environmental reservoir. Clin. Infect. Dis. 22:222–227 [DOI] [PubMed] [Google Scholar]
- 9. Guarro J., Del Palacio A., Gené J., Cano J. J., González C. G. 2009. A case of colonization of a prosthetic mitral valve by Acremonium strictum. Rev. Iberoam. Micol. 26:146–148 [DOI] [PubMed] [Google Scholar]
- 10. Guarro J., Gams W., Pujol I., Gené J. 1997. Acremonium species: new emerging fungal opportunists—in vitro antifungal susceptibilities and review. Clin. Infect. Dis. 25:1222–1229 [DOI] [PubMed] [Google Scholar]
- 11. Gupta A., Gupta V., Gupta A., Dogra M. R., Pandav S. S. 2003. Risk factors for post-cataract surgery endophthalmitis with poor outcome. North Zone J. Ophthalmol. 13:1–4 [Google Scholar]
- 12. Kendirli T., et al. 2008. Acremonium spp. peritonitis in an infant. Mycoses 51:455–457 [DOI] [PubMed] [Google Scholar]
- 13. Khan Z. U., et al. 2010. Cryptococcus randhawai sp. nov., a novel anamorphic basidiomycetous yeast isolated from tree trunk hollow of Ficus religiosa (peepal tree) from New Delhi, India. Antonie Van Leeuwenhoek 97:253–259 [DOI] [PubMed] [Google Scholar]
- 14. Krcmery V., Jr., et al. 1996. Invasive mold infections in cancer patients: 5 years' experience with Aspergillus, Mucor, Fusarium and Acremonium infections. Support. Care Cancer 4:39–45 [DOI] [PubMed] [Google Scholar]
- 15. Lacaz C. S., Porto E., Carneiro J. J., Pazianni I. O., Pimenta W. P. 1981. Endocarditis in dura mater prosthesis caused by Acremonium kiliense. Rev. Inst. Med. Trop. Sao Paulo 23:274–279 (In Portuguese.) [PubMed] [Google Scholar]
- 16. Lacaz C. S., Pereira A. D., Castro L. G. M., Nunes R. S., Arriganda G. L. H. 1999. Podalic eumycetoma caused by Acremonium kiliense: report of one case. An. Bras. Dermatol. 74:591–595 [Google Scholar]
- 17. Lacroix C., et al. 1988. Septicemie a Acremonium kiliense avec dissemination secondaire chez une patiente d'un myelome a forte masse tumorale. Bull. Soc. Fr. Mycol. Med. 17:93–98 [Google Scholar]
- 18. Landay M. E., Greenwald J. H., Stemer A. A., Ashbach D. L. 1982. Cephalosporium (Acremonium) in dialysis-connected peritonitis. J. Indiana State Med. Assoc. 75:391. [PubMed] [Google Scholar]
- 19. Le Guen P., Blancard A., Brisou P., Yvetot J., Muzellec Y. 1997. Kératomycose à Acremonium kiliense. Med. Malad. Infect. 27:738–739 [Google Scholar]
- 20. Lopes J. O., Kolling L. C., Neumaier W. 1995. Kerionlike lesion of the scalp due to Acremonium kiliense in a noncompromised boy. Rev. Inst. Med. Trop. Sao Paulo 37:365–368 [DOI] [PubMed] [Google Scholar]
- 21. Lopes J. O., et al. 1995. Acremonium kiliense peritonitis complicating continuous ambulatory peritoneal dialysis: report of two cases. Mycopathologia 131:83–85 [DOI] [PubMed] [Google Scholar]
- 22. Manzano-Gayosso P., Hernández-Hernández F., Méndez-Tovar L. J., González-Monroy J., López-Martínez R. 2003. Fungal peritonitis in 15 patients on continuous ambulatory peritoneal dialysis (CAPD). Mycoses 46:425–429 [DOI] [PubMed] [Google Scholar]
- 23. Mattei D., et al. 2003. Successful treatment of Acremonium fungemia with voriconazole. Mycoses 46:511–514 [DOI] [PubMed] [Google Scholar]
- 24. Messer S. A., et al. 2007. Evaluation of disk diffusion and Etest compared to broth microdilution for antifungal susceptibility testing of posaconazole against clinical isolates of filamentous fungi. J. Clin. Microbiol. 45:1322–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nedret Koc A., Utas C., Oymak O., Schmen E. 1998. Peritonitis due to Acremonium strictum in a patient of continuous ambulatory peritoneal dialysis. Nephron 79:357–358 [DOI] [PubMed] [Google Scholar]
- 26. Negroni R., López Daneri G. G., Arechavala A., Bianchi M. H., Robles A. M. 2006. Clinical and microbiological study of mycetomas at the Muñiz hospital of Buenos Aires between 1989 and 2004. Rev. Argent. Microbiol. 38:13–18 [PubMed] [Google Scholar]
- 27. Novicki T. J., et al. 2003. Genetic diversity among clinical isolates of Acremonium strictum determined during an investigation of a fatal mycosis. J. Clin. Microbiol. 41:2623–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nucci M., Anaissie E. J. 2006. Emerging fungi. Infect. Dis. Clin. North Am. 20:563–577 [DOI] [PubMed] [Google Scholar]
- 29. Pastorino A. C., et al. 2005. Acremonium kiliense infection in a child with chronic granulomatous disease. Braz. J. Infect. Dis. 9:529–534 [DOI] [PubMed] [Google Scholar]
- 30. Perdomo H., et al. 2011. Spectrum of clinically relevant Acremonium species in the United States. J. Clin. Microbiol. 49:243–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rakeman J. L., et al. 2005. Multilocus DNA sequence comparisons rapidly identify pathogenic molds. J. Clin. Microbiol. 43:3324–3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rotowa N. A., Shadomy H. J., Shadomy S. 1990. In vitro activities of polyene and imidazole antifungal agents against unusual opportunistic fungal pathogens. Mycoses 33:203–211 [DOI] [PubMed] [Google Scholar]
- 33. Saldarreaga A., et al. 2004. Antifungal susceptibility of Acremonium species using E-test and Sensititre. Rev. Esp. Quimioter. 17:44–47 (In Spanish.) [PubMed] [Google Scholar]
- 34. Sener A. G., et al. 2008. A case of Acremonium strictum peritonitis. Med. Mycol. 46:495–497 [DOI] [PubMed] [Google Scholar]
- 35. Simon G., Rákóczy G., Galgóczy J., Verebély T., Bókay J. 1991. Acremonium kiliense in oesophagus stenosis. Mycoses 34:257–260 [DOI] [PubMed] [Google Scholar]
- 36. Tuon F. F., Pozzi C., Penteado-Filho S. R., Benvenutti R., Contieri F. L. 2010. Recurrent Acremonium infection in a kidney transplant patient treated with voriconazole: a case report. Rev. Soc. Bras. Med. Trop. 43:467–468 [DOI] [PubMed] [Google Scholar]
- 37. Venugopal P. V., Venugopal T. V. 1995. Pale grain eumycetomas in Madras. Australas. J. Dermatol. 36:149–151 [DOI] [PubMed] [Google Scholar]
- 38. Walsh T. J., Groll A. H. 2001. Overview: non-fumigatus species of Aspergillus: perspectives on emerging pathogens in immunocompromised hosts. Curr. Opin. Investig. Drugs 21:366–367 [PubMed] [Google Scholar]
- 39. Weissgold D. J., Maguire A. M., Brucker A. J. 1996. Management of postoperative Acremonium endophthalmitis. Ophthalmology 103:749–756 [DOI] [PubMed] [Google Scholar]