Abstract
Orbiviruses infect a wide range of hosts, including humans. The ability to detect them has been hampered by their diversity. Here we present a simple consensus reverse transcription (RT)-PCR method targeting the polymerase gene for orbivirus recognition and characterization. Phylogenetic assignment is achieved by automated Web-based sequence analysis of amplification products.
TEXT
In current taxonomy, the genus Orbivirus, family Reoviridae, comprises 21 species; 11 additional “tentative species” are recognized by the International Committee on Taxonomy of Viruses (ICTV) (8, 9). Species have high sequence similarity and potential for segmental reassortment. Interspecies diversity, however, has confounded the development of diagnostic PCR assays.
Orbivirus VP1 consensus PCR primers were designed using SCPrimer software (http://scprimer.cpmc.columbia.edu/SCPrimerApp.cgi) (5) using the 42 sequences available in that region in public databases (see Table S1 in the supplemental material). The program uses a greedy algorithm to identify the most conserved sequences and to create the minimum primer set needed for amplification of all sequences in the alignment. Four forward primers (OrbiVP1-F2494-1, TCTGAGATGTAYGTYGGAGATGATA; OrbiVP1-F2494-2, TCTGAGATGTAYGTYGGTGATGACA; OrbiVP1-F2494-3, TCGGAACARTAYGTVGGNGAYGATA; OrbiVP1-F2494-4, TCNGARCARTAYGTKGGNGAYGACA) and one reverse primer (OrbiVP1-R2682, CCYTGYTTNGCRTGNGTYTGYGTYTTYTC) were used. The assay was standardized using synthetic DNA standards containing Middle Point Orbivirus (MPOV) and Stretch Lagoon orbivirus (SLOV) VP1 gene sequences cloned into the pGEM-T Easy vector system (Promega, Fermantas, Lithuania). Total RNA was purified from 100 μl of sample. cDNA was generated using random hexamers. PCR parameters (MgCl2, annealing temperature, primer concentration, and enzymatic system) were optimized individually. A 2.5 mM concentration of MgCl2, temperature of 53.5°C, a 5 μM concentration of each primer, and Qiagen HotStarTaq DNA polymerase (Qiagen) were found to be optimal. Templates for dideoxy sequencing were obtained by excising the band of interest after size fractionation of products using 1% agarose gel electrophoresis. Where dual infections were detected, the band of interest was cloned before sequencing. The sensitivity of the assay was 50 to 500 RNA copies per assay.
Assay performance was validated with a large number of orbiviruses, including several previously uncharacterized viruses and clinical samples. First, 58 reference strains, including prototype Changuinola, Orungo, Eubenangee, Wongorr, Great Island, Corriparta, and Umatilla viruses, were used to test sensitivity and specificity (see Table S2 in the supplemental material). This set represents 13 of the 21 recognized orbivirus species. We also tested three viruses that were not classified by serology and are considered tentative members of the Orbivirus genus based on morphology (Matucare [MATV], Itupiranga [ITUV], and Tracambe [TRCV] viruses) (6, 13). In all cases, strong bands of the expected size were obtained (data not shown) and confirmed by sequence analysis.
To test sensitivity in clinical specimens, white-tailed deer (Odocoileus virginianus) were experimentally infected by subcutaneous inoculation with a bluetongue virus 8 (BTV8) strain originally isolated from an infected cow in the Netherlands. Whole blood was collected on days 3, 6, 7, 8, 9, 10, 12, 15, 17, 22, 24, and 28 postinfection. A total of 26 whole-blood samples were tested. In deer 1, BTV8 was detected from day 6 to 24 postinfection; in deer 2, BTV8 was detected from day 6 to 15 postinfection.
A third set of samples comprising two collections of arboviruses that had not been characterized other than to genus was tested: (i) 145 not identified by conventional serological testing (109 viruses from insects, 35 from cattle, and 1 from a deer); (ii) 5 unidentified orbiviruses obtained from the World Reference Center for Emerging Viruses and Arboviruses, of which four had been previously identified as ungrouped orbiviruses by electron microscopy (CSIRO51, CSIRO1740, CSIRO1747, and JML05088). Of these 150 viruses, 21 yielded products in VP1 consensus PCR (cPCR), including all four of the ungrouped orbiviruses. Two of the isolates contained sequences from two different orbiviruses; hence, a total of 23 orbiviruses were identified (see Table S2 in the supplemental material).
Phylogenetic analysis (MEGA package, version 4) (7) was performed using a set of 123 orbivirus sequences (132 nucleotides [nt] in length) comprising 58 newly sequenced strains (52 from well-characterized prototype strains), 42 sequences from GenBank (through July 2010), and 23 previously uncharacterized strains. Phylogenetic trees were generated from the nucleotide sequence alignment (Fig. 1). Pairwise comparisons of the orbivirus database were done by global alignment using the Needleman-Wunsch algorithm (10), implemented by the EMBOSS program (http://www.cii.columbia.edu) (12).
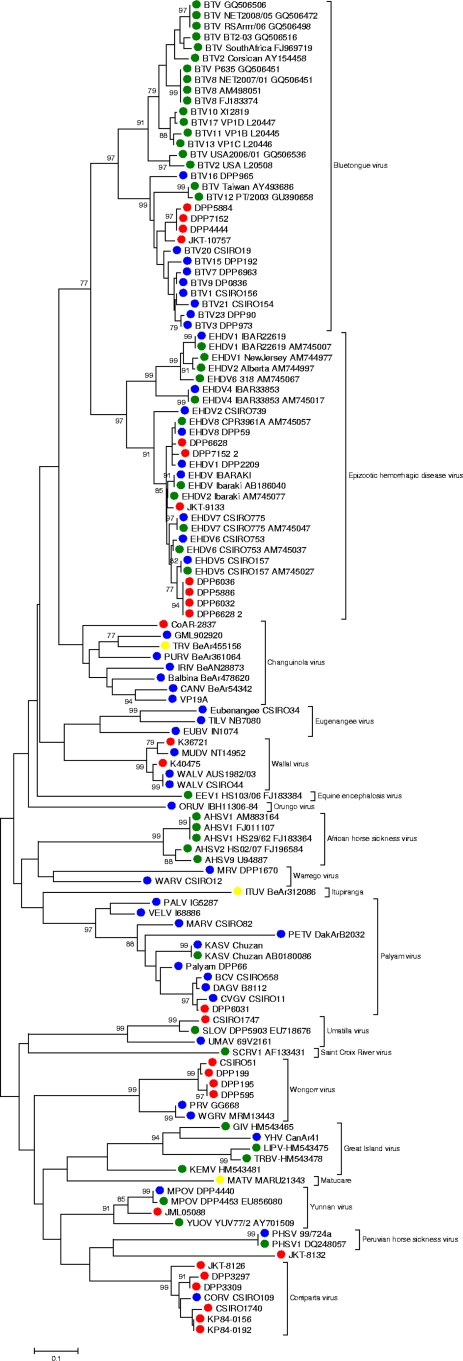
Fig. 1.
Phylogenetic tree of the genus Orbivirus generated from a partial region of the VP1 (nucleotide level). Phylogenetic analysis was performed using the Kimura model of nucleotide substitution, with trees reconstructed through the neighbor-joining (NJ) method using the MEGA package (version 4). The statistical significance of the tree topology was evaluated by bootstrap resampling of the sequences 1,000 times. Red dots, unknown viruses characterized in this study; blue dots, reference strains used to validate the assay; green dots, sequences recovered from GenBank; yellow dot, “tentative” virus species according to the ICTV.
Our analysis included representatives of 13 of 21 accepted orbivirus species. Clustering at both the nucleotide and amino acid level was consistent with existing orbivirus taxonomy. Only limited phylogenetic insights are feasible given the short length of the sequences available for analysis. Nonetheless, some interesting facts should be highlighted. (i) The BTV sequences formed two distinct clusters. One of these included all of the Australian isolates plus a Taiwanese isolate (GenBank accession no. AY493686), while the other cluster consisted of the remaining international isolates, supporting the concept that BTVs fall into distinct geographical topotypes (2). (ii) Elsey virus was 100% identical to Peruvian horse sickness virus (PHSV) at the nucleotide level (1). (iii) Although Mitchell river virus (MRV) is considered a strain of Warrego virus (WARV), it clustered poorly with that virus, suggesting that it may be more divergent than previously assumed. (4) TRCV, ITUV, and MATV viruses are classified as tentative members of the Orbivirus genus; while TRCV was clearly identified in our approach as a member of the Changuinola virus group, neither ITUV nor MATV appears to belong to any previously described orbivirus group. (v) The isolates CSIRO1747 and SLOV both clustered with Umatilla virus. Complete genome characterization of ITUV and MATV isolates is under way to assess their taxonomic classifications.
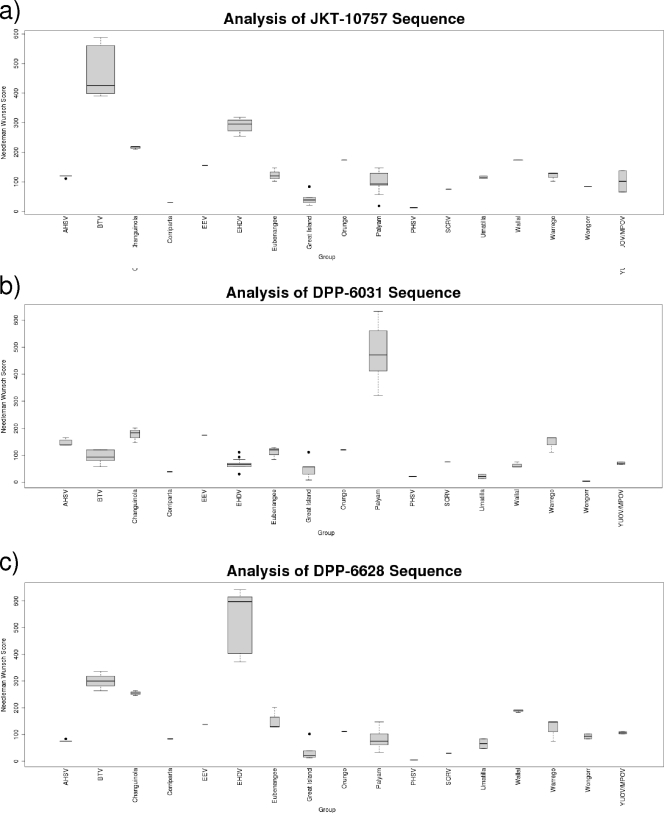
A pairwise sequence comparison was done within orbivirus sequences to assess the potential for establishing a simple program for classification of orbiviruses similar to programs previously devised for mumps virus (11) and dengue virus 1 genotyping (4) or filovirus and adenovirus identification (3, 14). This analysis allowed virus identification in all instances (Fig. 2).
Fig. 2.
Use of pairwise analysis for identification of three “unknown” orbiviruses. Samples are JKT-10757 from the BTV group, DPP-6031 from the Palyam virus group, and DPP6628 from the epizootic hemorrhagic disease virus (EHDV) group. A pairwise Needleman-Wunsch score for the test sequence against all other members of the database was determined. The validity of the method was confirmed by analysis of variance (ANOVA), with a comparison of the scores of sequences within species to interspecies scores. The average score (with the corresponding standard deviation, maximum, and minimum values) against the members of each genotype is plotted.
The method described here is capable of identifying an unknown isolate to the level of the genus Orbivirus. When the method is combined with sequencing, it can also be used to identify the species or to indicate whether the isolate is likely to be a new species requiring further characterization. Applications include virus discovery and the screening of existing collections of arboviruses that have resisted identification by other methods and clinical diagnosis from suspected cases, particularly during outbreak situations. Over 150 serologically distinct orbiviruses have been described to date. This list is likely to increase as arbovirus collections are expanded and revisited with recently developed research methods. Use of this technology is anticipated to provide insight into the appearance and distribution of known and novel orbiviruses, to rapidly identify them, and to enhance a rapid response to these and other emerging pathogens.
Supplementary Material
Acknowledgments
We thank Ross Lunt at CSIRO AAHL, Susan Walsh at the Berrimah Veterinary Laboratories, Northern Territory, and Annette Broom, Cheryl Johansen, and Michael Lindsay at the University of Western Australia for providing viral isolates and information about them. We also thank Richard Bowen at Colorado State University for providing samples from infected deer.
This work was supported by Google.org, National Institutes of Health award AI57158 (Northeast Biodefense Center; W.I.L.), and USAID Predict funding source code 07-301-7119-52258 (Center for Infection and Immunity). Robert B. Tesh and Amelia Travassos da Rosa were supported by NIH contract NO1-AI-30027. Charles H. Calisher was supported by Google.org.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 30 March 2011.
REFERENCES
- 1. Attoui H., et al. 2009. Peruvian horse sickness virus and Yunnan orbivirus, isolated from vertebrates and mosquitoes in Peru and Australia. Virology 394:298–310 [DOI] [PubMed] [Google Scholar]
- 2. Balasuriya U. B., et al. 2008. The NS3 proteins of global strains of bluetongue virus evolve into regional topotypes through negative (purifying) selection. Vet. Microbiol. 126:91–100 [DOI] [PubMed] [Google Scholar]
- 3. Casas I., et al. 2005. Molecular identification of adenoviruses in clinical samples by analyzing a partial hexon genomic region. J. Clin. Microbiol. 43:6176–6182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Domingo C., et al. 2006. Use of a short fragment of the C-terminal E gene for detection and characterization of two new lineages of dengue virus 1 in India. J. Clin. Microbiol. 44:1519–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jabado O. J., et al. 2006. Greene SCPrimer: a rapid comprehensive tool for designing degenerate primers from multiple sequence alignments. Nucleic Acids Res. 34:6605–6611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Justines G., Kuns M. L. 1970. Matucare virus, a new agent recovered from Ornithodoros (Alectorobius) boliviensis. Am. J. Trop. Med. Hyg. 19:697–702 [DOI] [PubMed] [Google Scholar]
- 7. Kumar S., Tamura K., Jakobsen I. B., Nei M. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244–1245 [DOI] [PubMed] [Google Scholar]
- 8. Libikova H., Heinz F., Ujhazyova D., Stunzner D. 1978. Orbiviruses of the Kemerovo complex and neurological diseases. Med. Microbiol. Immunol. 166:255–263 [DOI] [PubMed] [Google Scholar]
- 9. Mertens P., Maan S., Samuel A., Attoui H. 2005. Genus Orbivirus. In Fauquet C. M., Mayo M. A., Maniloff J., Desselberger U., Ball L. A. (ed.), Virus taxonomy: eighth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, London, United Kingdom [Google Scholar]
- 10. Needleman S. B., Wunsch C. D. 1970. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J. Mol. Biol. 48:443–453 [DOI] [PubMed] [Google Scholar]
- 11. Palacios G., et al. 2005. Molecular identification of mumps virus genotypes from clinical samples: standardized method of analysis. J. Clin. Microbiol. 43:1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rice P., Longden I., Bleasby A. 2000. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 16:276–277 [DOI] [PubMed] [Google Scholar]
- 13. Travassos da Rosa J. F., et al. 1998. Arboviruses isolated in the Evandro Chagas Institute, including some described for the first time in the Brazilian Amazon region, their known hosts, and their pathology for man. Instituto Evandro Chagas, Pará, Brazil [Google Scholar]
- 14. Zhai J., et al. 2007. Rapid molecular strategy for filovirus detection and characterization. J. Clin. Microbiol. 45:224–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.