Abstract
There is a need for accurate diagnosis of intestinal parasite infections in humans, but currently available copromicroscopic techniques have shortcomings, such as low sensitivity. We compared the diagnostic accuracy of a further modified version of the recently developed Flotac technique with that of the widely used formalin-ether concentration technique (FECT) for the detection of intestinal protozoa in human stool samples. Formaldehyde-preserved stool samples from 108 individuals in Côte d'Ivoire were subjected to the Flotac-400 dual technique, using two different flotation solutions (FS), and to the FECT. Stool samples were examined according to computer-generated random lists by an experienced laboratory technician blinded for the results of each method. Both methods detected the same eight intestinal protozoon species. While the Flotac-400 dual technique (results from both FS combined) found higher prevalences of Entamoeba coli (77.8% versus 71.3%, P < 0.001), Blastocystis hominis (20.4% versus 13.0%, P = 0.458), and Giardia intestinalis (8.3% versus 6.5%, P < 0.001), the FECT detected higher prevalences of Entamoeba histolytica/Entamoeba dispar (27.8% versus 20.4%, P = 0.019) and four species of nonpathogenic intestinal protozoa. The diagnostic agreement between the two methods differed considerably depending on the intestinal protozoon investigated (Cohen's kappa measures; range, 0.01 to 0.46). Our study confirmed that the Flotac-400 dual technique can be utilized for the diagnosis of intestinal protozoon infections in humans. Since Flotac is a sensitive technique for the detection of soil-transmitted helminths and Schistosoma mansoni, it might become a viable copromicroscopic technique for the concurrent diagnosis of helminths and intestinal protozoon infections.
INTRODUCTION
Numerous protozoon species parasitize the intestine of humans, and some of them, e.g., Giardia intestinalis (synonyms: G. duodenalis and G. lamblia) and Entamoeba histolytica, cause a remarkable but presently not fully quantified disease burden, particularly in the humid tropics (26, 38). Epidemiologic studies and public health interventions, however, more often focus on helminths (e.g., Schistosoma mansoni, Ascaris lumbricoides, Trichuris trichiura, and hookworm) than intestinal protozoon infections (27). This may be partially explained by the fact that the microscopic diagnosis of intestinal protozoa is more difficult, hence requiring experienced, highly skilled technicians for correct identification and differentiation of intestinal protozoon cysts, and is more time consuming than the microscopic detection of helminth eggs in human stool samples.
Various fecal egg count techniques, including stool concentration methods based on either flotation or sedimentation of parasitic elements, are employed for the diagnosis and epidemiologic surveillance of helminth and intestinal protozoon infections in humans and animals. By making use of centrifugal flotation or density gradient solutions, flotation techniques facilitate the floating of parasitic elements (e.g., larvae, ova, and cysts) to the surface of a fecal suspension, from where they can be readily transferred onto a microscope slide for direct examination. Sedimentation techniques, in contrast, enable the parasitic elements in a fecal sample to concentrate on the bottom (12).
The formalin-ether concentration technique (FECT) is a widely used sedimentation technique for the diagnosis of intestinal protozoa in preserved stool samples (2). The most commonly used fixatives for stool preservation are either formalin or sodium acetate-acetic acid-formalin (SAF) (3, 24, 40). Of note, the FECT has some drawbacks. For example, a recent comparison between European reference laboratories showed that, even when adhering to a standard protocol, the diagnostic agreement between the reference centers was only moderate for the two pathogenic intestinal protozoon species E. histolytica/Entamoeba dispar and G. intestinalis (37). This observation underlines the difficulty of an accurate diagnosis of intestinal protozoon infections and, hence, that microscopic identification is challenging even in reference laboratories.
In developing countries, polyparasitism is the norm (29, 30). Because of polyparasitism being so common and a recent trend toward integrated control of multiple parasitic diseases (19, 23), there is a need for sensitive diagnostic tools that are simple to apply and concurrently detect different intestinal parasite species in the same stool sample. However, the FECT lacks sensitivity for the diagnosis of helminths, and its diagnostic accuracy is inferior to that of the widely used Kato-Katz technique (18) for the diagnosis of S. mansoni and the common soil-transmitted helminths (A. lumbricoides, T. trichiura, and hookworm) (34).
The Flotac technique is a newly developed stool flotation method which is gaining interest in human and veterinary public health circles (7, 8). It is facilitated by the Flotac apparatus, developed by one of the authors (G. Cringoli, University of Naples, Naples, Italy). The method's principle is based on the centrifugation of stool samples in a flotation solution (FS) with a given specific gravity and the subsequent translation of the upper part of the fecal suspension containing the helminth eggs and intestinal protozoon cysts for microscopy. Studies carried out thus far indicate that the Flotac technique detects human helminth infections with a higher sensitivity than the Kato-Katz technique (20, 21, 39). Recently, a study from southern Italy—designed to assess the prevalence of parasitic infections among immigrants—showed that the Flotac technique is able to diagnose not only helminths but also intestinal protozoon infections (16); hence, further studies are warranted to compare the diagnostic accuracy of Flotac with those of currently more widely used techniques.
The objective of the study presented here was to validate the Flotac-400 dual technique for the diagnosis of human intestinal protozoon infections in stool samples obtained from an area in sub-Saharan Africa where they are highly endemic. Hence, we compared the diagnostic accuracy of the Flotac-400 dual technique and the FECT, which is broadly considered the diagnostic standard technique for the identification of intestinal protozoon species infections in humans. Stool samples were obtained from a random sample of 108 individuals from Côte d'Ivoire and were subjected to the Flotac-400 dual technique, using two different FSs, and the FECT, adhering to standard protocols.
MATERIALS AND METHODS
Study area and population.
The study was carried out between May and September 2009 and consisted of a field and a laboratory component. In May and June 2009, stool samples were collected in Léléblé, a rural setting of south-central Côte d'Ivoire, located approximately 160 km north of the country's economic capital Abidjan. Our study was embedded in a cross-sectional epidemiologic baseline survey assessing people's infection status with helminths and other parasites in the recently established health demographic surveillance system in Taabo (Taabo HDSS). In late 2008, a population database was created in the Taabo HDSS, which provides demographic, health, and socioeconomic data on approximately 38,000 inhabitants.
In order to obtain baseline epidemiologic data in Léléblé, the existing Taabo HDSS database was used to draw up a computer-generated random list of 351 individuals as a representation for the whole Taabo HDSS population in the village, thus including children and female and male adults of all age groups.
Ethical considerations.
The study was approved by the institutional research commissions of the Swiss Tropical and Public Health Institute (Basel, Switzerland) and the Centre Suisse de Recherches Scientifiques en Côte d'Ivoire (Abidjan, Côte d'Ivoire). Ethical approval was granted by the ethics committee of Basel (EKBB, reference number 316/08) and Côte d'Ivoire, and a research authorization for the first author of this paper (S. L. Becker) was provided by the Ministry of Higher Education and Scientific Research in Côte d'Ivoire (authorization number: 124/MESRS/DGRSIT/YKS/sac). Written informed consent was obtained from all participants aged 16 years and above at the beginning of the study. The parents or legal guardians of participating children (aged <16 years) gave their written informed consent prior to the collection of stool samples. At the end of the study, free treatment with albendazole (400 mg) was offered to all study participants, and individuals infected with Schistosoma haematobium and/or S. mansoni were treated with praziquantel (40 mg/kg of body weight) according to the national treatment guidelines of Côte d'Ivoire.
Field and laboratory procedures.
The purpose and procedures of the study were explained to all participants. After written informed consent was obtained, stool collection containers were distributed, and participants were invited to provide a lime-sized sample of their own morning stool the following day. Stool samples were collected between 08:00 and 10:00 a.m. and transferred to a laboratory in Taabo Cité, 28 km east of Léléblé.
All stool samples were examined with standard methods (Kato-Katz, Baermann, and Koga agar plate) (14, 18, 22) for soil-transmitted helminths, including Strongyloides stercoralis, and for S. mansoni infections. Sufficiently large stool samples were preserved in 5% formaldehyde and subjected to the Flotac-400 dual technique and the FECT to (i) diagnose helminth infections for prevalence assessment in the frame of the cross-sectional epidemiologic baseline survey for the Taabo HDSS and (ii) diagnose protozoon infections for the validation of the Flotac-400 dual technique for diagnosis of human intestinal protozoon infections. The latter objective is the primary focus of the work presented here. First, about half of the preserved stool samples were utilized for preliminary investigations to standardize the Flotac-400 preparation protocol for the diagnosis of intestinal protozoa. Second, the remaining stool samples were analyzed by Flotac-400 and FECT, adhering to the standard protocols described below. The diagnostic accuracy of both techniques was assessed.
Stool preparation procedures for intestinal protozoon diagnosis using Flotac-400 and FECT were as follows. A portion of 2 to 3 g of each sample was placed into a tube containing 10 ml of 5% formaldehyde. The stool material was stirred with a wooden spatula, and the tube was vigorously shaken in order to achieve a homogenized suspension of fecal material. Tubes were labeled with unique identifiers, and the set of formaldehyde-preserved stool samples was forwarded to the Regional Center for Monitoring Parasites (CREMOPAR) in Eboli, Italy.
After a preservation time of 10 to 12 weeks at room temperature, the stool samples were prepared and examined under a light microscope (Olympus CX21LED; Volketswil, Switzerland) by an experienced laboratory technician, adhering to standard protocols for FECT and the Flotac-400 dual technique. Each sample was resuspended by shaking and strained through a fine-mesh (250-μm) wire sieve. Subsequently, the strained suspension was put into a tube and centrifuged in a Hettich Universal 320 centrifuge (Tuttlingen, Germany) for 1 min at 500 × g. Following decantation of the supernatant, the fecal material was weighed and afterwards resuspended in 5% formaldehyde. Next, every mixture was split into three parts; the first was assigned to FECT and the second and third to the Flotac-400 dual technique using FS4 (315 g NaNO3 plus 685 ml H2O; specific gravity, 1.20 [sodium nitrate, catalog no. A3911; AppliChem]) and FS7 (685 g ZnSO4 plus 685 ml H2O; specific gravity, 1.35 [zinc sulfate, catalog no. A1000; AppliChem]). Of note, FS4 and FS7 were selected from a set of 14 currently available FSs with specific gravities ranging between 1.20 and 1.45 (8, 9). FS4 and FS7 were chosen because they produced the most accurate results for the diagnosis of soil-transmitted helminth and S. mansoni infections in previous studies (8, 20, 21, 39).
Standard protocol for FECT.
Each tube containing a homogenized stool sample preserved in 5% formaldehyde was centrifuged for 1 min at 500 × g. The supernatant was discarded, and 7 ml of 0.85% sodium chloride and 3 ml of diethyl ether were added to the remaining pellet, consisting of 0.5 to 1 ml. The tube was sealed and rigorously shaken in order to bring the diethyl ether in contact with all parts of the remaining fecal material. Following another centrifugation step of 5 min at 500 × g, four different layers had formed, as follows: (i) a sediment at the bottom, (ii) saline, (iii) fecal debris, and (iv) diethyl ether on the top. The upper three layers were decanted so that only the sediment remained in the tube. This layer was resuspended in a drop of 0.85% sodium chloride and subsequently placed on a slide, which was examined under a microscope for intestinal protozoa at high magnification (10× ocular lens, 40× objective) using oil immersion microscopy.
Standard protocol for Flotac-400 dual technique.
In the laboratory, FS4 was prepared by dissolving 315 g of NaNO3 in 500 ml tap water, followed by further addition of tap water until a final volume of 1 liter was reached. FS7 was prepared by dissolving 685 g of ZnSO4 · 7H2O in 685 ml tap water. The specific gravities, 1.20 and 1.35, respectively, were checked with a hydrometer. Subsequently, 10 ml of the fecal suspension was transferred into two tubes each, and the tubes were centrifuged for 3 min at 170 × g. The supernatant was discarded, and the pellet of one of the tubes was resuspended with 7 ml of 0.85% sodium chloride and 3 ml of diethyl ether and again centrifuged for 3 min at 170 × g. This ether washing step was performed to retain fecal debris. Subsequently, the supernatant was discarded and each tube was filled with 5 ml of 0.85% sodium chloride and centrifuged for 3 min at 170 × g. Following decantation of the supernatant, each tube was filled to the 6-ml mark using FS7.
The other tube, containing the second part of the fecal sample, was filled to the 6-ml mark with FS4 without a prior ether washing step. Five milliliters of each stool-FS suspension was placed into one of the two 5-ml chambers of the Flotac-400 apparatus, which was subsequently centrifuged for 5 min at 120 × g. Following centrifugation, a simultaneous 45° rotation of the translation disc and the reading disc of the apparatus cut the apical portion of the suspension in both chambers transversally. Finally, the reading disk was examined under a microscope at high magnification (10× ocular lens, 40× objective) using oil immersion microscopy to identify intestinal protozoa. For both Flotac-400 observation grids, intestinal protozoa were recorded separately for each species and each FS.
Blinding of microscopic examinations.
To guarantee the independence of each method's results, the microscopic examination of all stool samples was carried out according to two independent, computer-generated randomization lists, one for each diagnostic method. All samples were examined by one laboratory technician having long-standing experience with the diagnosis of intestinal protozoa and being familiar with the procedures of both techniques. For quality control, approximately 10% of the samples analyzed during the standardization process for the Flotac-400 preparation protocol were reexamined by experienced laboratory technicians from the Swiss Tropical and Public Health Institute (Basel, Switzerland) and the University of Naples (Naples, Italy).
Whenever the determination of an intestinal protozoon species could not be ascertained unambiguously, the observation was classified as negative, i.e., absence of an infection.
Statistical analysis.
All data were double entered and cross-checked in Excel, version 10.0 (2002 edition; Microsoft Corporation). For statistical analysis, STATA (version 10.0; StataCorp, College Station, TX) was utilized. Every sample found to be positive for a specific intestinal protozoon species by one of the two diagnostic techniques employed was considered true positive, leading to the prevalence results of each method. The combined results of the FECT and the Flotac-400 dual technique served as the diagnostic gold standard (with an assumed 100% specificity) and were used as an estimate of the “true” prevalence. The sensitivity and negative predictive value (NPV) were calculated for each method in relation to this diagnostic gold standard, including 95% confidence intervals (CIs) to quantify statistical uncertainty. We used Cohen's kappa measure (κ) to assess and interpret the agreement between the two diagnostic techniques for the detection of individual intestinal protozoon species (6, 36). Kappa measures were interpreted as follows: κ < 0, no agreement; κ = 0 to 0.20, poor agreement; κ = 0.21 to 0.40, fair agreement; κ = 0.41 to 0.60, moderate agreement; κ = 0.61 to 0.80, substantial agreement; and κ = 0.81 to 1.0, nearly perfect agreement. We checked for marginal distributions of 2-by-2 contingency tables and employed a test of marginal homogeneity (1). Since we did not find any significant differences (P > 0.05), we used κ values rather than raked κ, which was employed in a previous study comparing Flotac with the FECT and the Kato-Katz technique for the diagnosis of S. mansoni and soil-transmitted helminths (15).
RESULTS
Study cohort.
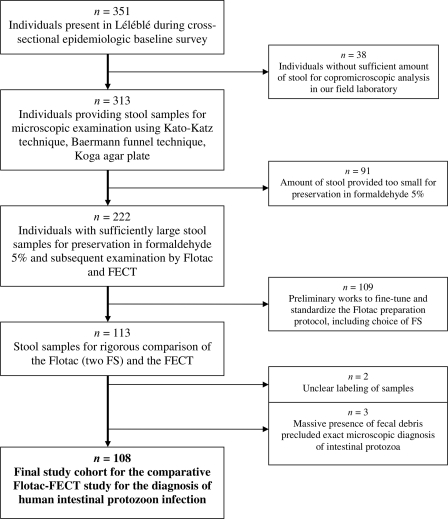
From the 351 study participants selected for the epidemiologic baseline data collection pertaining to parasitic infections in the Taabo HDSS, 222 individuals provided sufficiently large stool specimens for the preservation of 2 to 3 g of stool in 5% formaldehyde (Fig. 1). After the standardization process for the Flotac-400 dual technique, 113 remaining stool samples were subjected to both the Flotac-400 dual technique and the FECT, with 108 specimens being suitable for comparison of the methods. There were slightly more female than male participants (55 versus 53). The median age of our study cohort was 13 years (mean age, 18.7 years; standard deviation, 16.3 years; range, 1 to 69 years).
Fig. 1.
Compliance and final cohort for a study comparing the diagnostic accuracy of the Flotac-400 dual technique and the FECT for the detection of intestinal protozoa in stool samples obtained from participants in Léléblé, south-central Côte d'Ivoire. The original group was a computer-generated random list of 351 individuals that was drawn up as a representation for the whole population in the village.
Prevalence of intestinal protozoon infections.
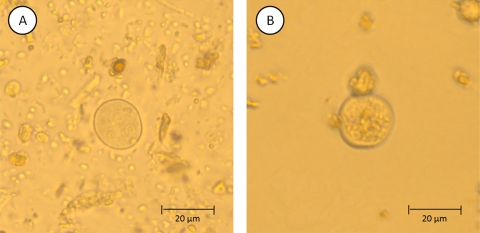
Table 1 shows the prevalence of all eight species of intestinal protozoa identified in the current study according to the FECT, the Flotac-400 dual technique (shown are combined results using both FS4 and FS7, as well as separate results obtained from FS4 or FS7 only), or the combination of both methods, including kappa measures of agreement between the two methods. According to our diagnostic gold standard, Entamoeba coli was the most frequent intestinal protozoon, with an overall prevalence of 87.0% (95% CI, 79.2 to 92.7%). The levels of prevalence of E. histolytica/E. dispar and Blastocystis hominis were 38.9% (95% CI, 29.7 to 48.8%) and 30.6% (95% CI, 22.1 to 40.2%). G. intestinalis was found in 11.1% (95% CI, 5.9 to 18.6%) of the 108 stool samples subjected to both methods. During the microscopic examination of the Flotac reading disk, we regularly observed deformed cysts of E. coli (Fig. 2). However, species identification was still possible due to the uniformity of this phenotypic transformation in all E. coli-positive stool samples. Helminth eggs were found by both methods (data not shown).
Table 1.
Prevalence of intestinal protozoon infections determined by the formalin-ether concentration technique (FECT), the Flotac-400 dual technique (results obtained from FS4 and FS7 combined and results from each FS singly), and a combination of both techniques (considered the diagnostic gold standard)
| Intestinal protozoon | Combined results |
FECT results |
Flotac results |
Kappa valuea | P valueb | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FS4 and FS7 combined |
FS4 only |
FS7 only |
|||||||||||||||
| No. | % | 95% CI | No. | % | 95% CI | No. | % | 95% CI | No. | % | 95% CI | No. | % | 95% CI | |||
| Entamoeba coli | 94 | 87.0 | 79.2–92.7 | 77 | 71.3 | 61.8–79.6 | 84 | 77.8 | 68.8–85.2 | 74 | 69.0 | 58.9–77.1 | 66 | 61.1 | 51.3–70.3 | 0.35 | <0.001 |
| Entamoeba histolytica/E. dispar | 42 | 38.9 | 29.7–48.8 | 30 | 27.8 | 19.6–37.2 | 22 | 20.4 | 13.2–29.2 | 12 | 11.0 | 5.9–18.6 | 17 | 15.7 | 9.5–24.0 | 0.20 | 0.019 |
| Blastocystis hominis | 33 | 30.6 | 22.1–40.2 | 14 | 13.0 | 7.3–20.8 | 22 | 20.4 | 13.2–29.2 | 20 | 19.0 | 11.7–27.1 | 7 | 6.5 | 2.7–12.9 | 0.01 | 0.458 |
| Entamoeba hartmanni | 14 | 13.0 | 7.3–20.1 | 12 | 11.1 | 5.9–18.6 | 3 | 2.8 | 0.6–7.9 | 3 | 2.8 | 0.6–7.9 | 1 | 0.9 | 0.02–5.1 | 0.09 | 0.107 |
| Giardia intestinalis | 12 | 11.1 | 5.9–18.6 | 7 | 6.5 | 2.6–12.9 | 9 | 8.3 | 3.9–15.2 | 5 | 4.6 | 1.5–10.5 | 7 | 6.5 | 2.6–12.9 | 0.46 | <0.001 |
| Endolimax nana | 12 | 11.1 | 5.9–18.6 | 12 | 11.1 | 5.9–18.6 | 2 | 1.9 | 0.2–6.5 | 2 | 1.9 | 0.2–6.5 | 0 | 0 | 0.26 | <0.001 | |
| Chilomastix mesnili | 8 | 7.4 | 3.3–14.1 | 8 | 7.4 | 3.3–14.1 | 1 | 0.9 | 0.1–5.1 | 1 | 0.9 | 0.02–5.1 | 0 | 0 | 0.21 | <0.001 | |
| Iodamoeba buetschlii | 4 | 3.7 | 1.0–9.2 | 3 | 2.7 | 0.6–7.9 | 2 | 1.9 | 0.2–6.5 | 0 | 0 | 2 | 1.9 | 0.2–6.5 | 0.39 | <0.001 | |
The Cohen kappa statistic indicates the agreement between the two diagnostic methods (n = 108).
P values were obtained by comparison of FECT and Flotac (results from FS4 and FS7 combined).
Fig. 2.
The intestinal protozoon E. coli as seen under a light microscope when examining human stool samples using FECT (A) and the Flotac-400 dual technique (B).
Comparison of diagnostic methods.
Comparing the FECT and the Flotac in terms of absolute numbers of identified positive cases (shown as two-way contingency tables in Table 2), the Flotac-400 dual technique—comprising the combined results obtained by using FS4 and FS7—detected a higher number of individuals infected with G. intestinalis (Flotac, n = 9; FECT, n = 7), while E. histolytica/E. dispar was diagnosed more often by the FECT (n = 30) than the Flotac (n = 22). The agreement between both methods was moderate for G. intestinalis (κ = 0.46) but only poor for E. histolytica/E. dispar (κ = 0.20). The Flotac-400 detected more infections with B. hominis (n = 22) than FECT (n = 14), with poor agreement between the two methods (κ = 0.01). Importantly, even when comparing the results of each FS chamber separately to the results of the FECT (hence comparing equal amounts of stool), FS7 revealed as many G. intestinalis cases as FECT (Flotac FS7, n = 7; FECT, n = 7) (Table 1) and FS4 diagnosed more B. hominis infections than FECT (Flotac FS4, n = 20; FECT, n = 14). Of note, for most of the nonpathogenic intestinal protozoon species, namely, Entamoeba hartmanni, Endolimax nana, Chilomastix mesnili, and Iodamoeba buetschlii, the FECT consistently revealed higher prevalences than the Flotac-400 dual technique, while the opposite was observed for the commensal E. coli. The agreement between the two techniques was fair for most of these commensal protozoa.
Table 2.
Two-way contingency table showing the number of identified positives and the agreement between the formalin-ether concentration technique (FECT) and the Flotac-400 dual technique for the diagnosis of G. intestinalis, E. histolytica/E. dispar, and B. hominis in stool specimens from south-central Côte d'Ivoirea
| Species | Result in Flotac | No. of isolates with result in FECT |
Total | |
|---|---|---|---|---|
| Positive | Negative | |||
| Giardia intestinalis | Positive | 4 | 5 | 9 |
| Negative | 3 | 96 | 99 | |
| Total | 7 | 101 | 108 | |
| Entamoeba histolytica/E. dispar | Positive | 10 | 12 | 22 |
| Negative | 20 | 66 | 86 | |
| Total | 30 | 88 | 108 | |
| Blastocystis hominis | Positive | 3 | 19 | 22 |
| Negative | 11 | 75 | 86 | |
| Total | 14 | 94 | 108 | |
A total of 108 stool samples were examined. The combined results from FS4 and FS7 for the Flotac-400 dual technique are included.
Table 3 reports the sensitivity and NPV for both techniques for the different species of intestinal protozoa detected under a microscope in relation to our diagnostic gold standard. With regard to pathogenic protozoa, the FECT was more sensitive for the diagnosis of E. histolytica/E. dispar (sensitivity, 71.4%; 95% CI, 55.4 to 84.3%) than the Flotac-400 dual technique (sensitivity, 52.4%; 95% CI, 36.4 to 68.0%), whereas the opposite was found for G. intestinalis (Flotac sensitivity, 75.0%, and 95% CI, 72.8 to 94.5%; FECT sensitivity, 58.3%, and 95% CI, 27.7 to 84.8%).
Table 3.
Species-specific sensitivity and NPV of the formalin-ether concentration technique (FECT) and the Flotac-400 dual technique for the diagnosis of intestinal protozoon infections in stool specimens from south-central Côte d'Ivoirea
| Species and test | Sensitivity (95% CI) | NPV (95% CI) |
|---|---|---|
| Entamoeba coli | ||
| FECT | 81.9% (72.6–89.1%) | 45.2% (27.3–64.0%) |
| Flotac | 88.3% (80.0–94.1%) | 54.2% (32.9–74.5%) |
| Entamoeba histolytica/E. dispar | ||
| FECT | 71.4% (55.4–84.3%) | 84.6% (74.7–91.8%) |
| Flotac | 52.4% (36.4–68.0%) | 76.7% (66.4–85.2%) |
| Blastocystis hominis | ||
| FECT | 42.4% (25.5–60.8%) | 79.8% (70.3–87.4%) |
| Flotac | 66.7% (48.2–82.0%) | 87.2% (78.3–93.4%) |
| Entamoeba hartmanni | ||
| FECT | 85.7% (57.2–98.2%) | 97.9% (92.7–99.8%) |
| Flotac | 21.4% (4.7–50.8%) | 89.5% (82.0–94.7%) |
| Giardia intestinalis | ||
| FECT | 58.3% (27.7–84.8%) | 95.1% (88.8–98.4%) |
| Flotac | 75.0% (72.8–94.5%) | 97.0% (91.4–99.4%) |
| Endolimax nana | ||
| FECT | 100% (73.5–100%) | 100% (96.2–100%) |
| Flotac | 16.7% (2.1–48.4%) | 90.6% (88.3–95.4%) |
| Chilomastix mesnili | ||
| FECT | 100% (63.6–100%) | 100% (96.4–100%) |
| Flotac | 12.5% (0.3–52.7%) | 93.5% (87.0–97.3%) |
| Iodamoeba buetschlii | ||
| FECT | 75.0% (19.4–99.4%) | 99.1% (94.8–99.9%) |
| Flotac | 50.0% (6.8–93.2%) | 98.1% (93.4–99.8%) |
A total of 108 stool specimens were examined. The combined results from FS4 and FS7 for the Flotac-400 dual technique are included. The pooled results of the FECT and the Flotac-400 dual technique were considered the diagnostic gold standard (sensitivity = 100%, NPV = 100%).
DISCUSSION
There is a growing awareness of the clinical importance and public health relevance of intestinal parasitic infections, particularly those caused by helminths (17). Less attention is given to intestinal protozoon infections (28), despite a report some 20 years ago by the World Health Organization (WHO) Collaborating Centre for the Epidemiology of Intestinal Parasitic Infections emphasizing that the accurate diagnosis of intestinal protozoon infections is of pressing necessity in order to implement cost-effective control measures for intestinal parasitic infections (5).
We investigated the accuracy of the Flotac-400 dual technique for the diagnosis of intestinal protozoon infections in 108 stool samples obtained from a random sample of people living in rural south-central Côte d'Ivoire. Comparison was made with the FECT, which is a widely used technique for detection of intestinal protozoon cysts, both in epidemiologic surveys and reference laboratories (25, 27, 30, 37). Adhering to standard protocols, both techniques revealed the same eight intestinal protozoon species. The number of individuals identified as positive by each technique, however, varied considerably from one species to another. While the Flotac-400 dual technique showed a higher sensitivity and, thus, identified more fecal samples as positive for the pathogenic protozoon species G. intestinalis, as well as the potentially pathogenic B. hominis (33, 35) and the nonpathogenic E. coli, FECT detected E. histolytica/E. dispar and four nonpathogenic commensal protozoon species (i.e., E. hartmanni, E. nana, I. buetschlii, and C. mesnili) with a higher sensitivity. Our study, therefore, confirms that the Flotac-400 dual technique is useful for the diagnosis of human intestinal protozoon infections, which adds further weight to preliminary findings of an investigation with stool samples obtained from immigrants in southern Italy (16). Our study constitutes the first rigorous comparison of diagnostic accuracy between the Flotac-400 dual technique and the FECT. We adhered to standard protocols and analyzed stool samples according to sequences of computer-generated random lists. The microscopist was blinded to the results of the other technique, and approximately 10% of the readings were quality controlled. Our results are encouraging, as both methods achieved comparable recovery rates for the diagnosis of intestinal protozoa.
It is important to note that the study presented here utilized stool samples obtained from an African population in an area where intestinal protozoa and other intestinal parasites are highly endemic. The use of a single diagnostic technique that is able to diagnose most of the intestinal parasitic pathogens would represent an important step forward for more accurate differential diagnosis. Indeed, previous studies showed that the Flotac technique is able to detect common soil-transmitted helminths and S. mansoni with an equal or higher sensitivity than currently more widely used methods, such as Kato-Katz and FECT (8, 15, 20, 21, 39). In the framework of the present study (baseline prevalence assessment for parasitic diseases in the Taabo HDSS), FECT and the Flotac technique were also successfully employed for the diagnosis of helminth infections. Hence, Flotac might become a useful addition to the suite of diagnostic techniques, particularly those that are able to concurrently detect helminths and intestinal protozoa. Of note, the Flotac-400 dual technique consists of the examination of the same stool sample in the two chambers of the Flotac apparatus, each filled with a different FS. In the present investigation, FS4 and FS7, which are commonly employed for the diagnosis of human helminth infection (8, 15, 20, 21, 39), detected intestinal protozoa with sensitivities comparable to that of the FECT, even when comparing the results of the latter technique to those obtained by separate analysis of only one Flotac chamber (using either FS4 or FS7).
From a clinical point of view, the accurate and reliable identification of pathogenic parasites is of paramount importance, while the diagnosis of simple commensal species is of lesser interest. Hence, the evaluation of a diagnostic technique for intestinal protozoa should focus primarily on the diagnostic accuracy for G. intestinalis, E. histolytica/E. dispar, and perhaps, B. hominis. At present, no decisive statement in favor of either diagnostic technique employed here can be made, as the Flotac-400 dual technique detected infections with G. intestinalis and B. hominis with a higher sensitivity than the FECT but was inferior to the FECT with regard to the diagnosis of E. histolytica/E. dispar infections. It is noteworthy that no currently available copromicroscopic technique is able to reliably differentiate between the pathogenic E. histolytica and the nonpathogenic commensal E. dispar (11, 31). Hence, other laboratory techniques, such as PCR assays, must be employed for an accurate diagnosis of E. histolytica (13). The pressing need to further augment the available diagnostic tools for intestinal protozoa is underlined by our results; even the FECT, which is widely used in the diagnosis of intestinal protozoa, failed to detect a considerable number of infections, regardless of the species investigated.
The agreement between the two diagnostic techniques, as determined by Cohen's kappa statistic, was generally low to moderate. The highest kappa value was found for G. intestinalis (κ = 0.46), whereas for other intestinal protozoon species, the kappa values were below 0.40. Similar observations have been made in previous studies in which equal sets of stool samples were examined by different reference diagnostic centers adhering to standard protocols, and yet, unexpectedly low levels of agreement between laboratories were reported (4, 37). Moreover, stool consistency is an important consideration that may have negatively influenced the method agreement in our comparison, as the number of intestinal protozoon cysts in loose or watery stool specimens is considerably lower than in normally formed stool, hence rendering it more likely that light infections may be missed by microscopic techniques.
Our study suffers from limitations that may at least partially explain the observed discordance between the two methods investigated for the diagnosis of intestinal protozoa. Most importantly, it must be noted that in the absence of an objective diagnostic gold standard, the pooled results of the Flotac-400 dual technique and the FECT had to be considered the “true” prevalence in order to render a calculation of NPV and sensitivity possible. Hence, no definitive statement about false-positive or false-negative results can be made, as it was not feasible to verify the reported data by another independent technique, such as PCR results. The use of PCR assays would probably have revealed even higher infection rates of most intestinal protozoon species. In the absence of readily available PCR or another highly sensitive diagnostic assay, multiple stool examinations using FECT, Flotac, or another copromicroscopic technique would have been required to determine the “true” infection prevalence of intestinal protozoa and helminths (34). Whenever copromicroscopic diagnostic techniques are employed, it is important to ensure that all laboratory technicians involved in the examination process are well trained so that possible bias is reduced to a minimum. In our study, all microscope slides were read by the same experienced technician to avoid interpersonal discrepancy influencing the results.
Additional studies are warranted to confirm the promising results reported here and those obtained before for migrants in Italy (16) regarding the accuracy of the Flotac-400 dual technique for diagnosis of intestinal protozoa. Three observations from our study emphasize the need for validating additional FS to further improve the accuracy of the Flotac-400 dual technique. First, the microscopic analysis of the Flotac samples in the laboratory was often time consuming, as a considerable quantity of fecal debris had not been retained by the chemical agents used during the preparation phase. The visibility of the intestinal protozoon cysts or vegetative forms was therefore limited, and the identification of subcellular structures like nucleoli became difficult. As the differentiation of some Entamoeba species is based on correct nucleolus counts, the examination of the Flotac-400 reading disk under the microscope sometimes did not permit determination of the species of intestinal protozoa, so that these results had to be excluded, decreasing the method's sensitivity. Staining the fecal samples, e.g., by using Lugol's iodine, may help to improve the visibility and, hence, the differential diagnosis of Entamoeba species in future investigations. Second, E. coli was destroyed by both FS employed in the current study (Fig. 2), which might indicate that the chemicals used were too aggressive for some intestinal protozoon species. It is conceivable that the sensitivity of the Flotac-400 dual technique can be further enhanced when FSs are used that are able to retain debris more effectively and, hence, allow a more accurate identification of the parasites investigated. Indeed, a recent study suggests that FS3 may be more appropriate for the diagnosis of E. histolytica/E. dispar than the two FSs employed here (i.e., FS4 and FS7), yet it remains to be elucidated whether FS3 can be used for concurrent helminth diagnosis. Lately, it has been acknowledged that the choice of FS plays an important role in the results obtained by any diagnostic technique that is based upon flotation and sedimentation (8, 9). This observation is confirmed by our data, as FS4 and FS7 showed remarkably different sensitivities depending on the intestinal protozoon species investigated. Therefore, new research is needed to document the influence of different FSs on the visibility and differential diagnosis of intestinal protozoon cysts. Third, the effect of the fixative used for preservation of fecal samples has to be evaluated, as there is considerable debate whether a 5% or 10% formalin fixation or a SAF fixative is more appropriate for the preservation of stool samples pending Flotac, as well as FECT (8). With regard to FECT, it should be noted that in some laboratories, the use of ether is not allowed any longer, and it has been replaced by diethyl acetate, which is supposed to have no impact on diagnostic accuracy (37).
We conclude that there is a need for additional studies elucidating the influence of the chemical agents used for fecal fixation, the performance of different FSs, and the duration of stool preservation on the accuracy of intestinal protozoon and helminth diagnosis (8, 10, 15). New insights will help to facilitate, improve, and further standardize the Flotac preparation protocols, particularly for the diagnosis of intestinal protozoa. In considering the advantages and drawbacks of the Flotac technique compared to those of currently more widely used diagnostic techniques, it is too early to conclude whether broader application of the Flotac method would offer a real benefit. The observation that the intestinal protozoon E. coli, as well as the trematode S. mansoni (15), are somewhat deformed when subjected to the Flotac technique must be considered. Despite these constraints, the diagnostic performance of the Flotac-400 dual technique for detection of intestinal protozoa is promising, at least when compared to that of the widely used FECT. It seems worthwhile to further develop and validate the Flotac method, particularly when considering that the technical requirements of both diagnostic tools are almost equal and that the preparation and reading time for the Flotac-processed samples is comparable to the time needed for FECT and other standard diagnostic techniques (32). The concurrent diagnosis of both helminths and intestinal protozoa would represent a major step forward, as it would facilitate the epidemiologic surveillance of polyparasitism and spur public health interventions throughout the world (29, 30). With regard to clinical aspects, a multivalent diagnostic approach such as the Flotac method may be employed as a screening test for individuals whose clinical symptoms are not sufficient to reliably determine a specific infection.
ACKNOWLEDGMENTS
We thank all the study participants from Léléblé, south-central Côte d'Ivoire, for their participation in the present study and for providing fecal samples. We express our gratitude to the laboratory technicians Ida Guariglia, Giovanna Cappelli, Maria Paola Morelli, and Maria Elena Morgoglione in Eboli, Italy, and to Elisabeth Escher and Michelle Dobler from the Swiss Tropical and Public Health Institute in Basel, Switzerland, for their help with laboratory examinations and taking photographs of E. coli. Many thanks are addressed to Annibale Biggeri (University of Florence, Italy) for help with the statistical analysis.
This study received financial support from the Swiss National Science Foundation (project no. PPOOB-102883 and PPOOB-119129) and the nongovernmental organization Fairmed (Bern, Switzerland). S. Knopp was supported by the Emanuel Burckhardt Foundation Basel (personal stipend for the final year of her Ph.D.).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions were as follows: S.L.B., E.K.N., and J.U. conceived and designed the epidemiologic study; S.L.B., B.S., L.R., G.C., and J.U. conceived and designed the comparison of the diagnostic methods; S.L.B., L.K.L., and B.S. collected the data; S.L.B., L.R., S.K., G.C., and J.U. analyzed and interpreted the data; S.L.B. wrote the paper; and S.L.B., B.S., L.R., S.K., E.K.N., G.C., and J.U. critically revised the manuscript and read and approved the final version.
G.C. is the inventor and current patent holder of the Flotac apparatus. In the case that the current validation of the Flotac technique for human helminths and intestinal protozoa is successful, the method will be licensed free of charge to the WHO and interested public noncommercial research centers. None of the other authors has any conflict of interest concerning the work reported in this paper.
Footnotes
Published ahead of print on 27 April 2011.
REFERENCES
- 1. Agresti A., Ghosh A., Bini M. 1995. Raking kappa: describing potential impact of marginal distribution of measures of agreement. Biom. J. 7:811–820 [Google Scholar]
- 2. Allen A. V. H., Ridley D. S. 1970. Further observations on the formol-ether concentration technique for faecal parasites. J. Clin. Pathol. 23:545–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ben Musa N. A., Ibrahim R. 2007. Long term formalin-preserved stool specimens for detection of intestinal parasites from school-aged children in Tripoli, Libya. J. Egypt. Soc. Parasitol. 37:1049–1054 [PubMed] [Google Scholar]
- 4. Bogoch I. I., Raso G., N′Goran E. K., Marti H. P., Utzinger J. 2006. Differences in microscopic diagnosis of helminths and intestinal protozoa among diagnostic centres. Eur. J. Clin. Microbiol. Infect. Dis. 25:344–347 [DOI] [PubMed] [Google Scholar]
- 5. Bundy D. A. P., Hall A., Medley G. F., Savioli L. 1992. Evaluating measures to control intestinal parasitic infections. World Health Stat. Q. 45:168–179 [PubMed] [Google Scholar]
- 6. Cohen J. 1960. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 20:213–220 [Google Scholar]
- 7. Cringoli G. 2006. FLOTAC, a novel apparatus for a multivalent faecal egg count technique. Parassitologia 48:381–384 [PubMed] [Google Scholar]
- 8. Cringoli G., Rinaldi L., Maurelli M. P., Utzinger J. 2010. FLOTAC: new multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat. Protoc. 5:503–515 [DOI] [PubMed] [Google Scholar]
- 9. Cringoli G., Rinaldi L., Veneziano V., Capelli G., Scala A. 2004. The influence of flotation solution, sample dilution and the choice of McMaster slide area (volume) on the reliability of the McMaster technique in estimating the faecal egg counts of gastrointestinal strongyles and Dicrocoelium dendriticum in sheep. Vet. Parasitol. 123:121–131 [DOI] [PubMed] [Google Scholar]
- 10. Dacombe R. J., et al. 2007. Time delays between patient and laboratory selectively affect accuracy of helminth diagnosis. Trans. R. Soc. Trop. Med. Hyg. 101:140–145 [DOI] [PubMed] [Google Scholar]
- 11. Diamond L. S., Clark C. G. 1993. A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J. Eukaryot. Microbiol. 40:340–344 [DOI] [PubMed] [Google Scholar]
- 12. Foreyt W. J. 1989. Diagnostic parasitology. Vet. Clin. North Am. Small Anim. Pract. 19:979–1000 [DOI] [PubMed] [Google Scholar]
- 13. Fotedar R., et al. 2007. Laboratory diagnostic techniques for Entamoeba species. Clin. Microbiol. Rev. 20:511–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. García L. S. 2001. Diagnostic medical parasitology, p. 791 ASM Press, Washington, DC [Google Scholar]
- 15. Glinz D., et al. 2010. Comparing diagnostic accuracy of Kato-Katz, Koga agar plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soil-transmitted helminths. PLoS Negl. Trop. Dis. 4:e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gualdieri L., et al. 2011. Intestinal parasites in immigrants in the city of Naples (southern Italy). Acta Trop. 117:196–201 [DOI] [PubMed] [Google Scholar]
- 17. Hotez P. J., et al. 2008. Helminth infections: the great neglected tropical diseases. J. Clin. Invest. 118:1311–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katz N., Chaves A., Pellegrino J. 1972. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 14:397–400 [PubMed] [Google Scholar]
- 19. Keiser J., et al. 2002. Polyparasitism with Schistosoma mansoni, geohelminths, and intestinal protozoa in rural Côte d'Ivoire. J. Parasitol. 88:461–466 [DOI] [PubMed] [Google Scholar]
- 20. Knopp S., et al. 2009. FLOTAC: a promising technique for detecting helminth eggs in human faeces. Trans. R. Soc. Trop. Med. Hyg. 103:1190–1194 [DOI] [PubMed] [Google Scholar]
- 21. Knopp S., et al. 2009. A single FLOTAC is more sensitive than triplicate Kato-Katz for the diagnosis of low-intensity soil-transmitted helminth infections. Trans. R. Soc. Trop. Med. Hyg. 103:347–354 [DOI] [PubMed] [Google Scholar]
- 22. Koga K., et al. 1991. A modified agar plate method for detection of Strongyloides stercoralis. Am. J. Trop. Med. Hyg. 45:518–521 [DOI] [PubMed] [Google Scholar]
- 23. Lammie P. J., Fenwick A., Utzinger J. 2006. A blueprint for success: integration of neglected tropical disease control programmes. Trends Parasitol. 22:313–321 [DOI] [PubMed] [Google Scholar]
- 24. Marti H. P., Escher E. 1990. SAF: an alternative fixation solution for parasitological stool specimens. Schweiz. Med. Wochenschr. 120:1473–1476 (In German.) [PubMed] [Google Scholar]
- 25. Nematian J., Gholamrezanezhad A., Nematian E. 2008. Giardiasis and other intestinal parasitic infections in relation to anthropometric indicators of malnutrition: a large, population-based survey of schoolchildren in Tehran. Ann. Trop. Med. Parasitol. 102:209–214 [DOI] [PubMed] [Google Scholar]
- 26. Okhuysen P. C. 2001. Traveler's diarrhea due to intestinal protozoa. Clin. Infect. Dis. 33:110–114 [DOI] [PubMed] [Google Scholar]
- 27. Ouattara M., N′Guessan N. A., Yapi A., N′Goran E. K. 2010. Prevalence and spatial distribution of Entamoeba histolytica/dispar and Giardia lamblia among schoolchildren in Agboville area (Côte d'Ivoire). PLoS Negl. Trop. Dis. 4:e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ouattara M., et al. 2008. Prevalence and polyparasitism of intestinal protozoa and spatial distribution of Entamoeba histolytica, E. dispar and Giardia intestinalis from pupils in the rural zone of Man in Côte d'Ivoire. Santé 18:215–222 (In French.) [PubMed] [Google Scholar]
- 29. Pullan R., Brooker S. 2008. The health impact of polyparasitism in humans: are we under-estimating the burden of parasitic diseases? Parasitology 135:783–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raso G., et al. 2004. Multiple parasite infections and their relationship to self-reported morbidity in a community of rural Côte d'Ivoire. Int. J. Epidemiol. 33:1092–1102 [DOI] [PubMed] [Google Scholar]
- 31. Singh A., Houpt E., Petri W. A. 2009. Rapid diagnosis of intestinal parasitic protozoa, with a focus on Entamoeba histolytica. Interdiscip. Perspect. Infect. Dis. 2009:547090 doi:10.1155/2009/547090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Speich B., et al. 2010. Comparative cost assessment of the Kato-Katz and FLOTAC techniques for soil-transmitted helminth diagnosis in epidemiological surveys. Parasit. Vectors 3:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stark D., van Hal S., Marriott D., Ellis J., Harkness J. 2007. Irritable bowel syndrome: a review on the role of intestinal protozoa and the importance of their detection and diagnosis. Int. J. Parasitol. 37:11–20 [DOI] [PubMed] [Google Scholar]
- 34. Steinmann P., et al. 2008. Extensive multiparasitism in a village of Yunnan province, People's Republic of China, revealed by a suite of diagnostic methods. Am. J. Trop. Med. Hyg. 78:760–769 [PubMed] [Google Scholar]
- 35. Stensvold C. R., et al. 2009. Blastocystis: unravelling potential risk factors and clinical significance of a common but neglected parasite. Epidemiol. Infect. 137:1655–1663 [DOI] [PubMed] [Google Scholar]
- 36. Thrusfield M. 1995. Veterinary epidemiology, p. 280–282 Blackwell, London, United Kingdom [Google Scholar]
- 37. Utzinger J., et al. 2010. Microscopic diagnosis of sodium acetate-acetic acid-formalin-fixed stool samples for helminths and intestinal protozoa: a comparison among European reference laboratories. Clin. Microbiol. Infect. 16:267–273 [DOI] [PubMed] [Google Scholar]
- 38. Utzinger J., N′Goran E. K., Marti H. P., Tanner M., Lengeler C. 1999. Intestinal amoebiasis, giardiasis and geohelminthiases: their association with other intestinal parasites and reported intestinal symptoms. Trans. R. Soc. Trop. Med. Hyg. 93:137–141 [DOI] [PubMed] [Google Scholar]
- 39. Utzinger J., et al. 2008. FLOTAC: a new sensitive technique for the diagnosis of hookworm infections in humans. Trans. R. Soc. Trop. Med. Hyg. 102:84–90 [DOI] [PubMed] [Google Scholar]
- 40. Yang J., Scholten T. 1977. A fixative for intestinal parasites permitting the use of concentration and permanent staining procedures. Am. J. Clin. Pathol. 67:300–304 [DOI] [PubMed] [Google Scholar]