Abstract
Considerable efforts have been directed toward the identification of small-ruminant prion diseases, i.e., classical and atypical scrapie as well as bovine spongiform encephalopathy (BSE). Here we report the in-depth molecular analysis of the proteinase K-resistant prion protein core fragment (PrPres) in a highly scrapie-affected goat flock in Greece. The PrPres profile by Western immunoblotting in most animals was that of classical scrapie in sheep. However, in a series of clinically healthy goats we identified a unique C- and N-terminally truncated PrPres fragment, which is akin but not identical to that observed for atypical scrapie. These findings reveal novel aspects of the nature and diversity of the molecular PrPres phenotypes in goats and suggest that these animals display a previously unrecognized prion protein disorder.
INTRODUCTION
Classical scrapie is a fatal contagious disease that naturally affects sheep and goats. Together with Creutzfeldt-Jakob disease (CJD) and bovine spongiform encephalopathy (BSE) in cattle, it figures among the transmissible spongiform encephalopathies (TSE) or prion diseases (25). These are lethal neurodegenerative disorders that show neuropil spongiosis as well as intraneuronal vacuolation, astrocytic gliosis, and the deposition of a pathological conformer (PrPd) of the physiological, host-encoded prion protein (PrPc) in the central nervous system (CNS).
While there is clear evidence that BSE is a zoonosis that causes variant CJD in humans, there are no such indications for classical scrapie (10, 16, 33). Nevertheless, TSEs in small ruminants have drawn the attention of veterinary and political authorities because of concerns that BSE might also affect sheep and goats. Moreover, a previously unrecognized type of TSE, termed atypical scrapie or Nor98, was reported first in Norway in 1998 (7) and later in many other countries (for a review, see reference 6), but its significance for public and animal health is uncertain. As a consequence, active TSE surveillance of small ruminants has been considerably enhanced. This resulted in the identification not only of a substantial number of classical and atypical scrapie cases but also of two goats that were affected by a TSE indistinguishable from BSE (11, 18).
Many efforts have been directed toward establishing and validating diagnostic techniques for the identification and discrimination of atypical scrapie, classical scrapie, and small-ruminant BSE. At present, a multistage serial testing strategy using screening tests as well as confirmatory and discriminatory tests is applied in many countries. These tests are based on the postmortem detection of PrPd or its proteinase K (PK)-resistant core fragment, PrPres, in the CNS or the lymphoreticular system. Discriminatory tests rely on differences in the proteolytic processing of PrPd between the three types of small-ruminant TSEs, which result in PrPres fragments of distinct molecular masses and specific antibody binding properties by Western immunoblotting (WB) (5, 17, 22, 28). This complex strategy obviously requires profound knowledge of the molecular phenotypic diversity of naturally occurring TSEs in sheep and goats. In this respect, considerable work has been done with sheep; however, only a few cases of classical goat scrapie have been described in the literature (for a review, see reference 32), and reports of the PrPres characteristics determined by WB for goats are rare.
Recently, we reported the outbreak of classical scrapie in a Greek goat flock with a high incidence of infection (8). In the present study, we analyze specimens of animals from this flock to generate baseline data on the molecular PrPres phenotype diversity in natural goat scrapie. While most of the goats present the WB PrPres banding pattern of classical scrapie, as known for sheep, we uncover a previously unknown truncated PK-resistant prion protein fragment in a series of animals. These findings extend the view of the phenotypic diversity of prion protein disorders in goats and challenge current strategies for their diagnosis and classification.
MATERIALS AND METHODS
Animals.
The flock under investigation was identified in Northern Greece in 2007 upon laboratory confirmation of classical scrapie in three index cases. All animals were clinically examined and culled according to European Union legislation. From a total of 98 adult goats (age, >12 months), brain tissue samples of 86 adult goats were available for the purpose of the present study. Of these animals, five displayed clinical signs of scrapie, while all others were clinically healthy at the time of culling.
Sample preparation.
Immediately after death, medulla oblongata samples were removed via the foramen magnum and forwarded to the Greek national laboratory for statutory testing. These samples were later returned to the laboratories of the Aristotle University of Thessaloniki. During storage they were accidentally exposed to an ambient temperature for a prolonged time, which resulted in severe tissue autolysis, and these samples were found to be unsuitable for histopathological processing and examination. In contrast, the heads of the goats were transported directly to the University of Thessaloniki and stored at 4°C until further sampling, which took place between day 1 and day 3 (23 to 60 h) postmortem in the order of sequential identifications (goat 1 [G1] to G98), and therefore, these brain structures were relatively well conserved. The brains were removed from the heads and cut sagitally in halves. One half of the brain, together with tonsil samples, was fixed in formalin, and the second half served for the collection of fresh tissue samples from the diencephalon, the cerebellar cortex, and the cerebral frontal cortex, which were immediately frozen at −80°C. Similarly, samples of the obex were taken from the frozen medulla oblongata. Tissue homogenates (20% [wt/vol]; 1.8 ml) were prepared by using a Ribolyser apparatus according to the protocol of the Bio-Rad TeSeE enzyme-linked immunosorbent assay (ELISA). These homogenates and the formalin-fixed brains were then forwarded to the NeuroCentre, University of Berne, for biochemical and histopathological examination. All biochemical tests used the same homogenates as the starting material.
Control tissues.
Classical scrapie control tissues originated from Greece (sheep S93 and goat G40) and from Switzerland (sheep C). Atypical scrapie material was available from a goat (G2/FS) (26), and three medulla oblongata samples of experimentally BSE-infected sheep of the ARQ/ARQ genotype (ovine BSE [ovBSE]) were kindly provided by P. Berthon and F. Lantier from the INRA, Tours-Nouzilly, France.
Western immunoblotting.
The confirmatory NeuroCentre WB (NC-WB) was described previously (21). Briefly, homogenates were digested with PK and purified with a commercial test kit (TeSeE sheep and goat purification kit; Bio-Rad). The resulting PrPres pellet was resuspended in Laemmli buffer (Bio-Rad) and subjected to SDS-PAGE using 1.5-mm-thick hand-cast discontinuous 16.5% or 13.5% Tris-HCl acrylamide gels (8 by 7 cm). Proteins were then blotted onto polyvinylidene difluoride (PVDF) membranes, and PrPres was detected with monoclonal antibodies (MAbs) P4 (0.4 μg/ml in phosphate-buffered saline–Tween [PBST]) (epitope according to the caprine prion protein sequence 93WGQGGSH99; R-Biopharm) and L42 (148YEDRYY153, 1 μg/ml; R-Biopharm). A biotinylated molecular mass standard (B2787; Sigma) was included in the outer lanes of each gel and visualized by the addition of a streptavidin-peroxidase conjugate (Sigma). The molecular masses of individual bands were determined with Quantity-One software (version 4.6.2; Bio-Rad).
Histopathology and immunohistochemistry.
Histopathological examination and PrPd immunohistochemistry (IHC) were performed on standard brain sections (excluding the caudal medulla oblongata). IHC was routinely performed with MAb F99/97.6.1 (VMRD, Inc.) (2 μg/ml) as described previously (21). Positive- and negative-control sections were analyzed in parallel in each run.
Screening tests.
The TeSeE ELISA (Bio-Rad), which is approved for TSE surveillance of small ruminants in the European Union, and the PrioStrip SR assay (Prionics), an immunochromatographic assay which is approved for the same purpose in Switzerland, were performed according to the instructions provided by the manufacturers. For the PrioStrip SR assay, we adjusted 20% (wt/vol) homogenates to 10% (wt/vol) homogenates by the addition of an equal amount of 2× PrioStrip SR homogenization buffer.
Epitope mapping.
For epitope mapping, we used the NC-WB procedure with MAbs P4 and L42 and the following panel of PrP-specific MAbs: BG4 (54PQGG57, 1 μg/ml; TSE Resource Center, the Roslin Institute, University of Edinburgh) (14), SAF32 (59[4xQPHGGGW]89, 0.4 μg/ml; SPI-Bio, France) (12), 12B2 (93WGQGG97, 1.1 μg/ml) (20), 9A2 (102WNK104, 2 μg/ml) (20), and Sha31 (148YEDRYYRE155, 1:10, TeSeE Western kit; Bio-Rad) (12).
Autolysis study.
Tissue autolysis in formalin-fixed brains was assessed semiquantitatively by histopathological examination on a scale from 0 (no autolysis) to 4 (severe autolysis), as described previously (27). In addition, frozen brain tissue samples from two experimentally classical-scrapie-infected sheep (Sccl1 and Sccl2), two atypical-scrapie-affected sheep (Scat1 and Scat2), and a TSE-negative but severely autolytic goat were prepared as 50% (wt/vol) macerates in sterile, distilled, and nuclease-free water by chopping tissues finely and by repeated vortexing using 4-mm ceramic beats. Four aliquots of TSE-negative macerates (4 ml) were then spiked with the TSE-positive macerates (1 ml) and incubated in a humid chamber at 37°C for a total of 7 days. Nonspiked TSE-negative macerates served as a control. Each day, 500 μl of macerates was removed and stored at −20°C until analysis by NC-WB.
PRNP genotyping and statistical analysis.
The genotype for each animal was determined as previously reported (8). The chi-squared test (SAS, version 9.1, 1999; SAS Institute) was used to assess genotype frequencies between animals displaying the ∼12-kDa fragment and those which did not. Goats from these two groups were required to be within the same birth cohorts. Since all animals that showed the ∼12-kDa fragment were older than 24 months of age, the same age threshold was applied to the other animals. This edit removed 7 animals from the ∼12-kDa-fragment-free group, resulting in a total of 79 animals for statistical analysis.
RESULTS
Molecular PrPres phenotype in classical-scrapie-affected goats.
To determine the molecular PrPres phenotype, brain tissue homogenates of at least three different neuroanatomical regions per animal were analyzed by NC-WB. Of a total of 86 goats, 20 animals revealed a three-band PrPres profile in the range of 19 to 29 kDa at least in the medulla oblongata. For most animals this was also detected in other brain structures, like the cerebellar cortex and the cerebral cortex. Among these were the five goats that showed clinical signs of scrapie antemortem. The molecular masses of the unglycosylated PrPres peptides were similar to those in classical-scrapie-affected sheep and different from those in ovine BSE and atypical scrapie controls, irrespective of the brain structure. The PrPres deposits were consistently most prominent in the medulla oblongata (Fig. 1). Taken together, these findings corroborate our previous conclusion that this flock was affected by classical scrapie and that the classical scrapie PrPres phenotype in these goats is similar to that in sheep.
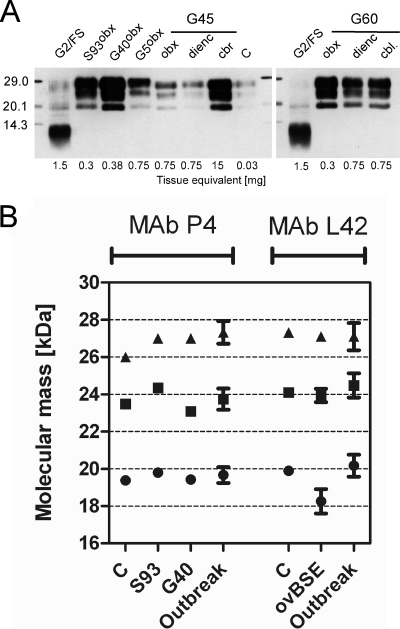
Fig. 1.
Molecular PrPres phenotype in classical-scrapie-affected goats. (A) Western immunoblot analysis of three exemplary goats (G5, G45, and G60), with MAb P4 demonstrating a uniform PrPres banding pattern, which is similar to those of classical sheep and goat scrapie controls (S93, G40, and C) and clearly different from that of atypical goat scrapie (G2/FS). Abbreviations: obx, obex region in the medulla oblongata; dienc, diencephalon; cbl, cerebellar cortex. Molecular mass standards are indicated on the left in kilodaltons. (B) Comparison of the molecular masses of unglycosylated (circles), monoglycosylated (squares), and diglycosylated (triangles) PrPres moieties in classical-scrapie-affected goats of the Greek outbreak with those of classical scrapie controls and ovine BSE (ovBSE) using MAbs P4 and L42. Scores are depicted as average values, and error bars indicate standard deviations (SDs). Note that in ovine BSE the molecular mass of the unglycosylated PrPres is ∼1 kDa lower than those for the goats under investigation and the classical scrapie controls.
Identification of a MAb P4-reactive peptide.
For 10 clinically healthy goats we observed a single PK-resistant peptide with a molecular mass of ∼12 kDa, which strongly reacted with MAb P4 by NC-WB. Its molecular mass was clearly different from that of PrPres in classical scrapie but indistinguishable from the predominant PrPres band in atypical scrapie (Fig. 2A). While this peptide was most abundant in the medulla oblongata in seven goats, it was found only in the cerebral cortex and not in the other brain structures in three animals (Table 1). The average age of these animals was 37 months (range, 28 to 60 months) and was not significantly different from that of the classical-scrapie-affected goats, with an average age of 32.5 months (range, 24 months to 48 months). For one goat (G22) a three-band PrPres pattern of the classical scrapie type was recognized in the diencephalon, while the single ∼12-kDa peptide emerged in the medulla oblongata (Fig. 2B). This coexistence of classical scrapie PrPres and the ∼12-kDa band was not observed for any of the other goats.
Fig. 2.
N- and C-terminally truncated PK-resistant prion proteins. (A) Western immunoblot showing a single aberrant peptide in goats (MAb P4), which migrates at a molecular mass indistinguishable from that of the predominant PrPres in atypical goat scrapie (G2/FS). Abbreviations: C, classical scrapie control; obx, obex region in the medulla oblongata; dienc, diencephalon; cbr, cerebral cortex. Tissue equivalents loaded onto the gels are indicated at the bottom. (B) Details of one goat (G22) that showed the aberrant peptide in the medulla oblongata and the classical scrapie PrPres signature as well as a faint signal for the aberrant peptide in the diencephalon. (C) Epitope mapping. Shown is a comparison of PrPres reactivities using a panel of prion-protein-specific antibodies (SAF32, P4, 12B2, 9A2, L42, and Sha31) in classical scrapie (lane 1, 0.03 mg), atypical scrapie (G2/FS) (lane 2, 1.5 mg), TSE-negative goats (lane 3, 5 mg), and a Greek goat that revealed the aberrant PrPres peptide (G34) (lane 4, 5 mg). Molecular mass standards are indicated on the left in kilodaltons.
Table 1.
PRNP genotypes at codons 211, 222, and 240 and test performances for goats that displayed an aberrant truncated PrPres by Western immunoblotting
| Animal | Age (mo) | PRNP genotyped at codon 211/222/240 | NeuroCentre Western blot result (∼12-kDa band)a |
TeSeE ODb | RDU by PrioStrip SR in 1st/2nd/3rd runc | |||
|---|---|---|---|---|---|---|---|---|
| obx | dienc | cbl | cbr | |||||
| G17 | 32 | RR/QQ/PP | + | NT | + | − | 0.023 | 51/0/0 |
| G18 | 40 | RR/QQ/PS | +++ | + | + | − | 0.023 | NT |
| G22 | 38 | RR/QQ/PS | ++ | +++ cl | − | − | 0.024 | 0 |
| G34 | 38 | RR/QK/PS | − | − | − | +++ | 0.023 | 84/0/40 |
| G50 | 24 | RR/QQ/PS | +++ | (+) | ++ | − | 0.024 | 4 |
| G53 | 36 | RQ/QQ/PS | − | − | − | + | 0.022 | 0 |
| G72 | 38 | RR/QQ/PS | + | − | − | − | 0.030 | 0 |
| G78 | 60 | RR/QK/PS | + | − | − | − | 0.022 | 97/92/150 |
| G81 | 36 | RQ/QQ/PS | − | − | − | + | 0.023 | 130/199/269 |
| G84 | 28 | RQ/QK/SS | +++ | + | + | − | 0.022 | 84/15/50 |
Abbreviations: obx, obex region in the medulla oblongata; dienc, diencephalon; cbl, cerebellar cortex; cbr, cerebral cortex; −, +, ++, and +++, no, weak, moderate, and strong signals, respectively; cl, classical scrapie pattern; NT, not tested. Parentheses indicate signals visible only after overexposure of the film.
In optical density (OD) units; cutoff, OD of 0.205 (medulla oblongata).
In relative density units (RDU); cutoff, 60 RDU (medulla oblongata).
Letters correspond to single-letter amino acid code.
Epitope mapping.
To investigate whether the observed ∼12-kDa peptide corresponds to a PrPres fragment, we applied a panel of MAbs that bind to defined epitopes in different regions of the caprine prion protein in the NC-WB to tissues of two goats (G34 cerebral cortex and G18 medulla oblongata) as well as to atypical and classical scrapie control tissues. The ∼12-kDa fragment was detected by MAbs SAF32, 12B2, P4, and 9A2 but not by MAbs L42 and Sha31. In turn, all these MAbs readily linked to the prominent band of a similar molecular mass in atypical scrapie (Fig. 2C). Neither the peptide under investigation nor atypical or classical scrapie PrPres reacted with N-terminal MAb BG4, which, however, bound PrPc in non-PK-treated negative controls (data not shown). Based on the binding sites of the applied antibodies, we predict that the N and C termini of the ∼12-kDa peptide lie in the range of PrP residues 55 to 87 and 105 to 152, respectively, which corresponds to a minimum molecular mass of 4.2 kDa (residues 87 to 105) and a maximum of 9.8 kDa (residues 55 to 152). In turn, the predominant fragment in atypical scrapie extends at least to residue 155 at its C terminus (Fig. 3). This indicates that the observed ∼12-kDa peptide corresponds to a C- and N-terminally truncated PrPres fragment, which is akin but not identical to the predominant one in atypical scrapie.
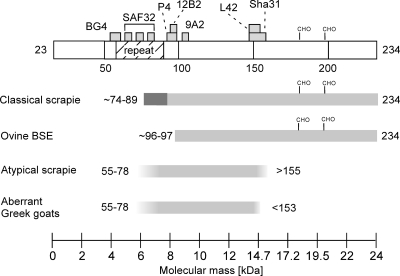
Fig. 3.
Model of PrPres fragments predicted for TSEs in small ruminants. The illustration at the top shows the full-length mature caprine prion protein and indicates the binding sites of the antibodies used for epitope mapping and the octapeptide repeat region between residues 54 and 89. The predicted PrPres fragments for classical scrapie and ovine BSE were adopted from data described previously by Thuring and coworkers (30), while those for atypical scrapie and the aberrant Greek goats are estimated based on data from the present study. Additional glycosylated PrPres fragments that migrate in the range of 19 to 30 kDa occur in atypical scrapie, but these are not included in this figure. The predicted N- and C-terminal residues are indicated for each fragment; gray boxes represent alternative terminal residues. To facilitate molecular mass assessments, a scale is indicated at the bottom. Glycosylation sites are shown at positions 182 and 199 (CHO). All numbered residues refer to the caprine prion protein (GenBank accession number X91999).
Immunohistochemistry.
When we analyzed formalin-fixed brain tissues by IHC, we found prominent PrPd deposits in those animals that displayed the classical scrapie phenotype by WB (see Fig. S1 in the supplemental material). Although the target nuclei for the diagnosis of classical scrapie in the obex region were not available, adjacent structures in the medulla oblongata and the pons were most severely affected (see Table S1 in the supplemental material). PrPd deposits were identified in the gray matter and were of different morphological types: intraneuronal, perineuronal, punctuate, glial, stellate, granular, linear, perivascular, subpial, and plaque-like. Again, these findings were consistent with the phenotype of classical scrapie in sheep and goats. In contrast, in the goats that displayed the truncated ∼12-kDa PrPres by WB, we could not detect PrPd deposits in any of the brain structures by our standard protocol using MAbs F99/97.6.1, P4, and 12B2 (Table S1 and data not shown).
Impact of tissue autolysis on the molecular PrPres phenotype.
By histopathological examination of formalin-fixed brain tissues (see Table S1 in the supplemental material) and by visual inspection of the frozen tissues (including the medulla oblongata samples), we found no significant differences in the degrees of tissue autolysis between the goats affected by classical scrapie and those with the ∼12-kDa PrPres peptide. To address this point experimentally, we spiked severely autolytic TSE-negative brain tissues with classical- and atypical-scrapie-positive tissue macerates and subjected these to further autolysis. Neither in classical nor in atypical scrapie PrPres did the banding pattern change after 1 week of forced autolysis under laboratory conditions. In particular, there was no evidence that classical and atypical scrapie PrPres fragments were degraded (Fig. 4).
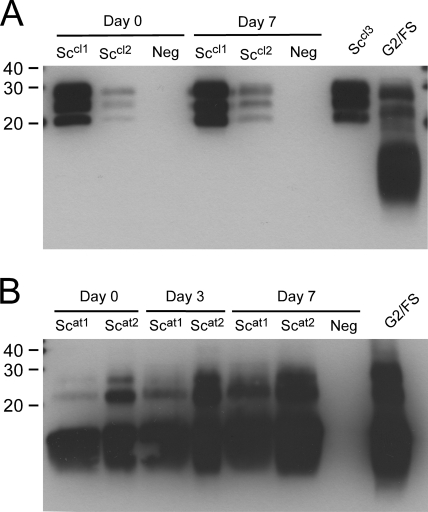
Fig. 4.
Tissue autolysis and PrPres phenotype. A severely autolytic TSE-negative brain tissue macerate (Neg) was spiked with classical scrapie (Sccl1 and Sccl2) (A) and atypical scrapie (Scat1 and Scat2) (B) brain tissue macerates and subjected to further autolysis for 7 days. Samples were analyzed by NC-WB with MAb P4. Severely autolytic tissue samples from a Swiss classical sheep scrapie case (Sccl3) and an atypical goat scrapie case (S2/FS) are included as controls.
PRNP genotyping.
Five out of the 10 goats with the aberrant PrPres fragment carried Q211 and/or K222 polymorphisms (Table 1). The proportions of these animals in the examined population were 8 and 12 out of 86, respectively. A statistical comparison revealed that the genotypic frequencies for Q/K222 and S/P240 did not differ significantly between goats that showed the ∼12-kDa PrPres and those that did not (P > 0.05). In contrast, the Q211 polymorphism was statistically associated with goats that displayed the single ∼12-kDa PrPres (P = 0.0258).
DISCUSSION
Here we show that for the majority of the affected goats in the flock under investigation, the PrPres WB pattern and the neuroanatomical PrPd deposition are similar to those described for classical scrapie in sheep. However, for a series of goats we found a unique PrPres fragment of ∼12 kDa that shares some features of a PrPres fragment observed for atypical scrapie. Both fragments were indistinguishable in their apparent molecular masses but differed in their C-terminal truncations. Based on epitope mapping, we calculated the molecular mass of the aberrant PrPres fragment in the Greek goats to be 4.2 kDa to 9.8 kDa. In the literature, there is some disagreement regarding the molecular mass of the prominent PrPres fragment in atypical scrapie. While in some studies, as in the present one, it was estimated to be ∼10 to 12 kDa (2, 13, 14), in others (19, 21, 24) it was found to migrate at ∼7 to 8 kDa. It is likely that these estimates are biased by the use of different molecular mass standards and electrophoresis conditions. Indeed, the ∼12-kDa band in the atypical scrapie control sample (G2/FS) migrated at ∼7 to 8 kDa when we used the same standard as that applied previously (21). This finding argues for an overestimation of the molecular masses in our experimental setup. The finding that the fragment in atypical scrapie extents more to the PrPres C terminus than the one in the aberrant Greek goats, but reveals a similar molecular mass, indicates that this difference is composed of only a few amino acids and could not be resolved by the SDS-PAGE system that we used. Alternatively, both fragments may also differ in their N-terminal truncations; however, this was difficult to investigate by epitope mapping, because the N terminus is predicted to fall in the PrP octapeptide repeat region (residues 54 to 89), which provides three bindings sites for MAb SAF32 (Fig. 3). Atypical scrapie has been shown to involve additional PrPres bands in the range of 15 to 30 kDa, representing unglycosylated and glycosylated forms of at least one more PrPres fragment of a higher molecular mass (2, 21), but these were not detected in the aberrant Greek goats. Moreover, in atypical goat scrapie, PrPres was found to accumulate primarily in cerebral structures and only to a minimal extent in the medulla oblongata (26). This situation was reflected in three of the these goats, in which the aberrant PrPres fragment was identified only in the cerebral cortex, but in the others, it was most prominent in the medulla oblongata, which is rather characteristic for classical scrapie.
One limitation of our study is that other phenotypic features, such as histopathological lesions and IHC PrPd deposition patterns, could not be properly assessed, because formalin-fixed medulla oblongata tissues were not available, and for the remaining brain structures, the interpretation of histopathological lesions was hampered by tissue autolysis. While for most of the classical-scrapie-affected goats the disease was unambiguously confirmed by IHC of brain tissue sections, we failed to confirm disease for the goats that displayed the ∼12-kDa band. In atypical scrapie, in contrast to classical scrapie, we observed previously that extended tissue storage in formalin eventually results in a loss of the PrPd IHC signal (A. Oevermann, unpublished data). The tissues available for the present study had been stored for ∼2 years in formalin prior to our analysis, and therefore, a similar effect might have occurred.
A crucial point is whether tissue autolysis led to the observed PrPres truncation. NC-WB was validated previously in our laboratory by using 100 confirmed TSE-negative medulla oblongata samples of fallen goats that had been collected in the frame of active TSE surveillance (15). The majority of these samples were at least moderately autolytic (autolysis score, ≥3). However, the ∼12-kDa band emerged in none of them (data not shown), indicating that tissue autolysis does not compromise the specificity of the NC-WB assay in this respect. However, the aberrant goats may also be atypical scrapie cases in which the PrPres pattern has changed or classical scrapie cases in which the ∼12-kDa isoform was generated from the longer isoforms by degradation. In our experimental setup, PrPres degradation was not observed under laboratory conditions. In addition, several lines of evidence indicate that these scenarios are unlikely. First, the degrees of tissue autolysis were similar for samples of classical-scrapie-affected goats and those with the ∼12-kDa PrPres; second, the truncated fragment was also identified in brain structures that were sampled on the first day postmortem; third, with the exception of one particular goat (G22), in none of the samples did the truncated fragment co-occur with the PrPres triplet of classical scrapie; and fourth, the ∼12-kDa fragment was also detected in brain regions other than the medulla oblongata, which were only moderately autolytic. Altogether, this argues against the truncated ∼12-kDa PrPres fragment being an autolysis artifact and supports our notion that the disease phenotype in these animals is inconsistent with the current phenotype definition of classical and atypical scrapie.
The absence of residues 153 to 155 in the truncated PrPres fragment of the aberrant Greek goats has consequences for its detectability in small-ruminant TSE surveillance, because many tests use MAbs that bind to epitopes that are not covered by the truncated fragment. This counts for MAbs L42 and Sha31, both of which were used in the present study, and others that bind to more C-terminal PrPres regions. Reference laboratories may take this situation into account when either of these MAbs is used for confirmatory WB procedures. Information on the binding sites of antibodies applied in the screening tests is not available in the public domain, but our data suggest that the PrioStrip SR assay in principle detects this fragment, while the TeSeE ELISA, one of the tests used most widely in the European Union, does not. Therefore, such animals may escape active surveillance, depending on the screening tests and the confirmatory tests applied.
The disease phenotype in TSEs depends on host- and agent-related factors. One prominent host factor is the PRNP genotype. Interestingly, 5 out of the 10 aberrant Greek goats carried the K222 and/or the Q211polymorphism, with the latter being associated with those animals with the 12-kDa fragment. These findings deserve attention, as both polymorphisms have been associated with resistance to classical scrapie in goats (1, 4, 8, 31). Due to the low numbers of animals in the aforementioned studies and in the present report, the statistical significance is weak, and it would be premature to draw any further conclusions on this.
From the epidemiological perspective it is noteworthy that the truncated PrPres fragment was found in a series of animals that were held in close contact in the same flock and that this flock comprised a high proportion of classical-scrapie-affected goats. This condition may therefore be contagious and additionally may be etiologically related to classical scrapie. The diversity of TSE phenotypes also depends on the occurrence of different prion strains in a given host population. For classical scrapie it was postulated that at least 20 strains can be discriminated based on their phenotypic features in mouse transmission studies (9). Recently, transmission studies of scrapie isolates in transgenic mice indicated that some isolates contained multiple prion strains, which resulted in different disease phenotypes, including distinct PrPres banding patterns (29, 34). Therefore, the Greek flock may have been infected by an isolate that was composed of more than one prion strain or by two different strains simultaneously, and either of these strains emerged in individual goats. Interestingly, we identified one goat (G22) that showed both the classical scrapie and the truncated PrPres banding patterns in different brain regions. Whether this resulted from a coinfection or from prion strain mutation remains difficult to assess. In the latter case, this animal may be at the origin of the phenotypic diversity of this TSE outbreak.
Several studies have pointed to similarities in the molecular PrPres phenotypes between atypical scrapie in small ruminants and Gerstmann-Sträussler-Scheinker disease (GSS), a genetic TSE in humans (3, 7, 14, 19, 21). Similar to the Greek goats, in some GSS cases this is the only detectable PrPres moiety. Parchi and coworkers previously determined by amino acid sequencing and mass spectrometry that the N terminus of the ∼7- to 8-kDa PrPres fragment in patients with GSS varies between residues 78 and 82 and that its C terminus lies in the range of residues 147 to 153 (23). Therefore, the truncated PrPres fragments in atypical scrapie, the aberrant Greek goats, and GSS appear to map to the same region of the prion protein. The underlying pathogenic mechanisms for the formation of these fragments, however, need to be addressed by further work.
In conclusion, the present study reveals novel aspects of the nature and diversity of the molecular PrPres phenotypes in TSE-affected goats. The identification of a distinct C- and N-terminally truncated PrPres fragment in a proportion of goats suggests that these animals display a previously unrecognized prion protein disorder, with consequences for small-ruminant TSE surveillance. However, potential implications for public and animal health remain to be established. Transmission studies have now been initiated to confirm that this condition involves a transmissible agent.
Supplementary Material
ACKNOWLEDGMENTS
We thank Michaela Gsponer and Valerie Juillerat for their excellent technical assistance.
Florian Lörtscher was supported by the BNF Swiss qualification program. The NeuroCentre is partially funded by the Swiss Federal Veterinary Office.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 30 March 2011.
REFERENCES
- 1. Acutis P. L., et al. 2006. Identification of prion protein gene polymorphisms in goats from Italian scrapie outbreaks. J. Gen. Virol. 87:1029–1033 [DOI] [PubMed] [Google Scholar]
- 2. Arsac J. N., et al. 2007. Similar biochemical signatures and prion protein genotypes in atypical scrapie and Nor98 cases, France and Norway. Emerg. Infect. Dis. 13:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arsac J. N., Biacabe A. G., Nicollo J., Bencsik A., Baron T. 2007. Biochemical identification of bovine spongiform encephalopathies in cattle. Acta Neuropathol. 114:509–516 [DOI] [PubMed] [Google Scholar]
- 4. Barillet F., et al. 2009. Identification of seven haplotypes of the caprine PrP gene at codons 127, 142, 154, 211, 222 and 240 in French Alpine and Saanen breeds and their association with classical scrapie. J. Gen. Virol. 90:769–776 [DOI] [PubMed] [Google Scholar]
- 5. Baron T. G., Madec J. Y., Calavas D., Richard Y., Barillet F. 2000. Comparison of French natural scrapie isolates with bovine spongiform encephalopathy and experimental scrapie infected sheep. Neurosci. Lett. 284:175–178 [DOI] [PubMed] [Google Scholar]
- 6. Benestad S. L., Arsac J. N., Goldmann W., Noremark M. 2008. Atypical/Nor98 scrapie: properties of the agent, genetics, and epidemiology. Vet. Res. 39:19. [DOI] [PubMed] [Google Scholar]
- 7. Benestad S. L., et al. 2003. Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Vet. Rec. 153:202–208 [DOI] [PubMed] [Google Scholar]
- 8. Bouzalas I. G., et al. 2010. Caprine PRNP polymorphisms at codons 171, 211, 222 and 240 in a Greek herd and their association with classical scrapie. J. Gen. Virol. 91:1629–1634 [DOI] [PubMed] [Google Scholar]
- 9. Bruce M. E., et al. 2002. Strain characterization of natural sheep scrapie and comparison with BSE. J. Gen. Virol. 83:695–704 [DOI] [PubMed] [Google Scholar]
- 10. Bruce M. E., et al. 1997. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 389:498–501 [DOI] [PubMed] [Google Scholar]
- 11. Eloit M., et al. 2005. BSE agent signatures in a goat. Vet. Rec. 156:523–524 [DOI] [PubMed] [Google Scholar]
- 12. Feraudet C., et al. 2005. Screening of 145 anti-PrP monoclonal antibodies for their capacity to inhibit PrPSc replication in infected cells. J. Biol. Chem. 280:11247–11258 [DOI] [PubMed] [Google Scholar]
- 13. Gavier-Widen D., et al. 2004. Recognition of the Nor98 variant of scrapie in the Swedish sheep population. J. Vet. Diagn. Invest. 16:562–567 [DOI] [PubMed] [Google Scholar]
- 14. Gretzschel A., Buschmann A., Langeveld J., Groschup M. H. 2006. Immunological characterization of abnormal prion protein from atypical scrapie cases in sheep using a panel of monoclonal antibodies. J. Gen. Virol. 87:3715–3722 [DOI] [PubMed] [Google Scholar]
- 15. Hausermann C., et al. 2010. Surveillance and simulation of bovine spongiform encephalopathy and scrapie in small ruminants in Switzerland. BMC Vet. Res. 6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hill A. F., et al. 1997. The same prion strain causes vCJD and BSE. Nature 389:448–450, 526 [DOI] [PubMed] [Google Scholar]
- 17. Hope J., et al. 1999. Molecular analysis of ovine prion protein identifies similarities between BSE and an experimental isolate of natural scrapie, CH1641. J. Gen. Virol. 80:1–4 [DOI] [PubMed] [Google Scholar]
- 18. Jeffrey M., et al. 2006. Immunohistochemical features of PrP(d) accumulation in natural and experimental goat transmissible spongiform encephalopathies. J. Comp. Pathol. 134:171–181 [DOI] [PubMed] [Google Scholar]
- 19. Klingeborn M., et al. 2006. Characterization of proteinase K-resistant N- and C-terminally truncated PrP in Nor98 atypical scrapie. J. Gen. Virol. 87:1751–1760 [DOI] [PubMed] [Google Scholar]
- 20. Langeveld J. P., et al. 2006. Rapid and discriminatory diagnosis of scrapie and BSE in retro-pharyngeal lymph nodes of sheep. BMC Vet. Res. 2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nentwig A., et al. 2007. Diversity in neuroanatomical distribution of abnormal prion protein in atypical scrapie. PLoS Pathog. 3:e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nonno R., et al. 2003. Molecular analysis of cases of Italian sheep scrapie and comparison with cases of bovine spongiform encephalopathy (BSE) and experimental BSE in sheep. J. Clin. Microbiol. 41:4127–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parchi P., et al. 1998. Different patterns of truncated prion protein fragments correlate with distinct phenotypes in P102L Gerstmann-Straussler-Scheinker disease. Proc. Natl. Acad. Sci. U. S. A. 95:8322–8327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Polak M. P., et al. 2010. Diagnosis of the first cases of scrapie in Poland. Vet. J. 186:47–52 [DOI] [PubMed] [Google Scholar]
- 25. Prusiner S. B. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136–144 [DOI] [PubMed] [Google Scholar]
- 26. Seuberlich T., et al. 2007. Atypical scrapie in a Swiss goat and implications for transmissible spongiform encephalopathy surveillance. J. Vet. Diagn. Invest. 19:2–8 [DOI] [PubMed] [Google Scholar]
- 27. Seuberlich T., et al. 2009. Field performance of two rapid screening tests in active surveillance of transmissible spongiform encephalopathies in small ruminants. J. Vet. Diagn. Invest. 21:97–101 [DOI] [PubMed] [Google Scholar]
- 28. Stack M. J., Chaplin M. J., Clark J. 2002. Differentiation of prion protein glycoforms from naturally occurring sheep scrapie, sheep-passaged scrapie strains (CH1641 and SSBP1), bovine spongiform encephalopathy (BSE) cases and Romney and Cheviot breed sheep experimentally inoculated with BSE using two monoclonal antibodies. Acta Neuropathol. 104:279–286 [DOI] [PubMed] [Google Scholar]
- 29. Thackray A. M., Hopkins L., Lockey R., Spiropoulos J., Bujdoso R. 26 January 2011, posting date Emergence of multiple prion strains from single isolates of ovine scrapie. J. Gen. Virol. doi:10.1099/vir.0.028886-0 [DOI] [PubMed] [Google Scholar]
- 30. Thuring C. M., et al. 2005. Immunohistochemical distinction between preclinical bovine spongiform encephalopathy and scrapie infection in sheep. J. Comp. Pathol. 132:59–69 [DOI] [PubMed] [Google Scholar]
- 31. Vaccari G., et al. 2006. Identification of an allelic variant of the goat PrP gene associated with resistance to scrapie. J. Gen. Virol. 87:1395–1402 [DOI] [PubMed] [Google Scholar]
- 32. Vaccari G., et al. 2009. State-of-the-art review of goat TSE in the European Union, with special emphasis on PRNP genetics and epidemiology. Vet. Res. 40:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Will R. G., et al. 1996. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347:921–925 [DOI] [PubMed] [Google Scholar]
- 34. Yokoyama T., et al. 2010. Intraspecies prion transmission results in selection of sheep scrapie strains. PLoS One 5:e15450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.