Abstract
The human polyomavirus JC virus (JCV) is the agent of progressive multifocal leukoencephalopathy (PML). It has also recently been involved in cerebellar atrophy. Factors involved in this entity are elusive. We present a case of a human immunodeficiency virus (HIV)-infected patient with PML and cerebellar atrophy. In addition to a compartmentalization of JCV strains between urine, cerebrospinal fluid, and cerebellum, specific rearrangements in the JCV regulatory region were observed in the cerebellum, resulting in alterations of transcription factor binding sites. Our data underline the importance of searching for JCV in HIV-infected patients with cerebellar disorders and suggest that mutations in the regulatory region may be involved in cerebellar degeneration.
INTRODUCTION
Cerebellar disorders are uncommon among patients infected with human immunodeficiency virus (HIV) and include various etiologies (2). The polyomavirus JC virus (JCV) is the causative agent of progressive multifocal leukoencephalopathy (PML), with lesions usually restricted to the white matter. In a few cases, PML has also been associated with alterations in the gray matter. JCV has recently been involved in a neuronal infection restricted to the granule layer of the cerebellum (3, 9, 11) and is now considered the etiology of a new entity named JC virus granule cell neuronopathy (GCN). The factors involved in the development of JCV-associated neuronal disorders are poorly understood. In patients with PML, sequence variations in the JC virus capsid protein VP1 and in the regulatory region (RR) which regulates early and late viral gene expression have been shown to occur in cerebral JCV isolates (7, 14, 17, 18, 19). Viral characteristics associated with JCV cerebellar disorders remain to be explored. We describe here the case of an HIV-infected patient presenting with PML and cortical atrophy of the cerebellum associated with JCV infection. We present data on the genetic rearrangements occurring in the regulatory region of the specific cerebellum JCV isolate which result in transcription factor binding sites alterations.
CASE REPORT
A 38-year-old HIV-positive man was admitted on 27 June 2008 for subacute onset of a cerebellar disorder. HIV infection had been diagnosed 2 years earlier with cachexia due to disseminated Mycobacterium avium infection. His medical history included several episodes of cytomegalovirus reactivation, oropharyngeal candidiasis, and anal condyloma. He had no history of alcohol intake. Since the initiation of highly active antiretroviral therapy (HAART) (lopinavir, ritonavir, emtricitabine, and tenofovir) in 2007, plasma HIV RNA had remained undetectable for 8 months but his absolute CD4+ lymphocyte count always remained below 50/mm3 and he had uncontrolled mycobacterial intra-abdominal infection.
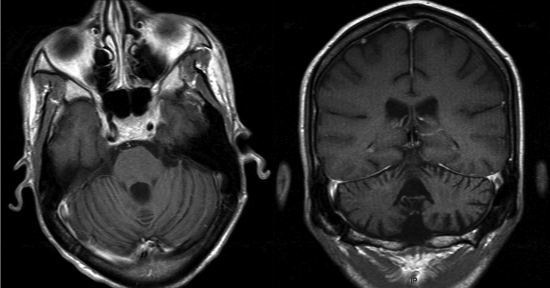
On 27 June, the clinical examination showed evidence of a pancerebellar syndrome, including severe ataxia, kinetic tremor, central nystagmus, and major dysarthria. The patient was unable to stand unassisted. Muscle stretch reflexes were brisk, and plantar responses were flexor bilaterally. Sensory examination was normal. Brain magnetic resonance imaging (MRI) revealed mild, generalized cerebral atrophy with prominent cerebellar atrophy involving midline and hemispheric structures, with an enlarged fourth ventricle (Fig. 1). The fluid attenuated inversion recovery (FLAIR) sequence revealed few white matter abnormalities. No focal abnormalities were observed. His absolute CD4+ lymphocyte count was 39/mm3, and his plasma HIV RNA level was <50 copies/ml. Molecular analysis for viral etiologies showed in cerebrospinal fluid (CSF) the absence of HIV, cytomegalovirus, varicella-zoster virus, herpes simplex virus type 1 and type 2, Epstein-Barr virus, human herpesvirus 6, and BK virus, along with the absence of syphilis antibodies. In contrast, JCV DNA detection in CSF was positive by a real-time PCR assay targeting the large T antigen (6). In CSF, neuronal autoantibodies were negative. Vitamin plasma levels were within the normal range. JCV DNA was also detected in urine. Quantities of JCV DNA, performed in CSF (27 August 2008), in urine (2 October 2008), and in a postmortem cerebellum biopsy specimen (14 October 2008), were 4.7, 6.80, and 8.08 log10 copies per 150,000 cells, respectively. Despite the continuation of HAART, his CD4+ lymphocyte count remained unchanged and the patient's clinical status progressively worsened with cachexia, major asthenia, and confusion. He died a few months later.
Fig. 1.
Cerebellar MRI. Axial no-contrast T1-weighted (left panel) and coronal contrast-enhanced T1-weighted (right panel) MR images show atrophy of the vermis and cerebellar hemispheres with enlargement of the 4th ventricle.
MATERIALS AND METHODS
Neuropathologic examination.
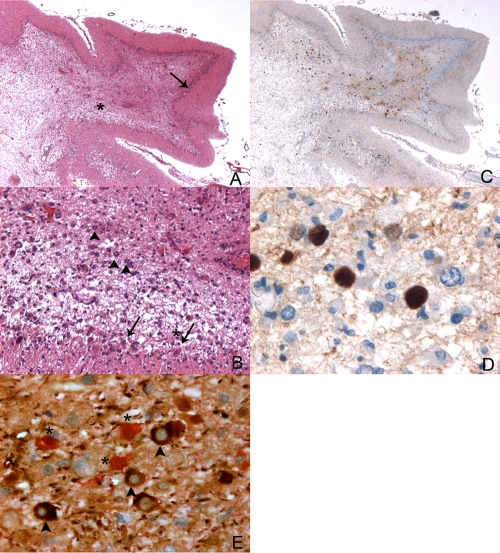
Autopsy was obtained according to current rules, including interrogation of the National Register (Agence de la Biomédecine), and information was given to next of kin. After fixation, sampling, paraffin embedding, and standard histology techniques, immunohistochemistry for polyomavirus was performed on cerebellar slides using a rabbit polyclonal antibody to large T antigen, provided by D. L. Walker, University of Wisconsin Medical School, Madison, WI (dilution, 1/500 overnight). A double labeling was performed using a monoclonal mouse anti-human, 2F11 clone (Dako), associated with the antipolyomavirus antibody, in a Ventana automat (Fig. 2E). Detection was made with a Ventana Ultraview DAB apparatus for neurofilaments and a Dako REAL detection system kit for polyomavirus.
Fig. 2.
Polyomavirus JC infection of cerebellar granule cell layer. (A) Cerebellum (hematoxylin-eosin). Magnification, ×20. Severe alteration of the white matter (*) associated with dramatic atrophy of the granular layer (arrow). (B) Same field as panel A at higher magnification (hematoxylin-eosin). Magnification, ×100. Purkinje cells are still recognizable (arrow), contrasting with the dramatic loss of granule cells (*). In the deepest part of the granular layer, some enlarged cells are reminiscent of modified oligodendrocytes (arrowheads). (C) Same field as panel A (polyomavirus immunohistochemistry). Magnification, ×20. The dot-like labeling is predominant in the white matter. (D) Same field as panels A and B (polyomavirus immunohistochemistry). Magnification, ×400. The enlarged nuclei of modified oligodendrocytes are labeled with the antipolyomavirus antibody. (E) Coimmunostaining of polyomavirus and neurofilaments. Magnification, ×400. Polyomavirus-infected cells are stained in red (*) and neurofilaments in brown (arrowheads).
Analysis of VP1 and RR sequences.
Nucleic acids were extracted from urine, CSF, and cerebellum samples using an EasyMag system (bioMérieux) and were used to amplify the VP1 and RR genes as previously described (1, 13, 18). Purified PCR products were sequenced using an ABI 3100 genetic analyzer (Applied Biosystems). Sequences were aligned using Sequence Navigator software (version 1.0.1; Applied Biosystems). This approach enables the detection of predominant variants in each site with a 20% limit of detection for minor variants.
RESULTS
Macroscopic examination of the cerebellum showed severe atrophy with dramatic cortical thinning in both the vermis and cerebellar hemispheres. Microscopically, there was a loss of granular neurons throughout the cerebellum with sparing of the Purkinje and the molecular layers. Moreover, the white matter presented severe lesions, with enlarged bizarre astrocytes and enlarged oligodendrocyte nuclei. Immunohistochemistry stains for polyomavirus proved the diagnosis of PML with nuclear positivity of astrocytes and oligodendrocytes (Fig. 2). Costaining of JCV and neuronal cells found no evidence of infected granule cell neurons (Fig. 2E) but showed a juxtaposition of infected JCV cells and neurofilament-positive cells, in the context of an extensive destruction of the granule cell layer. This observation favors cytotoxicity from satellite oligodendrocytes rather than direct infection of granule cell neurons, although the latter hypothesis cannot be ruled out due to the extent of the damage.
Sequence variations in VP1 and RR from urine, CSF, and cerebellum samples were analyzed to look at the compartmentalization of the virus and to assess a possible strain specificity associated with cerebellar atrophy. Phylogenetic analysis based on VP1 sequences indicated that the strain was related to genotype 5, while the RR sequence was closely related to genotype 1A (data not shown).
Considering the region of VP1 involved in cell entry, strains from three compartments were similar, except for a substitution of serine-23 with cysteine in the outer DE loop of the cerebellar strain. Such a mutation has previously been observed in PML cases but is not known to confer new phenotypic characteristics (18). A deletion associated with a frameshift in the C-terminal sequence of the VP1 gene has also been previously observed in a patient with cerebellar atrophy and was hypothesized to be associated with modifications in viral postentry events. In our case, the C-terminal region of VP1 showed no major difference with PML JC virus variants (1). Only two substitutions, methionine-339 with isoleucine and lysine-345 with arginine, which do not change amino acid properties, were present in all sequences.
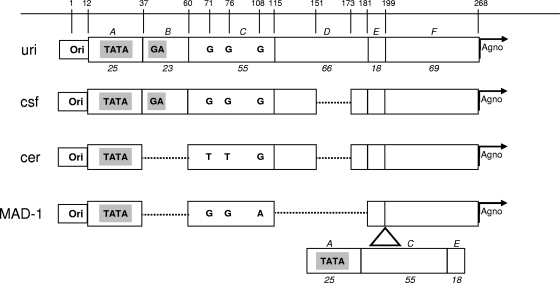
The sequence analysis of RR showed three distinct strains in urine, CSF, and cerebellum. The successive loss of genetic sequences suggests a genetic drift from urine to CSF and ultimately to the cerebellum (Fig. 3). The sequences in the cerebellum and CSF had a deletion of 22 bp compared to the sequence from urine, and the sequence in the cerebellum had another additional 23-bp deletion. According to the previously described classification of RR forms (8), the RR from urine was of type II-S with a single 98-bp unit and one 23-bp insert (block B) and one 66-bp insert (block D), as seen in the JCV archetype. The cerebellum and CSF RRs had a similar form but had one deletion of 22 bp in block D. The cerebellar sequence had an additional 23-bp deletion corresponding to block B 7 bp downstream of the TATA box (nucleotides 37 to 59), like the JCV Mad1 reference strain (4), and encompassing the GA box, which is the binding site of the Sp1 transcription factor. Interestingly, this deletion has resulted in a new motif (AGGGAAGGGA) called the lytic control element (LCE) (12), creating a binding site for Pur alpha and YB. In addition, two other mutations at nucleotides 71 (G → T) (archetype numbering) and 76 (G → T) disrupted the binding sites for the NF-1 nuclear factor and the GF-1 glial factor, respectively (15). The binding site for the AP-1 activating transcription factor was maintained. The three isolates showed the absence of tandem repeat of the 98-bp element, usually seen in JCV isolates recovered from the central nervous system of PML patients.
Fig. 3.
Sequencing results of JCV RR between strains detected in urine (uri), cerebrospinal fluid (csf), and cerebellum (cer). The nucleotide numbers indicated on top are based on the archetype sequence (8). The regulatory region sequence section contains the origin of DNA replication (Ori), followed by sequence sections designated A, B, C, D, E, and F in italics. The base-pair length of each section is noted in italic numbers (8). The TATA box and the GA box are represented by TATA and GA in gray boxes, respectively. The start of Agnogene is indicated by an arrow labeled “Agno.” Dotted lines represent deletions or regions not present. The representation of the Mad1 sequence (MAD-1) is shown at the bottom. The location of the 98-bp unit repeat insert (including sections A, C, and E) is indicated by a triangle. Single letters in boldface indicate nucleotides.
DISCUSSION
Our patient experienced extensive cerebellar atrophy involving the granule cell layer which was concomitant with the development of PML. Except for JCV infection, no other etiology was found to explain cerebellar disorders. The high cerebellum viral load, the unique pattern of genetic mutations in RR, and the JCV immunostaining on a cerebellar biopsy specimen suggested that JCV was associated with a granule cell neuronopathy. JCV is known to productively infect astrocytes and oligodendrocytes, resulting in demyelinated white matter. Cerebellar atrophy, which implies neuronal lesions, is absent in typical PML. It has recently been shown among HIV-infected patients that latent JCV infection of cerebellar GCNs was actually frequent (16). The mechanisms leading to JCV lytic replication of GCNs and then to cerebellar atrophy are not determined.
Amino acid substitutions (at positions 55, 60, 265, 267, and 269) on the surface of the VP1 capsid protein associated with typical PML (14) or the deletion in the C-terminal portion of VP1 described in a patient with PML and GCN (1) was not found in this case. In contrast, in the present case, the few substitutions observed in the cerebellar strain do not support a specific phenotype due to altered VP1 protein. The RR sequence analysis showed a compartmentalization of JCV and suggested a progressive genetic drift from urine to CSF and finally to cerebellum. RRs from CSF and cerebellum had partial deletion of block D, like some other RR sequences from HIV-infected patients with PML that have been associated with increased early gene expression and higher JCV replication in vitro (5), which is consistent with the high viral load observed in the cerebellum for this patient. In contrast to previous results from patients suffering from JCV granule cell neuronopathy, the cerebellum strain showed a specific deletion in the RR encompassing the binding site of Sp1, known to be a strong inducer of JCV expression in glial cells. The deletion has resulted in the creation of a binding site for Pur alpha and YB. Although these two factors also have the ability to interact with the 23-bp sequence element deleted in the cerebellar isolate, it has been shown that their interaction with the LCE had more of an effect on early and late gene transcription (12). Notably, it has been shown in vivo that block B deletion is associated with a higher cerebrospinal fluid JCV DNA level (10). Except in the Mad1 strain, this deletion has previously been reported only in one patient with PML with no evidence of cerebellar atrophy but pseudobulbar paralysis (19). In addition, two other mutations in the cerebellar JCV sequence abolished two binding sites for transcription factors, including the binding site for NF, considered a major transcription factor of JCV. Although in vitro studies have demonstrated that the number of NF binding sites is proportional to the level of JCV transcription in glial cell lines, an in vivo analysis including 45 patients has shown, in contrast, that the number of NF binding sites is negatively correlated with the JCV load (10). The sequencing results, without excluding the presence of minor variants, clearly demonstrate that the majority of cerebellar strains harbor mutations in the regulatory region not observed or underrepresented in other compartments. The specific effects of such rearrangements on cerebellar pathogenesis and GCN infection deserve further investigation. In conclusion, our observation underlines the importance of ascertaining whether JCV is present in HIV-infected patients with cerebellar disorders even when gray matter is mainly affected and shows that mutations in the regulatory region may be involved in cerebellar atrophy.
ACKNOWLEDGMENTS
There was no financial support.
We do not have any conflicts of interest to declare.
Footnotes
Published ahead of print on 23 March 2011.
REFERENCES
- 1. Dang X., Koralnik I. J. 2006. A granule cell neuron-associated JC virus variant has a unique deletion in the VP1 gene. J. Gen. Virol. 87:2533–2537 [DOI] [PubMed] [Google Scholar]
- 2. Del Valle L., Pina-Oviedo S. 2006. HIV disorders of the brain: pathology and pathogenesis. Front. Biosci. 11:718–732 [DOI] [PubMed] [Google Scholar]
- 3. Du Pasquier R. A., et al. 2003. Productive infection of cerebellar granule cell neurons by JC virus in an HIV+ individual. Neurology 61:775–782 [DOI] [PubMed] [Google Scholar]
- 4. Frisque R. J., Bream G. L., Cannella M. T. 1984. Human polyomavirus JC virus genome. J. Virol. 51:458–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gosert R., Kardas P., Major E. O., Hirsch H. H. 2010. Rearranged JC virus noncoding control regions found in progressive multifocal leukoencephalopathy increase virus early gene expression and replication rate. J. Virol. 84:10448–10456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Herman J., et al. 2004. Polyomavirus infection in pediatric renal transplant recipients: evaluation using a quantitative real-time PCR technique. Pediatr. Transplant. 8:485–492 [DOI] [PubMed] [Google Scholar]
- 7. Iida T., et al. 1993. Origin of JC polyomavirus variants associated with progressive multifocal leukoencephalopathy. Proc. Natl. Acad. Sci. U. S. A. 90:5062–5065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jensen P. N., Major E. O. 2001. A classification scheme for human polyomavirus JCV variants based on the nucleotide sequence of the noncoding regulatory region. J. Neurovirol. 7:280–287 [DOI] [PubMed] [Google Scholar]
- 9. Koralnik I. J., et al. 2005. JC virus granule cell neuronopathy: a novel clinical syndrome distinct from progressive multifocal leukoencephalopathy. Ann. Neurol. 57:576–580 [DOI] [PubMed] [Google Scholar]
- 10. Marzocchetti A., et al. 2007. Characterization of JC virus in cerebrospinal fluid from HIV infected patients with progressive multifocal leukoencephalopathy: insights into viral pathogenesis and disease prognosis. J. Neurovirol. 13:338–346 [DOI] [PubMed] [Google Scholar]
- 11. Messam C. A., Hou J., Gronostajski R. M., Major E. O. 2003. Lineage pathway of human brain progenitor cells identified by JC virus susceptibility. Ann. Neurol. 53:636–646 [DOI] [PubMed] [Google Scholar]
- 12. Safak M., Gallia G. L., Khalili K. 1999. Reciprocal interaction between two cellular proteins, Puralpha and YB, modulates transcriptional activity of JCVCY in glial cells. Mol. Cell. Biol. 19:2712–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sugimoto C., et al. 1998. Amplification of JC virus regulatory DNA sequences from cerebrospinal fluid: diagnostic value for progressive multifocal leukoencephalopathy. Arch. Virol. 143:249–262 [DOI] [PubMed] [Google Scholar]
- 14. Sunyaev S. R., Lugovskoy A., Simon K., Gorelik L. 2009. Adaptive mutations in the JC virus protein capsid are associated with progressive multifocal leukoencephalopathy (PML). PLoS Genet. 5:e1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vaz B., Cinque P., Pickhardt M., Weber T. 2000. Analysis of the transcriptional control region in progressive multifocal leukoencephalopathy. J. Neurovirol. 6:398–409 [DOI] [PubMed] [Google Scholar]
- 16. Wuthrich C., et al. 2009. Frequent infection of cerebellar granule cell neurons by polyomavirus JC in progressive multifocal leukoencephalopathy. J. Neuropathol. Exp. Neurol. 68:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yasuda Y., et al. 2003. Comparison of PCR-amplified JC virus control region sequences from multiple brain regions in PML. Neurology 61:1617–1619 [DOI] [PubMed] [Google Scholar]
- 18. Zheng H. Y., et al. 2005. Characterization of the VP1 loop mutations widespread among JC polyomavirus isolates associated with progressive multifocal leukoencephalopathy. Biochem. Biophys. Res. Commun. 333:996–1002 [DOI] [PubMed] [Google Scholar]
- 19. Zheng H. Y., et al. 2005. New sequence polymorphisms in the outer loops of the JC polyomavirus major capsid protein (VP1) possibly associated with progressive multifocal leukoencephalopathy. J. Gen. Virol. 86:2035–2045 [DOI] [PubMed] [Google Scholar]