Abstract
Fusarium is a ubiquitous mold that can cause superficial infections such as keratitis and onychomycosis in immunocompetent humans; however, infections in immunocompromised hosts can be fatal. We report an unusual case of epidural abscess and vertebral osteomyelitis in a patient with an autoimmune disorder who was on long-term glucocorticoids. Multilocus DNA sequence-based typing revealed that the infection was caused by a novel three-locus haplotype of Fusarium falciforme designated FSSC 3+4qqq.
CASE REPORT
A 53-year-old woman with a history of an overlap autoimmune disorder who has been treated with long-term prednisone (10 mg/day) for 9 years was admitted with worsening back pain and progressive inability to walk for several months. She reported trauma to her right posterior thorax during childhood while living in Jamaica. Nine years prior to this admission, she developed a paravertebral abscess associated with a bamboo splinter, which had remained in situ since her childhood injury. The bamboo splinter, which was about 2 in. in length, was removed, and the abscess was evacuated; however, culture data were unavailable.
Two years prior to this admission she noted progressive back pain for several months, and she received an epidural steroid injection. She continued with back pain and presented to an outside hospital. Magnetic resonance imaging (MRI) of the spine showed an epidural mass at the thoracolumbar spine. She underwent debridement of the epidural mass and partial laminectomy of T9 to L3. Intraoperative findings, pathology, and culture revealed an abscess caused by an unidentified species of Fusarium. She was treated with 1 week of intravenous (i.v.) amphotericin B-lipid complex (5 mg/kg every 24 h), followed by 6 months of 400 mg oral posaconazole every 12 h. She improved temporarily but continued to have persistent pain that resulted in the placement of a spinal cord stimulator and a morphine pump. A repeat spinal surgery was planned but was not performed because she sustained a myocardial infarction prior to the surgery. She continued to have pain, had a fall, and ultimately became nonambulatory about 5 months prior to admission and presented to our hospital. There was no bowel or urine incontinence, weight loss, fever, chills, or night sweats. Her medical history also included steroid-induced diabetes which was poorly controlled, hypertension, dyslipidemia, depression, gastroesophageal reflux disease, and morbid obesity.
Physical examination was significant for near paraplegia of the lower extremities, an old healed surgical scar at the thoracolumbar spine, and an unremarkable ophthalmologic and skin examination. Laboratory tests showed a white blood cell count (WBC) of 9,600 cells/μl (range, 4,000 to 10,000), a C-reactive protein level (CRP) of 55 mg/liter (range, 0.3 to 8), and an erythrocyte sedimentation rate (ESR) of 73 mm/h (range, 0 to 30). MRI of the spine revealed an epidural mass in the lower thoracic and upper lumbar spine, T12 and L1 osteomyelitis, myelitis, and arachnoiditis (MRI taken at a later date shown in Fig. 1A).
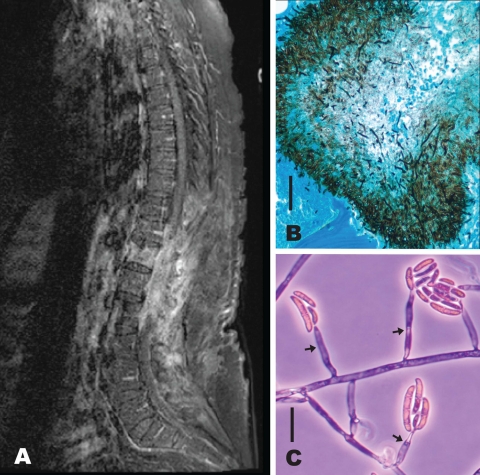
Fig. 1.
(A) MRI (T1 weighted, postcontrast) of the thoracic and lumbosacral spine (limited by motion infarct) shows edema and enhancement of T12 to L2 vertebral bodies, enhancement of soft tissue within the laminectomy defects from T9 through L3 extending into the epidural and intrathecal aspects of the spinal canal, and compression of the conus medullaris. (B) Gomori methenamine silver-stained vertebral body showing fungal elements in the pathology specimen. Bar, 50 μm. (C) Arrows identify monophialidic conidiophores of NRRL 54219 Fusarium falciforme FSSC 3+4qqq from a slide culture showing 0-to-1 septate microconidia and one 4-septate, thick-walled macroconidium. Bar, 20 μm.
The patient underwent a debridement of the epidural mass and a revision laminectomy of T9 to L3. Pathology revealed acute and chronic inflammation with abscess formation and fungal colonies within the bone and the abscess (Fig. 1B). Cultures of debrided tissue were positive for Fusarium (Fig. 1C).
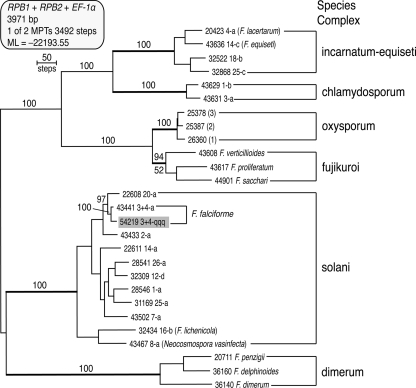
Her isolate was characterized further by subjecting it to two separate three-locus DNA sequence-based typing schemes. The first scheme for placement of an unknown within a species complex within Fusarium included portions of translation elongation factor 1α (EF-1α), the largest subunit of RNA polymerase (RPB1), and second-largest subunit of RNA polymerase (RPB2) (14). Maximum parsimony and maximum likelihood analyses of this data set established that her isolate (NRRL 54219) was nested within the Fusarium solani species complex (Fig. 2, solani). As a result, we employed a separate three-locus scheme for typing species/haplotypes within the solani complex (13), which included partial EF-1α, RPB2, and ITS + 28S rDNA sequences. Molecular phylogenetic analyses of the data revealed that the vertebral isolate represented a novel haplotype of Fusarium falciforme designated FSSC 3+4qqq. Maximum parsimony bootstrapping (BS) indicated that FSSC 3+4qqq was most closely related to two isolates from Florida (BS = 72%), FSSC 3+4tt from a human eye and FSSC 3+4ww from a turtle (reference 13 and unpublished data). Antifungal susceptibility testing using broth dilution according to NCCLS standard M38-A2 (performed at the Fungus Testing Laboratory, University of Texas Health Science Center at San Antonio) showed minimum inhibitory concentrations (MICs) for amphotericin B, voriconazole, and posaconazole of 2, 4, and >16 μg/ml, respectively.
Fig. 2.
One of two equally most-parsimonious trees (MPTs) inferred from a three-locus DNA data set consisting of partial RNA polymerase largest (RPB1) and second-largest (RPB2) subunit and translation elongation factor 1α sequences. The tree was rooted by the midpoint method (19). The best maximum likelihood tree received a negative-log likelihood (−lnL) score of −22193.55 (21) and was identical to the MPT shown. Numbers above nodes represent bootstrap support based on 1,000 maximum parsimony pseudoreplicates of the data. ML bootstrap support is indicated below nodes only if it differed by more than 5% of the maximum parsimony value. The pathogen in this case report (Fusarium falciforme FSSC 3+4qqq) is highlighted in gray. The five-digit strain accession numbers are those of the ARS Culture Collection, Peoria, IL. incarnatum-equiseti, F. incarnatum-equiseti species complex; chlamydosporum, F. chlamydosporum species complex; oxysporum, F. oxysporum species complex; fujikuroi, Gibberella fujikuroi species complex; solani, F. solani species complex; dimerum, F. dimerum species complex.
The patient's postoperative course was complicated by a methicillin-resistant Staphylococcus aureus (MRSA) wound infection that required additional debridements and eventually delayed primary closure. She regained some strength in her lower extremities after surgical decompression and aggressive physical therapy. She was treated with a combination of 500 mg i.v. liposomal amphotericin B (4.6 mg/kg) every 24 h for 12 weeks and 200 mg oral voriconazole every 12 h for 6 weeks. The oral voriconazole dose was increased to 400 mg every 12 h based on a borderline serum level of 2.6 μg/ml (normal range, 2 to 6 μg/ml). The patient inadvertently began taking oral voriconazole 400 mg three times daily. She developed decreased visual acuity and an elevated alkaline phosphatase level that was seven times the normal level. Her serum voriconazole level was 17 μg/ml (normal range, 1 to 5.5 μg/ml), and the oral dose was decreased to 100 mg every 12 h. A few weeks later, she presented with worsening back pain and weakness of the lower extremities. A repeat MRI showed persistent epidural and intrathecal phlegmon, enhancement of T12 to L3 vertebra, and compression of the conus medullaris (Fig. 1A). She was deemed not to be a surgical candidate and was prescribed long-term i.v. liposomal amphotericin B. However, she was unable to receive home i.v. amphotericin infusions due to nonmedical reasons and was placed on 100 mg oral voriconazole every 12 h indefinitely. With continued physical therapy, she regained some strength in her lower extremities and is ambulating short distances with the assistance of a cane.
Fusarium is a saprobic mold with a panglobal distribution; it represents the most important genus of toxin-producing plant pathogens (8). Although members of this genus rarely cause opportunistic infections in humans, at least 69 Fusarium species have been implicated in mycoses of humans and other animals (12, 14). Members of the F. solani species complex account for almost two-thirds of all fusarioses (13). Of the 20 mycosis-associated species within the F. solani complex, F. falciforme appears to be the most common and genetically diverse, in that it is represented by 68 unique three-locus haplotypes (13, 20). Because most of the species within this complex are morphologically cryptic and lack formal Latin binomials, Chang et al. (4) developed a standardized species complex/species/haplotype nomenclatural system in which capitalized roman letters were used to identify the species complex, Arabic numerals to distinguish each phylogenetically distinct species, and lowercase roman letters to identify each unique three-locus sequence type (ST). Following this system, the etiological agent in the present case report was identified as a novel ST within F. falciforme designated FSSC 3+4qqq.
Infection with Fusarium can mimic aspergillosis; hence, early diagnosis is key to successful treatment. In tissue, the hyphae and septate filaments may appear similar to Aspergillus. Fusarium species can be easily cultured from any site, and it is one of the rare molds that will grow readily from blood cultures. Most isolates can be identified as members of this genus by the production of diagnostic fusiform, multicellular macroconidia in culture; however, with the exception of several species within the Gibberella fujikuroi species complex, which can be identified morphologically (reference 15 and references therein), DNA sequencing of a phylogenetically informative locus 1α, such as translation elongation factor is essential for an accurate species-level identification (6, 14). Local debridement and biopsy of bone and culture with histopathologic examination showing septate hyphae are indicative of fungal osteomyelitis. Approximately 40 to 60% of blood cultures are positive for Fusarium in disseminated infections (3). Although a PCR-based method has been developed for the specific detection of Fusarium in tissues and blood (7), this test is not in routine clinical use and it has not been validated using a phylogenetically diverse set of isolates (2).
Fusarium infections in immunocompetent hosts usually result from direct inoculation of skin and soft tissue or ingestion or inhalation of conidia (10, 11). Infection with Fusarium can result in onychomycosis, keratitis, endopthalmitis, and mycetoma. Fusarium keratitis is the most common fungal keratitis worldwide and is typically associated with trauma (5). In contrast to traumatic introduction, the large outbreak of Fusarium keratitis reported in 2006 within the United States appeared to be due to contamination of one particular brand of contact lens solution by end users rather than a common point source during production (4). In our patient, trauma that resulted in a bamboo splinter in the thoracolumbar paraspinal area was the most likely route of inoculation.
Fusarium infections in immunocompromised patients can be acquired via direct inoculation or the sinopulmonary tract. In many cases, the source may remain elusive. Infections may include cellulitis, pneumonia, and fungemia with disseminated skin lesions. Patients at high risk for Fusarium infections include those with hematologic malignancies (3), hematopoietic stem cell transplant recipients with prolonged neutropenia, organ transplant recipients, and those with graft-versus-host disease (12). Our patient was immunocompromised due to prolonged glucocorticoid use and poorly controlled diabetes mellitus.
Fusarium osteomyelitis has been rarely described in the literature. Of the nine cases summarized by Sierra-Hoffman et al. (17), three cases were posttraumatic, four were caused by hematogenous dissemination in leukemic patients, one was postoperative, and one was due to a progressive diabetic foot ulcer. Four of nine cases occurred in immunocompetent hosts. Only one case of vertebral osteomyelitis was reported in a child with diabetes (9). Lower-extremity osteomyelitis caused by Fusarium was treated with a combination of surgical debridement, with or without amputation, and medical approaches (i.e., amphotericin or voriconazole antifungal therapy).
Species of Fusarium are generally resistant to most antifungals (16, 18); however, different species may exhibit various levels of susceptibility to antifungals (1). Fusarium spp. are usually susceptible to amphotericin B, but the MICs can be variable from a range of 0.5 to >16 μg/ml (13). Fusarium spp. generally exhibit high MICs to echinocandins, flucytosine, and azoles, although they may exhibit intermediate susceptibility to voriconazole (MIC, 1 to 8 μg/ml) and posaconazole (1). Correlation between in vitro drug susceptibility and a successful clinical response is unknown, and data on combination antifungal therapy are lacking. Host immune system is the most important factor predicting outcome (12), with high mortality observed in immunocompromised patients (50 to 80%). In all other cases of Fusarium osteomyelitis reported in the literature, death was observed only in immunocompromised leukemic patients (17).
Nucleotide sequence accession numbers.
GenBank accession numbers for the Fusarium falciforme FSSC 3+4qqq (NRRL 54219) sequence data are HQ401721 to HQ401723.
Acknowledgments
Special thanks are due to Stacy Sink for excellent technical assistance and Nathane Orwig for generating the DNA sequences in NCAUR's DNA core facility. At Emory University School of Medicine, special thanks go to Eileen Burd and Ruan Ramjit for assistance with photographs and George Marshall Lyon for helpful advice.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
There are no conflicts of interest or financial support for any authors.
Footnotes
Published ahead of print on 30 March 2011.
REFERENCES
- 1. Alastruey-Izquierdo A., Cuenca-Estrella M., Monzón A., Mellado E., Rodríguez-Tudela J. L. 2008. Antifungal susceptibility profile of clinical Fusarium spp. isolates identified by molecular methods. J. Antimicrob. Chemother. 61:805–809 [DOI] [PubMed] [Google Scholar]
- 2. Balajee S. A., et al. 2009. Sequence-based identification of Aspergillus, Fusarium, and Mucorales species in the clinical mycology laboratory: where are we and where should we go from here? J. Clin. Microbiol. 47:877–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boutati E. I., Anaissie E. J. 1997. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years' experience at a cancer center and implications for management. Blood 90:999–1008 [PubMed] [Google Scholar]
- 4. Chang D. C., et al. 2006. A multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA 296:953–963 [DOI] [PubMed] [Google Scholar]
- 5. Dignani M. C., Anaissie E. 2004. Human fusariosis. Clin. Microbiol. Infect. 10:67–75 [DOI] [PubMed] [Google Scholar]
- 6. Geiser D. M., et al. 2004. FUSARIUM-ID v. 1.0: a DNA sequence database for identifying Fusarium. Eur. J. Plant Pathol. 110:473–479 [Google Scholar]
- 7. Hue F.-X., Huerre M., Rouffault M. A., de Bievre C. 1999. Specific detection of Fusarium species in blood and tissues by a PCR technique. J. Clin. Microbiol. 37:2434–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leslie J. F., Summerell B. A. 2006. The Fusarium laboratory manual. Blackwell Publishing, Ames, IA [Google Scholar]
- 9. Moschovi M., et al. 2004. Subacute vertebral osteomyelitis in a child with diabetes mellitus associated with Fusarium. Pediatr. Int. 46:740–742 [DOI] [PubMed] [Google Scholar]
- 10. Nelson P. E., Dignani M. C., Anaissie E. J. 1994. Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 7:479–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nucci M., Anaissie E. 2002. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin. Infect. Dis. 35:909–920 [DOI] [PubMed] [Google Scholar]
- 12. Nucci M., Anaissie E. 2007. Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 20:695–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Donnell K., et al. 2008. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J. Clin. Microbiol. 46:2477–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Donnell K., et al. 2010. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J. Clin. Microbiol. 48:3708–3718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palmore T. N., Shea Y. R., Childs R. W., Sherry R. M., Walsh T. J. 2010. Fusarium proliferatum soft tissue infection at the site of plant trauma: recovery, isolation, and direct molecular identification. J. Clin. Microbiol. 48:338–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reuben A., et al. 1989. Antifungal susceptibility of 44 clinical isolates of Fusarium species determined by using a broth microdilution method. Antimicrob. Agents Chemother. 33:1647–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sierra-Hoffman M., Paltiyevich-Gibson S., Carpenter J. L., Hurley D. L. 2005. Fusarium osteomyelitis: case report and review of the literature. Scand. J. Infect. Dis. 37:237–240 [DOI] [PubMed] [Google Scholar]
- 18. Sutton D. A., Brandt M. E. Fusarium and other opportunistic hyaline fungi. In Versalovic J., Carroll K. C., Funke G., Jorgensen J. H., Landry M. L., Warnock D. W. (ed.), Manual of clinical microbiology. ASM Press, Washington, DC, in press [Google Scholar]
- 19. Swofford D. L. 2002. PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4. Sinauer Associates, Sunderland, MA [Google Scholar]
- 20. Zhang N., et al. 2006. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microbiol. 44:2186–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zwickl D. J. 2006. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence data sets under the maximum likelihood criterion. Ph.D. dissertation, the University of Texas at Austin, Austin, TX [Google Scholar]