Abstract
We report a case of scleral keratitis caused by Phomopsis phoenicicola. Pterygium surgery was a predisposing factor, and the patient was treated with natamycin and fluconazole eye drops and oral fluconazole. The fungus was identified by sequencing of the internal transcribed spacer (ITS) region of the fungal ribosomal DNA (rDNA) locus and confirmed on the basis of its typical pycnidia and conidia.
CASE REPORT
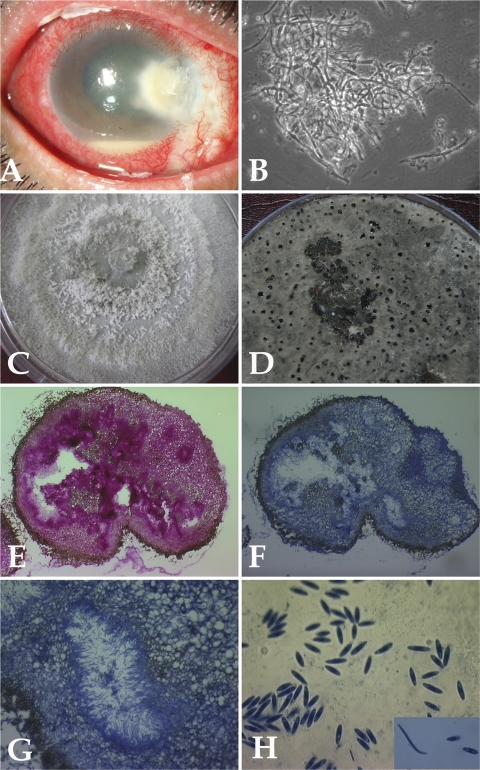
A 48-year-old postman presented with a history of pain, redness, and watering in his right eye since 6 weeks previously. He had undergone pterygium surgery in the same eye 6 weeks ago. The details of the surgical technique and the use of mitomycin C were not available. On examination, his uncorrected visual acuity was just light perception and projection in the right eye and 6/9 in the left eye. Slit lamp examination of the right eye showed a significant area of infiltration (3 to 5 mm) involving the peripheral cornea from the nasal side and extending toward the limbus temporally and superiorly. There was stromal infiltrate surrounding the descemetocele, blood pigments on the endothelium, and a corneal abscess with central thinning. Scleral necrosis and thinning nasally in the area of the pterygium excision were also seen. Diffused stromal haze and significant anterior chamber inflammation with 2-mm hypopyon were evident (Fig. 1A).
Fig. 1.
(A) Slit lamp photograph showing infected cornea involving regions of sclera. (B) Ten percent KOH mount of the scraping material showing fungal hyphae (magnification, ×400). (C) Growth of case isolate P. phoenicicola on PDA showing flat, spreading colony with gray-white sparse aerial mycelium. (D) Growth of case isolate P. phoenicicola on rice flake agar showing pycnidia (black dots) after 4 weeks at 25°C. (E to G) Histological sections of thick-walled pycnidium. (E) Staining with PAS (magnification, ×100). (F) Staining with lactophenol cotton blue (magnification, ×100). (G) Alpha and beta conidia lining the cavity (magnification, ×400). (H) Alpha and beta conidia (magnification, ×1,000).
Corneal scraping material obtained from the infected area was viewed microscopically using a potassium hydroxide (10% KOH)-calcofluor white preparation and Gram staining. It was inoculated on blood agar, potato dextrose agar (PDA), and tryptone soy broth (Himedia Labs Ltd., Mumbai, India) and incubated under appropriate atmospheric conditions. Examination of 10% KOH mounts showed numerous septate and branched hyphae (Fig. 1B).
Antifungal treatment was initiated with topical natamycin eye drops (Natamet; 5% suspension; Sun Pharmaceuticals Ind Ltd., Halol, India), moxifloxacin eye drops (Vigamox; 0.5% suspension; Alcon Laboratories Inc., Texas), and fluconazole eye drops (Zocon; 0.3% suspension; FDC Ltd., Aurangabad, India) hourly and atropine eye drops (0.1% suspension; Taj Pharma Ltd., Mumbai, India) three times a day. The patient was also prescribed Iopar tablets (250 mg to reduce intraocular pressure [IOP]) and an antifungal tablet (fluconazole; 150 mg) once daily.
The corneal scrapings inoculated onto the PDA and blood agar showed the presence of several colonies of a single fungus within 2 days. Fourteen days later, the lesion improved with a decrease in hypopyon. The natamycin and fluconazole therapy was reduced to every 2 h, and tablet fluconazole was continued. Further improvements were noted, and after 1 month the lesion had healed. The infection subsided in 2 months, following which all the medications were discontinued. The patient was left with a scar on the cornea and a best-corrected vision of 6/12.
Mycologic studies.
The isolate (ICIRC-CC58) was subcultured on PDA and Sabouraud dextrose agar (SDA) (Himedia Labs Ltd., Mumbai, India) and incubated at 25°C and 37°C. Growth was apparent within 2 days on both the agar plates at 25°C and 37°C, with optimum growth occurring at 25°C. The colonies were initially whitish-gray and folded. Subsequently, they turned dark gray and spread rapidly, filling the petri dish within 2 weeks (Fig. 1C). By 4 weeks, slightly greenish areas developed. No conidia or asexual fruiting bodies (pycnidia) were seen after 4 weeks of growth on PDA and SDA.
Molecular identification was done by sequencing of the internal transcribed spacer (ITS) and the D2-large subunit (LSU) region of the ribosomal DNA (rDNA) locus. The CTAB (cetyltrimethylammonium bromide) method for isolation of fungal genomic DNA was used (9). Sequencing of the ITS region (incorporating ITS1, the 5.8S rRNA, and ITS2) was done using primers ITS1 and ITS4 (23). DNA sequences were determined at First Base Laboratories Sdn Bhd, Malaysia. Sequencing of the LSU region using the MicroSeq D2-LSU rDNA fungal identification kit (Applied Biosystems) was done at a sequencing facility of the Gujarat State Biotechnology Mission (GSBTM), Government of Gujarat, India. Sequence analysis was carried out by BLASTN similarity search (htt://www.ncbi.nlm.nih.gov/BLAST). The ITS and D2-LSU sequences were submitted to the NCBI database. For identification, only complete ITS1-5.8S-ITS2 entries of reference isolates in the BLAST database were taken into consideration. The ITS sequence of the case isolate (ICIRC-CC58) showed a percent sequence similarity of 100% to Phomopsis phoenicicola (accession no. FJ 889452.1) with a BLAST search expect value of zero. The 100% match indicated the isolate to be a representative of Phomopsis phoenicicola. The D2-LSU sequence showed a maximum similarity score of 95% with the Phomopsis sp. strain MA194 28S rRNA gene (accession no. GU592018) with an expect value of 5E−12.
To confirm the identification, the fungal isolate was inoculated in a different agar medium to observe the production of pycnidia and conidia, a diagnostic characteristic of the genus Phomopsis. Subcultures on rice flake agar, prepared in-house (recommended by Glen Hartman, USDA, National Soybean Research Center, Urbana, IL; personal communication), showed the occurrence of black pycnidia after 4 weeks at 25°C (Fig. 1D). Mature pycnidia were cut from the rice flake agar, placed in 10% formalin, sectioned, and stained with periodic acid-Schiff (PAS) stain and lactophenol blue stain. A stained section revealed conidiomata (Fig. 1E to G) that were ostiolate, thick walled, immersed, and multilocular (more than one cavity) and measured 250 to 500 μm in diameter. Conidia oozing out of crushed pycnidia were collected in a drop of sterile water and stained with lactophenol cotton blue. Single-celled, clear, and oval to fusoid alpha conidia and filiform beta conidia with a characteristic curve were seen (Fig. 1H). The average length and width of 100 randomly selected alpha conidia measured using Biovis Image Plus Software v.4.11 (Expert Vision, Mumbai, India) from digitalized photographs at ×1,000 magnification were 5.52 ± 0.75 μm and 1.68 ± 0.4 μm, respectively. The length and width of 15 randomly selected beta conidia were 19.65 ± 1.5 μm and 0.7 ± 0.08 μm, respectively.
In vitro antifungal susceptibility testing.
Antifungal susceptibility testing for fluconazole, natamycin, and itraconazole was done using a macrodilution method (22). Due to the absence of conidia at the time of antifungal susceptibility testing, the inoculum was prepared spectrophotometrically, by covering the plate with saline, followed by gently probing the colonies with the tip of a pipette and adjusting the densities of the suspensions (read at 530-nm wavelength) to a final inoculum of 0.5 McFarland standard. The final drug concentration ranges were as follows: for fluconazole (Nuflucon; 0.3% suspension; NuLife Pharmaceuticals, Pune, India), 128 to 2,048 μg/ml; for itraconazole (Itral; 1% suspension; Jawa Pharmaceuticals, Gurgaon, India), 16 to 256 μg/ml; for natamycin (Natamet; 5% suspension; Sun Pharmaceuticals Ind Ltd., Halol, India), 2 to 32 μg/ml. All the antifungal agents were tested in RPMI 1640 (Himedia Labs Ltd., Mumbai, India). The results of the in vitro susceptibility tests indicated that the MICs for natamycin, fluconazole, and itraconazole were ≥32 μg/ml, ≥256 μg/ml, and ≥256 μg/ml, respectively.
Opportunistic mycoses are commonly caused by filamentous fungi (Aspergillus spp. and Fusarium spp.) that bear free conidia, but there are increasing reports of cutaneous/subcutaneous and invasive diseases due to coelomycetes, which produce conidia in a fruiting body called conidiomata (19). Mycotic scleral keratitis is a fungal infection of the cornea and sclera that causes ulceration, inflammation, and corneal melting usually following any corneal surgery, pterygium surgery, or surgical trauma. Here we report a case of mycotic scleral keratitis caused by a coelomycete, Phomopsis phoenicicola, following pterygium surgery.
The case was managed according to the protocol of the institute. Treatment was started with topical natamycin (5%) and fluconazole (0.3%). We prefer to use this antifungal agent because most of the isolates in this geographic region are filamentous fungi and natamycin is considered to be the most effective against this group of fungi (2). Based on the severity of infection, intensive oral therapy (fluconazole, 150 mg) was also commenced.
Natamycin and fluconazole showed the highest antifungal activity against the case isolate (ICIRC-CC58). The sensitivity of the present isolate is dissimilar to those of isolates of Phomopsis spp. causing osteomyelitis (20), in which itraconazole was found to be effective, and Phomopsis spp. causing keratitis, in which voriconazole and amphotericin B were found to be effective (7). Natamycin was not tested for its efficacy in both the above-mentioned reports. Since there are no breakpoints for antifungal susceptibility described for this organism, in retrospect we believe systemic amphotericin B would have given early and better prognosis, on the basis of breakpoints described for coelomycetes fungi (19). Fungal scleral keratitis after pterygium removal is not uncommon (1, 6, 10, 11, 17). It has been suggested that scleral necrosis and the avascular milieu increased the risk of fungal keratitis due to the possibility that the fungus thrives in the avascular necrotic tissue (11). Also, in 5 out of 6 reported cases of fungal origin the consequences were severe (1, 10, 11, 17). Thus, fungal keratitis and scleral melting are devastating complications of pterygium surgery, although in the present case, intensive therapy was successful in controlling and eradicating the infection.
Corneal phaeohyphomycosis caused by coelomycetous fungi is mainly due to Colletotrichum spp. (3, 4, 8), Phomopsis spp. (7), and Nattrassia mangiferae (5). The genus Colletotrichum is reported in 33 patients with keratitis from South India (4) and in one case from West India (4, 8). These ulcers were effectively treated with a combined therapy of natamycin and/or amphotericin B with azole, 5-flucytosine or ciprofloxacin, and natamycin and ciprofloxacin. Most authors suggest that since coelomycetes are slow to respond to antifungal therapy, long-term dosing regimens are required for eradication (19). The average duration of medical therapy with natamycin and ciprofloxacin for complete resolution in cases reported from India earlier was 47 ± 14 days (4). This is in concurrence with our treatment, which also lasted for 2 months. Coelomycetes fungi grow easily on various media, but they remain unidentified because ample time and appropriate medium are required for production of pycnidia (19). The difficulty and delay incurred in the identification of the present isolate (ICIRC-CC58) emphasize the importance of referring such isolates for identification using molecular techniques. The ITS regions of fungal ribosomal DNA (rDNA) are widely used for identification of fungal species (23). The case isolate Phomopsis phoenicicola ICIRC-CC58 was identified using ITS sequence and then further confirmed by its typical pycnidia, alpha conidia, and beta conidia. The dimensions of pycnidia and conidia of the case isolate were within the range described for Phomopsis spp. (19), but these morphological characteristics identified only the genus and the features were used only to confirm the sequencing results. Until recently, the genus Phomopsis was largely characterized on the basis of host association, contributing to a huge number of species names. The Species Fungorum database website (http://www.speciesfungorum.org/Index.htm), coordinated by CABI Bioservices, has data collected on more than 900 Phomopsis spp. The identification of Phomopsis by using only morphological characteristics is difficult, and it is now recognized that host is of minor importance (12). Currently, identification is based on molecular phylogenies, especially using sequences of the ITS region of rDNA (15, 21). Therefore, we agree with several authors that molecular techniques are the most promising approach for the identification of Phomopsis spp. (13, 15, 16, 24).
To date, only two reports are available on the fungus Phomopsis phoenicicola: (i) a description by Saccardo in 1913 (14) and (ii) a report on it as a pathogen causing fruit rot of arecanut in India (18). The latter isolate was submitted to the culture collection of Centraalbureau voor Schimmelcultures (CBS), Utrecht, Netherlands (18). This strain (CBS 161.64), the ex-isotype of P. phoenicicola and the actual isolate that represents the whole species, was only recently sequenced and given the GenBank accession number FJ889452 (16). Our case isolate (ICIRC-CC58) showed a 100% match with Phomopsis phoenicicola (CBS 161.64; GenBank accession number FJ889452). The identification of the case isolate as P. phoenicicola appears to be accurate, as it is clear in more than one phylogenetic tree that P. phoenicicola (CBS 161.64; FJ889452) is an individualized species and not an eventually misidentified isolate that belongs to another species (16).
To the best of our knowledge, 5 clinical isolates of unidentified Phomopsis spp. causing human infections, isolated from skin, scalp, sputum, cornea, and finger, have been reported (7, 19, 20). This highlights the need for molecular identification of all Phomopsis spp. causing human infections. The patient described here responded to topical natamycin and oral fluconazole, indicating their use in the management of keratomycosis caused by P. phoenicicola. The presented case of fungal keratitis is the first report of an ocular infection caused by P. phoenicicola and, furthermore, the first known case of disease in humans caused by this species.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the case isolate Phomopsis phoenicicola (ICIRC-CC58) are HQ650813 (ITS) and HQ288895 (D2-LSU).
Acknowledgments
We thank Glen Hartman, USDA, National Soybean Research Center, Urbana, IL; Deanna A. Sutton, Department of Pathology, Fungus Testing Laboratory, University of Texas Health Care Center; and Jorge M. Santos, Centro de Recursos Microbiológicos, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, Caparica, Portugal, for their valuable technical suggestions for identification of the fungal isolate.
D. Gajjar was supported by a research grant under the Women's Scientists Scheme (WOS-A), DST, Government of India (no. SR/WOS-A/LS-120/2008).
Footnotes
Published ahead of print on 30 March 2011.
REFERENCES
- 1. Hsiao C. H., et al. 1998. Intrascleral dissemination of infectious scleritis following pterygium excision. Br. J. Ophthalmol. 82:29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johns K. J., O'Day D. M. 1988. Pharmacologic management of keratomycoses. Surv. Ophthalmol. 33:178–188 [DOI] [PubMed] [Google Scholar]
- 3. Joseph J., Fernandes M., Sharma S. 2004. Colletotrichum dematium keratitis. J. Postgrad. Med. 50:309–310 [PubMed] [Google Scholar]
- 4. Kaliamurthy J., et al. 2004. Keratitis due to a coelomycetous fungus: case reports and review of the literature. Cornea 23:3–12 [DOI] [PubMed] [Google Scholar]
- 5. Kindo A. J., Anita S., Kalpana S. 2010. Nattrassia mangiferae causing fungal keratitis. Indian J. Med. Microbiol. 28:178–181 [DOI] [PubMed] [Google Scholar]
- 6. Kumar B., Crawford G. J., Morlet G. C. 1997. Scedosporium prolificans corneoscleritis: a successful outcome. Aust. N. Z. J. Ophthalmol. 25:169–171 [DOI] [PubMed] [Google Scholar]
- 7. Mandell K. J., Colby K. A. 2009. Penetrating keratoplasty for invasive fungal keratitis resulting from a thorn injury involving Phomopsis species. Cornea 28:1167–1169 [DOI] [PubMed] [Google Scholar]
- 8. Mendiratta D. K., Thamke D., Shukla A. K., Narang P. 2005. Keratitis due to Colletotrichum dematium—a case report. Indian J. Med. Microbiol. 23:56–58 [DOI] [PubMed] [Google Scholar]
- 9. Moller E. M., Bahnweg G., Sandermann H., Geiger H. H. 1992. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res. 20:6115–6116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moriarty A. P., Crawford G. J., McAllister I. L., Constable I. J. 1993. Fungal corneoscleritis complicating beta-irradiation-induced scleral necrosis following pterygium excision. Eye (Lond.) 7:525–528 [DOI] [PubMed] [Google Scholar]
- 11. Peponis V., Rosenberg P., Chalkiadakis S. E., Insler M., Amariotakis A. 2009. Fungal scleral keratitis and endophthalmitis following pterygium excision. Eur. J. Ophthalmol. 19:478–480 [DOI] [PubMed] [Google Scholar]
- 12. Rehner S., Uecker F. 1994. Nuclear ribosomal internal transcribed spacer phylogeny and host diversity in the coelomycete Phomopsis. Can. J. Bot. 72:1666–1674 [Google Scholar]
- 13. Rodeva R., Gabler J. 2004. First report of Phomopsis diachenii in Bulgaria. Mycol. Balcanica 1:153–157 [Google Scholar]
- 14. Saccardo P. 1913. Deuteromycetae, Sphaeriodacae, Phomopsis. Sylloge Fungorum XXII:903 [Google Scholar]
- 15. Santos J., Phillips A. 2009. Resolving the complex of Diaporthe (phomopsis) species occurring on Foeniculum vulgare in Portugal. Fungal Diversity 34:111–125 [Google Scholar]
- 16. Santos J. M., Correia V. G., Phillips A. J. 2010. Primers for mating-type diagnosis in Diaporthe and Phomopsis: their use in teleomorph induction in vitro and biological species definition. Fungal Biol. 114:255–270 [DOI] [PubMed] [Google Scholar]
- 17. Singh R. P., McCluskey P. 2005. Scedosporium prolificans sclerokeratitis 10 years after pterygium excision with adjunctive mitomycin C. Clin. Exp. Ophthalmol. 33:433–434 [DOI] [PubMed] [Google Scholar]
- 18. Srivastava H., Banu Z., Govindarajan V. 1962. Fruit rot of arecanut caused by a new fungus. Mycologia 54:5–11 [Google Scholar]
- 19. Sutton D. A. 1999. Coelomycetous fungi in human disease. A review: clinical entities, pathogenesis, identification and therapy. Rev. Iberoam. Micol. 16:171–179 [PubMed] [Google Scholar]
- 20. Sutton D. A., Timm W. D., Morgan-Jones G., Rinaldi M. G. 1999. Human phaeohyphomycotic osteomyelitis caused by the coelomycete Phomopsis saccardo 1905: criteria for identification, case history, and therapy. J. Clin. Microbiol. 37:807–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Rensburg J. C., Lamprecht S. C., Groenewald J. Z., Castlebury L. A., Crous P. W. 2006. Characterisation of Phomopsis spp. associated with die-back of rooibos (Aspalathus linearis) in South Africa. Stud. Mycol. 55:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vandenbossche I., Vaneechoutte M., Vandevenne M., De Baere T., Verschraegen G. 2002. Susceptibility testing of fluconazole by the NCCLS broth macrodilution method, E-test, and disk diffusion for application in the routine laboratory. J. Clin. Microbiol. 40:918–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. White T., Bruns T., Lee S., Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315–322 In Innis M. A., Gelfand D. H., Shinsky J. J., White T. J. (ed.), PCR protocols: a guide to methods and applications. Academic Press, Inc., New York, NY [Google Scholar]
- 24. Zhang A. W., Riccioni L., Pedersen W. L., Kollipara K. P., Hartman G. L. 1998. Molecular identification and phylogenetic grouping of Diaporthe phaseolorum and Phomopsis longicolla isolates from soybean. Phytopathology 88:1306–1314 [DOI] [PubMed] [Google Scholar]