Abstract
We present a novel electrical method for detecting viable bacteria in blood cultures that is 4 to 10 times faster than continuous monitoring blood culture systems (CMBCS) like the Bactec system. Proliferating bacteria are detected via an increase in the bulk capacitance of suspensions, and the threshold concentration for detection is ∼104 CFU/ml (compared to ∼108 CFU/ml for the Bactec system).
TEXT
Continuous monitoring blood culture systems (CMBCS), like the Bactec, BacT/Alert, and VersaTREK systems, currently serve as the “gold standard” for the detection of bacteremia and sepsis in the clinical setting. Blood cultures typically take between 12 and 72 h to yield positive results (3–5) and are usually continued for 120 h (5 days) before being deemed negative. For positive cultures, bacteria present are then identified (using various methods, ranging from traditional biochemical tests to PCR-based DNA analysis, that take an additional 3 to 24 h) before targeted antibiotics are administered. For every hour of delay in starting targeted antibiotic therapy, the risk of death for a given patient with sepsis increases by 6 to 10% (6). Since the blood culture step is by far the longer of the two diagnostic steps needed, cutting down the times to positivity (TTPs) of blood cultures is likely to reduce mortality and improve patient outcomes.
At the time the patient begins to show clinical symptoms of sepsis, the concentration of bacteria present in blood is very low (1 to 100 CFU/ml in adults [13] and <10 CFU/ml in neonates [9]). Currently available CMBCS (like the Bactec, BacT/Alert, and VersaTREK systems) require the user to introduce the drawn blood (∼10 ml for adults and ∼1 ml for neonates) into a bottle containing 20 to 40 ml of sterile bacterial growth medium and place it in a special incubation chamber. Here, the CMBCS monitor the levels of CO2 in the suspension. A significant increase in CO2 is taken to indicate the presence of viable bacteria in the suspension and hence in blood. Due to inherent limitations imposed by the metabolic rate of individual bacterial cells (e.g., one Escherichia coli bacterium consumes only ∼2 × 10−14 moles of O2 per hour [10]), the concentrations of bacteria in the suspension typically have to rise to ∼108 CFU/ml before they can be detected (12). Given the low initial loads, this implies that 20 to 30 doubling times must elapse before the bacteria can be detected, resulting in the long TTPs (12 to 72 h) typically observed. Newly emerging molecular diagnostic methods that seek to reduce TTPs, such as PCR-based methods, have been reported to suffer several technical limitations, such as (i) high detection limits (>103 CFU/ml) due to the presence of human DNA, (ii) false negatives due to presence of PCR inhibitors in blood, (iii) false positives due to contaminant bacterial DNA in reagents, and (iv) high expense (∼$250/test) due to the need for ultrapure chemicals (7). On the other hand, blood cultures, which cost only $20 to $30 per test, serve as a reliable, effective, and commercially viable way to screen a large number of patients. Hence, a method that retains the reliability and cost-effectiveness of blood cultures, while cutting down the TTP, could be very useful clinically. We believe the method reported here can, in the future, fulfill this need.
Our method for detecting viable bacteria in suspensions is based on the fact that, in the presence of high-frequency alternating-current (AC) electric fields, there occurs a buildup of charge at the cell membrane of viable cells, causing these cells to behave like electrical capacitors (1). Notably, when the integrity of the cell membrane is compromised (such as on cell death), this effect is lost. Aqueous solutions containing such viable cells can be electrically modeled by a circuit, one of whose elements (the bulk capacitance) reflects the amount of charge stored by elements dispersed in the interior of the suspension (as opposed to the electrode-solution interface, where most of the charge is typically stored) (11). One can estimate the value of the bulk capacitance by measuring the electrical impedance (Z) at a number of frequencies (ω) and fitting the Z-versus-ω data to a mathematical model of the circuit (10). If the number of viable bacteria increases, so does the bulk capacitance value. Conversely, an increase in the bulk capacitance over time can serve as a signature for the presence of proliferating (viable) bacteria. We have previously demonstrated our ability to detect multiple species of bacteria in food substrates (milk and apple juice) by using this approach (8). In the present work, we aim to show that it can be applied to blood culture broth as well.
We prepared, by serial dilution from a log culture of Escherichia coli DH5α (Invitrogen, Carlsbad, CA) grown in tryptic soy broth (TSB), suspensions of the bacteria in phosphate-buffered saline (PBS) with concentrations of ∼104, ∼103, ∼102, and ∼10 CFU/ml. One-hundred-microliter (0.1-ml) amounts of these suspensions were then added to 0.9 ml (each) of fresh (∼1 h after withdrawal) human blood (drawn, from healthy volunteers 25 to 35 years of age, into tubes coated with heparin anticoagulant) to obtain “seeded blood” with concentrations of ∼103, ∼102, ∼10, and ∼1 CFU/ml. One hundred microliters of this was plated onto TSB agar plates to verify the concentration of bacteria inoculated. Two 1-ml samples of seeded blood (each with the same known bacterial load) were taken; one set was introduced into tubes containing 10 ml (each) of Bactec Ped-Plus medium, and the other set was introduced into Bactec Ped-Plus bottles. The latter was loaded into a Bactec 9240 continuous monitoring blood culture system, and the TTP was recorded. The former was assayed electrically by using our method (8). Appropriate controls were also included with each set of experiments conducted. For Bactec readings, at least one bottle was inoculated with 1 ml of sterile blood (without bacteria).
The bottles were then incubated in the Bactec 9120 machine for 120 h and (per the established protocol) deemed negative when they failed to show signs of growth. Similarly, for our method, at least 1 vial with 10 ml of Bactec Ped-Plus medium was inoculated with 1 ml of sterile blood and monitored for 8 h. Every hour, including the zero hour (which would give us the initial concentration of the bacteria in the sample), two 100-μl aliquots from the sample being assayed by our method were drawn. One of them was plated on TSB agar (after serial dilution, when needed) to obtain an estimate of the bacterial load at that point in time. The other was loaded into a specially designed microfluidic cassette and assayed electrically. Details of the cassette design and electrical assay method are provided in our earlier work (8). Briefly, the key aspect of the cassette design is the confinement of the sample in a long, narrow channel with electrodes at the ends that enables the charge storage in the bacteria to contribute significantly to the measured impedance. The electrical assay involved measuring electrical impedance (Z) at multiple frequencies (ω) from 1 KHz to 100 MHz and using the Z-versus-ω data to obtain a parameter (the bulk capacitance) that reflected the capability of the interior of the solution to store charge. An increase in the number of bacteria due to proliferation is expected to result in an increase in this parameter. Conversely, a significant increase in the value of this parameter serves as a signature of the presence of viable bacteria in the suspension, and once such a significant change is recorded, our sample may be deemed positive.
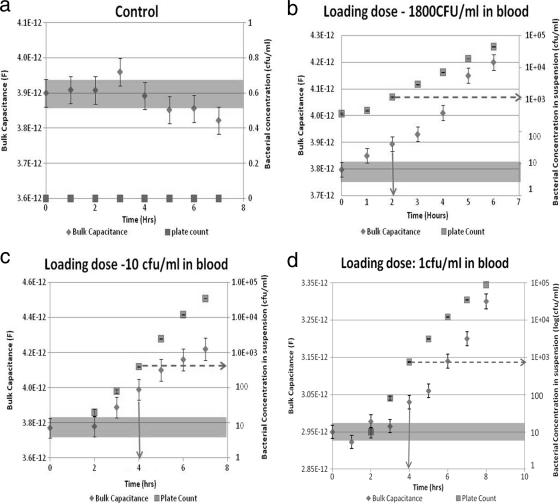
The change in the bulk capacitance value was considered significant if its range (estimate of value ± error, as specified by the ZView software that was used to analyze Z-versus-ω data) no longer overlapped with that of the value of the bulk capacitance at the start of the culture (zero-hour value). As shown in Fig. 1b, c, and d, with time, both the actual concentration of bacteria in the suspension (squares) and the suspension's bulk capacitance (diamonds) increased. As seen in Fig. 1b, a small increase in the number of bacteria (from 360 to 460 CFU/ml in 1 h) may result in an increase in bulk capacitance that is not significant (the error bar overlaps with that of the zero-hour value). At this point, based on electrical measurements alone, it is not possible to infer the presence of viable bacteria. However, a further increase in bacterial numbers (to 1,200 CFU/ml at the 2-h mark) resulted in a value of bulk capacitance that was significantly different. The time at which the significant increase was recorded is the TTP for our sample (solid arrow), and the concentration of bacteria in the suspension at this point is the threshold concentration (dotted arrow).
Fig. 1.
Typical plots showing how the calculated value of the constant phase element (CPE, the bulk capacitance parameter) changes with time for different samples (control [a] or samples with initial loads of ∼1,800 CFU/ml [b], ∼10 CFU/ml [c], or 1 CFU/ml [d]) (diamonds). Also shown are plate counts obtained from aliquots drawn at the same time at which the impedance measurements are taken (squares). Samples turn positive when the recorded CPE value is significantly different from the initial (zero-hour) value, and the solid arrows indicate the TTPs for each data set. It was also observed that bacterial concentrations at time to positivity are approximately 1,000 to 10,000 CFU/ml (represented by dotted arrows).
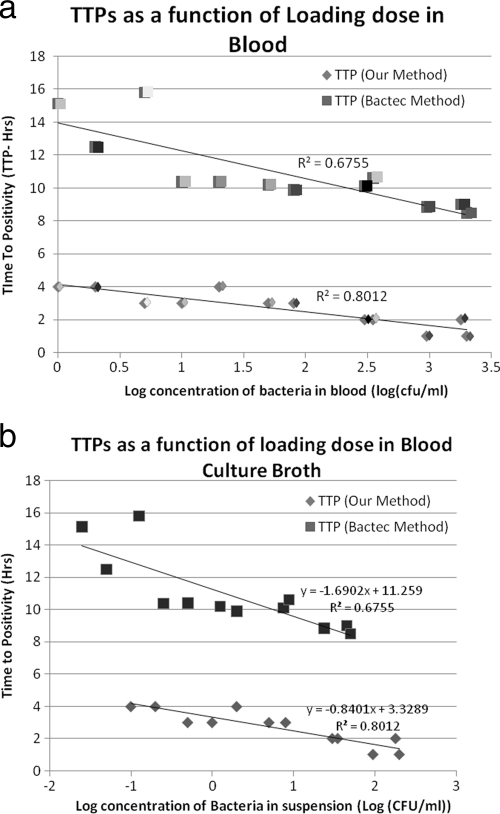
As seen, the threshold concentrations for all samples tested were similar (in the range of approximately 1,000 to 10,000 CFU/ml), but because different samples started with different loading concentrations of bacteria, it took different amounts of time for the bacterial loads to reach the threshold concentration. In general, the lower the initial load, the longer it will take for the sample to turn positive. This is borne out for our experiments summarized graphically in Fig. 2. In Fig. 2, our TTPs are also compared to those obtained using the Bactec system. As seen, for all initial loads, we were able to obtain a TTP that was 4 to 10 times less than that for the Bactec system. We believe that such significant decreases in TTP are made possible by the fact that we monitored a parameter (charge stored in the interior of a suspension) that is much more sensitive to bacterial proliferation than parameters like CO2/O2 levels, pH, and conductivity, etc., that are monitored by current instruments like the Bactec, BacT/Alert, and VersaTREK systems.
Fig. 2.
(a) Consolidated plot of TTPs obtained by our method of detection and those from the Bactec method for the same given initial concentrations of bacteria in blood. Pairs, consisting of one sample introduced into the Bactec system and another containing the same initial load assayed using our method, are represented by the same shading. (b) Consolidated plot of TTPs obtained by our method and the Bactec system for the initial concentrations of bacteria in blood culture broth (containing infected blood in Bactec Ped-Plus medium).
Like the current CMBCS, the method that we employ is self-normalizing. By this we mean that although different samples may have different initial bulk capacitance values (due to, for instance, different volumes of blood added into the blood culture broth and/or different hematocrits or white blood cell [WBC] counts in blood), the fact that we use a significant change from the zero-hour value as our criterion for flagging a culture as positive makes our method insensitive to such fluctuations in initial composition of the blood/blood culture broth. Commercial formulations of growth media contain additives (often proprietary) to counteract the effect of immunological cells and antimicrobials that retard bacterial growth. Our method would also have to rely on similar additives in the growth media for this purpose.
To conclude, we would like to highlight that in this work, we have demonstrated the feasibility of using a novel, electrically based technique to detect the presence of viable bacteria in blood cultures, 4 to 10 times faster than currently possible using CMBCS like the Bactec, BacT/Alert, and VersaTREK systems. The technique needs to be refined and made more user-friendly (by automating the aliquot collection, impedance measurement, and data analysis) and tested extensively against a panel of organisms encountered in bloodstream infections before it can be actually tested in a clinical setting. Our earlier work with multiple bacteria in food substrates (8) suggests that the threshold concentration is likely to be similar for other bacteria, but because their doubling times are different, their TTPs (for the same initial loads) will differ, with slower-growing bacteria having correspondingly longer TTPs (as is also the case for the Bactec system). However, since bacteria with lower metabolic rates also have correspondingly longer doubling times (2, 14), we expect to see a similar 4- to 10-fold decrease in TTP for other bacteria as well. While the above improvements need to be implemented and our conjectures regarding the applicability to other pathogens need to be verified, the work that we report here nevertheless represents a potential breakthrough technique that may dramatically reduce TTPs of blood cultures.
Acknowledgments
The research was funded using Startup Funds from the University of Missouri, granted to S.S. B.D.L. was also supported by Cheongbung Scholarship Foundation, South Korea.
We also acknowledge John A. Pardalos, Joan M. Yates, and Thomas Reilly for help with the project.
Footnotes
Published ahead of print on 6 April 2011.
REFERENCES
- 1. Asami K. 2002. Characterization of biological cells by dielectric spectroscopy. J. Non Cryst. Solids 305:268–277 [Google Scholar]
- 2. Chróst R. J., Faust M. A. 1999. Consequences of solar radiation on bacterial secondary production and growth rates in subtropical coastal water (Atlantic Coral Reef off Belize, Central America). Aquat. Microb. Ecol. 20:39–48 [Google Scholar]
- 3. Frank U., Malkotsis D., Mlangeni D., Daschner F. 1999. Controlled clinical comparison of three commercial blood culture systems. Eur. J. Clin. Microbiol. Infect. Dis. 18:248–255 [DOI] [PubMed] [Google Scholar]
- 4. Garcia-Prats J. A., Cooper T. R., Schneider V. F., Stager C. E., Hansen T. N. 2000. Rapid detection of microorganisms in blood cultures of newborn infants utilizing an automated blood culture system. Pediatrics 105:523–527 [DOI] [PubMed] [Google Scholar]
- 5. Haimi-Cohen Y., Vellozzi E., Rubin L. 2002. Initial concentration of Staphylococcus epidermidis in simulated pediatric blood cultures correlates with time to positive results with the automated, continuously monitored BACTEC blood culture system. J. Clin. Microbiol. 40:898–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumar A., et al. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 34:1589–1596 [DOI] [PubMed] [Google Scholar]
- 7. Mancini N., et al. 2010. The era of molecular and other non-culture-based methods in the diagnosis of sepsis. Clin. Microbiol. Rev. 23:235–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Puttaswamy S., Sengupta S. 2010. Rapid detection of bacterial proliferation in food samples using microchannel impedance measurements at multiple frequencies. Sens. Instrum. Food Qual. Saf. 4:108–118 [Google Scholar]
- 9. Reier-Nilsen T., Farstad T., Nakstad B., Lauvrak V., Steinbakk M. 2009. Comparison of broad range 16S rDNA PCR and conventional blood culture for diagnosis of sepsis in the newborn: a case control study. BMC Pediatr. 9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sengupta S., Battigelli D., Chang H. 2006. A micro-scale multi-frequency reactance measurement technique to detect bacterial growth at low bio-particle concentrations. Lab Chip 6:682–692 [DOI] [PubMed] [Google Scholar]
- 11. Sengupta S., Mahmud G., Chiou D., Ziaie B., Barocas V. 2005. Application of the lag-after-pulsed-separation (LAPS) flow meter to different protein solutions. Analyst 130:171–178 [DOI] [PubMed] [Google Scholar]
- 12. Smith J., Serebrennikova Y., Huffman D., Leparc G., García Rubio L. 2008. A new method for the detection of microorganisms in blood cultures: Part I. Theoretical analysis and simulation of blood culture processes. Can. J. Chem. Eng. 86:947–959 [Google Scholar]
- 13. Yagupsky P., Nolte F. 1990. Quantitative aspects of septicemia. Clin. Microbiol. Rev. 3:269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zacharof M., Lovitt R. 2010. Development of an optimised growth strategy for intensive propagation, lactic acid and bacteriocin production of selected strains of Lactobacilli genus. Int. J. Chem. Eng. Appl. 1:55–63 [Google Scholar]