Abstract
In low-income countries some infections caused by nontuberculous mycobacteria are misdiagnosed as multidrug-resistant tuberculosis. In most of these settings the observation of microscopic cords is the only technique used to identify Mycobacterium tuberculosis in the laboratory. In this article we definitively demonstrate that Mycobacterium abscessus, an emerging pulmonary pathogen, also forms microscopic cords.
TEXT
Mycobacterium tuberculosis complex (MTC) species, when grown in a liquid medium without detergent, form cords in tight bundles, consisting of bacilli aligned in parallel, end to end, and side to side, along the long axis of the cord (see reference 6 for a recent review). M. tuberculosis microscopic cords were first observed by Robert Koch in 1882, but their significance increased in 1947, when studies performed by Middlebrook et al. linked this phenotypic characteristic to the virulence of MTC organisms (16). From these pioneering studies to now, cord morphology has been considered to be a distinctive feature of the MTC, and its detection, in smears from liquid cultures, is a reliable criterion for the rapid presumptive identification of MTC isolates in many laboratories around the world (6, 12, 14, 20, 23). The first demonstration that cord formation could occur in other mycobacterial species was in 2008, when microscopic cords in a human clinical isolate of Mycobacterium marinum were described (21).
In 2010 we demonstrated that M. marinum produced true cords (bacilli arranged in parallel along the long axis of the cord) and not clumps (aggregates of bacilli in a random orientation), obtaining the first scanning electron microscopy (SEM) images of the mycobacterial cord ultrastructure (11). In the same work we demonstrated that the phenomenon of cording was also present in rough variants of other nontuberculous mycobacteria (NTM) such as Mycobacterium vaccae, Mycobacterium gilvum, Mycobacterium obuense, Mycobacterium chubuense, and Mycobacterium parafortuitum (11).
The study of the cording phenomenon by SEM aims to eliminate any doubt regarding the ability of NTM to form cords. This is necessary because the use of cording as a criterion for the identification of MTC isolates from clinical cultures has been adopted as a standard methodology by clinical microbiologists. As a result of this bias, when cordlike morphology is observed for NTM, these structures have been characterized as clumps, pseudocords, or loose aggregates (7, 14, 20). The resolution of the optical microscope does not allow the accurate visualization of the organization of the bacilli in these aggregates; thus, the idea that NTM cannot form true cords has persisted to the present time.
Mycobacterium abscessus is one of the most pathogenic and chemotherapy-resistant rapid-growing mycobacteria (15, 17). This mycobacterium has been reported to account for 10% of NTM lung infections in the United States and is the second most common cause of NTM lung disease in South Korea (13). M. abscessus causes lung disease in patients with underlying lung disorders but also in immunocompetent individuals. In addition to lung infections, this bacterium can also cause skin and soft tissue infections, meningitis, and disseminated infections (15, 17). The objective of this work was to use SEM to determine whether M. abscessus can also form true cords.
We have performed SEM studies of M. abscessus 390R, 390S, and 390V and the type strain of M. abscessus, DSMZ 44196T. Mycobacterium bovis strain BCG Japan has been included for comparison as a member of the MTC. M. abscessus strain 390R was isolated in a pure culture from an ileal granuloma of a patient with Crohn's disease. This strain displays rough colonies on 7H11 agar and was reported previously to form microscopic cords by optical microscopy methods (9). Strain 390S forms smooth colonies, does not produce cords as assessed by optical microscopy, and is a spontaneous mutant of strain 390R isolated in vitro. In a previous work, both strains were identified as being M. abscessus by biochemical and genetic analyses (3). In addition to forming nonpigmented colonies and having a rapid growth rate, results of biochemical analyses showed both these variants to be M. abscessus (nitrate reduction negative, positive for growth in 5% NaCl, iron uptake negative, citrate negative, inositol negative, and mannitol negative). Restriction enzyme analysis was performed after PCR amplification of a portion of the gene encoding the 65-kDa heat shock protein. By using the restriction endonucleases BstEII and HaeIII, both isolates were found to be identical to the M. abscessus control strain (3). Strain 390V was a spontaneous rough revertant obtained during serial passages of 390S, which regained the rough-colony phenotype and the capacity to form cords, as assessed by optical microscopy (9). It was found that the smooth phenotype is due to the expression of glycopeptidolipid (GPL), which is minimally expressed by rough variants. Strains 390R and 390V are both able to replicate in human macrophages and persist in the lungs of mice, while strain 390S lacks these capabilities (9).
For all the strains studied, one isolated colony was inoculated into Trypticase soy broth (TSB) (Scharlau Chemie, Spain), and the cultures were incubated (without shaking) for 2 weeks. The cells were obtained from uncentrifuged TSB cultures by gently removing the liquid medium; that is, liquid medium was removed with a Pasteur pipette, and cells were allowed to settle to the bottom of the tube. For fixation, cells were immersed in 2.5% (vol/vol) glutaraldehyde (electron microscopy [EM] grade; Merck, Darmstadt, Germany) in 0.1 M phosphate buffer at pH 7.4 (PB; Sigma, Steinheim, Germany) for 2 h. The samples were then processed according to conventional electron microscopy methods as previously described (11). Bacilli were observed with an S-570 scanning electron microscope (Hitachi Ltd., Japan) at an accelerating voltage of 30 kV.
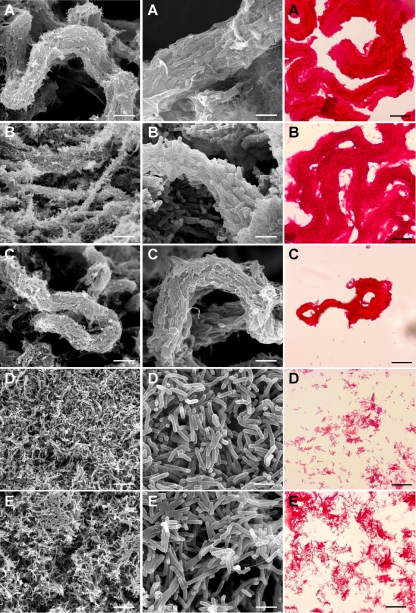
It is clearly shown in Fig. 1 that strain 390R forms true cords very similar to those formed by M. bovis. At a high magnification (also see Fig. S1 in the supplemental material), we observed the arrangement of the bacilli in the cord end to end and side to side in parallel along the long axis of the cord. We can also appreciate in Fig. 1 and in Fig. S2 in the supplemental material that strain 390V showed cords similar to those of strain 390R and M. bovis strains. On the contrary, the strains that made smooth colonies, 390S and DSMZ 44196T, did not form cords. Representative images of the Ziehl-Neelsen stains are also shown in Fig. 1. We conclude that M. abscessus forms true cords but that cord formation is restricted to strains forming rough colonies on solid medium.
Fig. 1.
Representative micrographs of SEM at medium (left) and high (middle) magnifications and photographs of Ziehl-Neelsen stains (right). Bar sizes, 7.5 μm (left), 1.9 μm (middle), and 20 μm (right). (A) M. abscessus 390R; (B) M. bovis BCG Japan; (C) M. abscessus 390V; (D) M. abscessus 390S; (E) M. abscessus DMSZ 44196T.
Recent clinical reports found an association of the rough-colony phenotype with invasive infection in patients with cystic fibrosis (4, 10, 19). This association is supported by recent basic science studies that have found that in contrast to the smooth phenotype, the rough phenotype is immunostimulatory and able to replicate in human macrophages (18). It would be of interest to determine whether cord formation is a general characteristic of M. abscessus rough variants.
The results obtained in this study and previous studies (11, 21) demonstrate that cord formation is not specific to members of the MTC and by itself cannot identify an isolate as belonging to that complex.
Two misconceptions in the area of clinical microbiology are that only MTC organisms form microscopic cords and that the incidence of NTM is extremely low in developing countries characterized by a high burden of M. tuberculosis infection (8, 22). It is possible that these two misconceptions have led to misdiagnoses of multidrug-resistant tuberculosis (TB) in low-income countries where the microbiological diagnosis of TB is based only on the observation of cording morphology (5, 6, 22, 23). In support of this, recent studies reported much higher rates of isolation of NTM in Africa when rigorous mycobacterial identification techniques were applied (1, 2, 5, 8, 22). Thus, our report demonstrates that laboratories that base the microbiological identification of members of the MTC solely on the observation of microscopic cords may mistakenly identify M. abscessus isolates as belonging to the MTC. Since M. abscessus is not sensitive to drugs used to treat TB, a lack of a clinical response to therapy could lead to the erroneous conclusion that a patient is infected with multidrug-resistant TB. As a result, the patient would be exposed to an ineffective drug regimen with many adverse side effects.
Supplementary Material
Acknowledgments
This work was supported by Catalan AGAUR (2009SGR 00108).
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 13 April 2011.
REFERENCES
- 1. Bonard D., et al. 2004. High incidence of atypical mycobacteriosis in African HIV-infected adults with low CD4 cell counts: a 6-year cohort study in Côte d'Ivoire. AIDS 18:1961–1964 [DOI] [PubMed] [Google Scholar]
- 2. Buijtels P., et al. 2009. Nontuberculous mycobacteria, Zambia. Emerg. Infect. Dis. 15:53–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Byrd T. F., Lyons C. R. 1999. Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection. Infect. Immun. 67:4700–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Catherinot E., et al. 2009. Acute respiratory failure involving an R variant of Mycobacterium abscessus. J. Clin. Microbiol. 47:271–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chihota V. N., et al. 2010. Liquid vs. solid culture for tuberculosis: performance and cost in a resource-constrained setting. Int. J. Tuberc. Lung Dis. 14:1024–1031 [PubMed] [Google Scholar]
- 6. Dos Santos Simeão F. C., et al. 2009. Cord factor detection and macroscopic evaluation of mycobacterial colonies: an efficient combined screening test for the presumptive identification of Mycobacterium tuberculosis complex on solid media. J. Bras. Pneumol. 35:1212–1216 [DOI] [PubMed] [Google Scholar]
- 7. Glickman M. S. 2008. Cording, cord factors, and trehalose dimycolate, p. 63–73 In Daffé M., Reyrat J. M. (ed.), The mycobacterial cell envelope. ASM Press, Washington, DC [Google Scholar]
- 8. Gopinath K., Singh S. 2010. Non-tuberculous mycobacteria in TB-endemic countries: are we neglecting the danger? PLoS Negl. Trop. Dis. 4:e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Howard S. T., et al. 2006. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology 152:1581–1590 [DOI] [PubMed] [Google Scholar]
- 10. Jönsson B. E., et al. 2007. Molecular epidemiology of Mycobacterium abscessus, with focus on cystic fibrosis. J. Clin. Microbiol. 45:1497–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Julián E., et al. 2010. Microscopic cords, a virulence-related characteristic of Mycobacterium tuberculosis, are also present in nonpathogenic mycobacteria. J. Bacteriol. 192:1751–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaminski D. A., Hardy D. J. 1995. Selective utilization of DNA probes for identification of Mycobacterium species on the basis of cord formation in primary BACTEC 12B cultures. J. Clin. Microbiol. 33:1548–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koh W.-J., et al. 2006. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest 129:341–348 [DOI] [PubMed] [Google Scholar]
- 14. McCarter Y. S., Ratkiewicz I. N., Robinson A. 1998. Cord formation in Bactec medium is a reliable, rapid method for presumptive identification of Mycobacterium tuberculosis complex. J. Clin. Microbiol. 36:2769–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Medjahed H., Gaillard J.-L., Reyrat J.-M. 2010. Mycobacterium abscessus: a new player in the mycobacterial field. Trends Microbiol. 18:117–123 [DOI] [PubMed] [Google Scholar]
- 16. Middlebrook G., Dobos R. J., Pierce C. 1947. Virulence and morphological characteristics of mammalian tubercle bacilli. J. Exp. Med. 861:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petrini B. 2006. Mycobacterium abscessus: an emerging rapid-growing potential pathogen. APMIS 114:319–328 [DOI] [PubMed] [Google Scholar]
- 18. Rhoades E. R., et al. 2009. Mycobacterium abscessus glycopeptidolipid masks underlying cell wall phosphatidyl-myo-inositol mannosides preventing their interaction with human monocyte TLR2. J. Immunol. 183:1997–2007 [DOI] [PubMed] [Google Scholar]
- 19. Sanguinetti M., et al. 2001. Fatal pulmonary infection due to multidrug-resistant Mycobacterium abscessus in a patient with cystic fibrosis. J. Clin. Microbiol. 39:816–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shen G. H., et al. 2009. Combining the Capilia TB assay with smear morphology for the identification of Mycobacterium tuberculosis complex. Int. J. Tuberc. Lung Dis. 13:371–376 [PubMed] [Google Scholar]
- 21. Staropoli J. F., Branda J. A. 2008. Cord formation in a clinical isolate of Mycobacterium marinum. J. Clin. Microbiol. 46:2814–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tortoli E., et al. 2009. Infection due to a novel mycobacterium, mimicking multidrug-resistant Mycobacterium tuberculosis. Clin. Microbiol. Infect. 16:1130–1134 [DOI] [PubMed] [Google Scholar]
- 23. Urbanczik R. 2010. Laboratory tests focusing on sputum. Int. J. Tuberc. Lung Dis. 14:1087–1093 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.