Abstract
Streptococcus pneumoniae serotype 6C, which was described in 2007, causes invasive disease in adults and children. We investigated the prevalence of 6C among pediatric isolates obtained from eight children's hospitals in the United States. S. pneumoniae isolates were identified from a prospective multicenter study (1993 to 2009). Fifty-seven serotype 6C isolates were identified by multiplex PCR and/or Quellung reaction. Five were isolated before 2000, and the prevalence increased over time (P < 0.000001). The median patient age was 2.1 years (range, 0.2 to 22.5 years). Clinical presentations included bacteremia (n = 24), meningitis (n = 7), pneumonia (n = 4), abscess/wound (n = 3), mastoiditis (n = 2), cellulitis (n = 2), peritonitis (n = 1), septic arthritis (n = 1), otitis media (n = 10), and sinusitis (n = 3). By broth microdilution, 43/44 invasive serotype 6C isolates were susceptible to penicillin (median MIC, 0.015 μg/ml; range, 0.008 to 2 μg/ml); all were susceptible to ceftriaxone (median MIC, 0.015 μg/ml; range, 0.008 to 1 μg/ml). By disk diffusion, 16/44 invasive isolates (36%) were nonsusceptible to erythromycin, 19 isolates (43%) were nonsusceptible to trimethoprim-sulfamethoxazole (TMP-SMX), and all isolates were clindamycin susceptible. Multilocus sequence typing (MLST) revealed 24 sequence types (STs); 9 were new to the MLST database. The two main clonal clusters (CCs) were ST473 and single-locus variants (SLVs) (n = 13) and ST1292 and SLVs (n = 23). ST1292 and SLVs had decreased antibiotic susceptibility. Serotype 6C causes disease in children in the United States. Emerging CC1292 expressed TMP-SMX resistance and decreased susceptibility to penicillin and ceftriaxone. Continued surveillance is needed to monitor changes in serotype prevalence and possible emergence of antibiotic resistance in pediatric pneumococcal disease.
INTRODUCTION
Streptococcus pneumoniae causes invasive pneumococcal disease (IPD) syndromes such as sepsis, meningitis, and pneumonia, as well as more common infections such as otitis media (9). S. pneumoniae is estimated to have caused 700,000 to 1,000,000 deaths annually in children aged 1 to 59 months worldwide, representing around 11% of all deaths in children in this age group (21). It is a significant cause of morbidity and mortality among children less than 2 years old and the elderly (27).
Ninety-two serotypes have been described to date, with serotypes 6C and 6D most recently discovered (3, 15, 24). Serotypes 6A, 6B, 6C, and 6D constitute serogroup 6 and have related capsular structures (3, 23). Serotype 6C is differentiated from serotype 6A by a 193-bp deletion in the wciN gene region of the capsular locus encoding a galactosyl transferase (23). As a result, a galactose of the serotype 6A capsular polysaccharide is substituted for a glucose in serotype 6C. Serotype 6C cross-reacts serologically with the 6A antiserum by the Quellung reaction; 6C-specific antiserum (factor antiserum 6d) was recently introduced to identify the 6C serotype (11, 13).
The 7-valent pneumococcal conjugate vaccine (PCV7), introduced in the United States in 2000, protects against serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F and has greatly reduced invasive disease and to a lesser degree diminished otitis media in children (1, 17). Data suggest that PCV7 provides cross-protection for 6A but not for 6C (19, 22). PCV13, introduced in the United States in 2010, is now recommended for routine administration starting at 2 months of age, providing coverage against the PCV7 serotypes plus serotypes 1, 3, 5, 6A, 7F, and 19A (2). Several studies have investigated the prevalence of serotype 6C infections in children by using PCR and/or serotyping (5, 6, 12, 14). The U.S. Pediatric Pneumococcal Multicenter Surveillance Study has prospectively identified pneumococcal infections in 8 children's hospitals since 1993 (17). We identified a group of isolates in this collection that were 6C by using first PCR and subsequently the Quellung reaction when 6C-specific antiserum became available, and we further characterized these isolates by multilocus sequence type (MLST).
MATERIALS AND METHODS
Patients and isolates.
From 1993 to 2009, investigators at eight pediatric hospitals prospectively identified cases of invasive pneumococcal disease and retrospectively completed standard case report forms (16). Isolates from noninvasive infections (e.g., otitis media or sinusitis) were collected from the centers from 1994 to 2007 and from Texas Children's Hospital during the entire study period. Patient data and bacterial isolates were sent to the Infectious Disease Laboratory at Texas Children's Hospital, Houston, TX, where a computer database is maintained. The isolates were serotyped using the Quellung reaction (Statens Serum Institut, Copenhagen, Denmark), tested for antibiotic susceptibility, and stored in horse blood at −80°C.
The study was approved by the institutional review boards at all participating institutions and was exempt from requirements to obtain patients' informed consent.
DNA extraction.
The isolates were grown on tryptic soy agar plates containing 5% sheep blood. DNA was isolated using a microbial DNA isolation kit (MO-BIO, Carlsbad, CA) or with the QIA cube (Qiagen, Valencia, CA) using Qiagen's DNeasy blood and tissue kit according to the manufacturer's specifications for Gram-positive bacteria.
Differentiation of 6C by PCR and serotyping.
DNA of isolates previously described as 6A was used in a multiplex PCR developed by Jacobs et al. to distinguish between serotypes 6A, B, and C (12). This method is based on deletions in the wciN gene. Positive controls (3-286 [6A], 1-60 [6B], and J-39 [6C]) were kindly provided by Michael R. Jacobs, Department of Pathology, University Hospitals, Case Medical Center and Case Western Reserve University, Cleveland, OH. As antiserum binding specifically to the serotype 6C capsule became available from Statens Serum Institute, isolates were serotyped by the Quelling method using factor antiserum 6d (13).
MLST.
DNAs from all serotype 6C isolates were typed by using the multilocus sequence typing (MLST) technique as specified at the MLST website (www.mlst.net) (10). Additionally, 20 serotype 6A isolates from the same time period were typed. Sequencing was performed at Beckman Coulter Genomics, Beverly, MA. The sequences were edited manually, and trimmed sequences were queried at www.mlst.net for allele and sequence type (ST) designations. eBURST (Based Upon Related Sequence Types) analysis was performed to compare STs using eBURST V3, available at www.mlst.net.
Antibiotic susceptibility.
Susceptibilities to penicillin and ceftriaxone were determined by broth microdilution assay using Clinical and Laboratory Standards Institute (CLSI) methods and interpretation guidelines (7). Susceptibilities to clindamycin, erythromycin, and trimethoprim-sulfamethoxazole (TMP-SMX) were determined using disk diffusion (CLSI) (7).
Statistical analysis.
The chi-square test for trend was used to analyze the increase of serotype 6C over time using True Epistat (Epistat Services, Richardson, TX). Analysis was two tailed, and a P value of <0.05 was considered statistically significant.
RESULTS
Isolates.
Fifty-seven S. pneumoniae serotype 6C isolates were identified either by multiplex PCR followed by serotyping once specific 6C antiserum became available or by serotyping alone. There was a 100% correlation between the multiplex PCR and Quellung reaction using specific 6C antiserum. Forty-four isolates were from invasive infections, and 13 were from otitis or sinusitis cultures. The earliest serotype 6C isolate in our population was recovered from a patient with otitis media in 1994, and only five serotype 6C isolates were isolated prior to 2000.
Demographics.
Thirty-nine (68%) of 57 children with serotype 6C isolates were male. The median patient age was 2.1 years (range, 0.2 to 22.5 years). There were 16 (28.1%) African-American, 28 (49.1%) Caucasian, and 10 (17.5%) Hispanic patients, and 3 patients (5.3%) were of another or unknown ethnicity.
Invasive disease.
Invasive infections included bacteremia (n = 24), meningitis (n = 7), pneumonia (n = 4), abscess/wound (n = 3), cellulitis (n = 2), mastoiditis (n = 2), peritonitis, and septic arthritis. Invasive infections caused by 6C increased modestly post-PCV7 as a proportion of the total number of invasive infections caused by serogroup 6 (P < 0.000001 by chi-square test for trend from 2000 to 2009). Nine of 44 invasive 6C infections presented in 2009 alone. Figure 1 shows the relative contribution of invasive serotype 6C among a total of 190 invasive serogroup 6 isolates from the pediatric multicenter study from 2000 to 2009. Serotype 6C represented 2/61 (3.3%) invasive serogroup 6 isolates in 2000 and 9/10 (90%) invasive isolates in 2009.
Fig. 1.
Prevalence of serotype 6A, 6B, and 6C invasive isolates from 2000–2009. Two invasive and three noninvasive serotype 6C isolates were collected prior to 2000. The noninvasive isolates from 2000 to 2009 were distributed over the years as follows: 2000, one isolate; 2002, one isolate; 2003, one isolate; 2008, seven isolates; and 2009, nine isolates.
Twenty-five patients had an underlying condition, i.e., malignancies (n = 6) or renal (n = 4), cardiovascular (n = 4), nervous system (n = 3), nonmalignant hematological (n = 3), or other (n = 5) conditions. One patient had a coinfection with influenza A virus.
The penicillin median MIC was 0.015 μg/ml (range, 0.008 to 2.0 μg/ml). All but one isolate were susceptible to penicillin using the respective guidelines for meningitis and nonmeningitis infections (7). The nonsusceptible isolate was from a meningitis infection (MIC = 0.25 μg/ml). All 44 invasive isolates were susceptible to ceftriaxone, with a median MIC of 0.015 μg/ml (range, 0.008 to 1 μg/ml). Sixteen isolates (36%) were nonsusceptible to erythromycin, 5 isolates (13%) were intermediate, and 11 isolates (25%) were resistant. Eight of 11 resistant isolates were from 2008 to 2009. All invasive isolates were susceptible to clindamycin. Nineteen isolates (43%) were nonsusceptible to TMP-SMX.
Noninvasive disease.
Thirteen isolates were from noninvasive infections, i.e., otitis media (n = 10) and sinusitis (n = 3). One patient had hydronephrosis, and the other 12 patients had no underlying condition. Since collection of noninvasive isolates was terminated at locations other than Texas Children's Hospital after 2007, changes in noninvasive infections over time were not analyzed. Antibiotic susceptibility patterns did not differ from those for invasive isolates: the penicillin median MIC was 0.03 μg/ml (range, 0.004 to 1 μg/ml), the ceftriaxone median MIC was 0.015 μg/ml (range, 0.008 to 0.25 μg/ml), 5 of 13 isolates (38.4%) were nonsusceptible to erythromycin, and 6/13 isolates (46.2%) were nonsusceptible to TMP-SMX.
MLST.
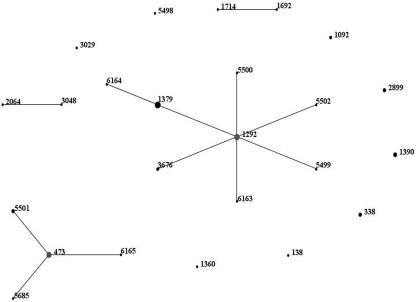
A total of 24 STs were identified among the 57 serotype 6C isolates (Fig. 2). Previously described STs include ST138, ST338 (n = 3), ST473 (n = 8), ST1092 (n = 2), ST1292 (n = 8), ST1360, ST1379 (n = 9), ST1390 (n = 4), ST1692, ST1714, ST2064, ST2899 (n = 3), ST3029, ST3048, and ST3676 (n = 2). A total of 9 new STs were identified: ST5498, ST5499, ST5500, ST5501 (n = 3), ST5502, ST5685, ST6163, ST6164, and ST6165. Five new alleles were discovered: gki-289, recP151, recP152, spi-249, and ddl-428. Two larger clonal clusters (CCs) of isolates were identified: ST473 and single-locus variants (SLVs) (ST5501, ST5685, and ST6165) and ST1292 and SLVs (ST1379, ST3676, ST5499, ST5500, ST5502, and ST6163). Additionally, a double-locus variant (DLV) of ST1292, ST6164, was identified. The two clusters were markedly different in antibiotic susceptibility patterns. ST473, ST5501, ST5685, and ST6165 represented a total of 13 isolates and had a penicillin median MIC of 0.008 μg/ml (range, 0.008 to 0.03 μg/ml) and a ceftriaxone median MIC of 0.008 μg/ml (range, 0.008 to 0.015 μg/ml). All isolates were susceptible to clindamycin and TMP-SMX, and only one isolate (an ST473 isolate from 2009) was nonsusceptible to erythromycin. The ST1292 and SLV cluster (ST1292, ST1379, ST3676, ST5499, ST5500, ST5502, and ST6163) consisted of 23 isolates. The penicillin median MIC was 0.25 μg/ml (range, 0.008 to 2 μg/ml), and the ceftriaxone median MIC was 0.125 μg/ml (range, 0.008 to 1 μg/ml). All isolates were susceptible to clindamycin, while only 4 isolates were susceptible to erythromycin and 3 were susceptible to TMP-SMX. TMP-SMX nonsusceptibility was also identified among ST138, ST338, ST1092, and ST5498 isolates, and one ST1390 isolate was nonsusceptible to erythromycin. Nine different STs were identified among the noninvasive isolates: ST473 (n = 3), ST1092, ST1292, ST1390, ST2899 (n = 2), ST3048, ST3676 (n = 2), ST5499, and ST5502.
Fig. 2.
eBURST analysis of serotype 6C isolates identified between 1993 and 2009. eBURST (Based Upon Related Sequence Types) analysis was performed to compare STs using eBURST V3, available at www.mlst.net.
Several of the STs identified among our isolates have previously been identified with other serotypes, most commonly 6A and 6B but also serotypes 18, 19A, 23A, and 23F. We analyzed 20 serotype 6A isolates by MLST to discern whether any of the STs observed among the serotype 6C isolates were circulating among the contemporary 6A isolates. Two new and 12 previously known STs were identified (ST327, ST376, ST460, ST473, ST660, ST672, ST1190, ST1876, ST2092, ST2144, ST2467, and ST3464). ST473 was the only ST identified among both serotype 6A and 6C isolates. Four serotype 6A isolates were ST473 and were among the last 6A isolates detected in our isolate collection before 6A was replaced by 6C.
DISCUSSION
There are few reports on S. pneumoniae serotype 6C infections or carriage in the United States, especially among the pediatric population. Serotype 6C was first described in 2007 but has been identified from isolates dating back to 1962 (18, 23). Our study identified a total of 57 pediatric serotype 6C isolates between 1993 and 2009, of which 44 were from invasive infections. The majority of the 6C isolates were collected after 2000. Invasive serotype 6C isolates represented 22% of the total number of invasive serogroup 6 isolates and 9/10 (90%) of the invasive serogroup 6 isolates collected in 2009. The majority of the isolates belonged to two main CCs. The largest cluster of 23 related isolates (ST1292 and SLVs) had higher median penicillin and ceftriaxone MICs and a high frequency of erythromycin and TMP-SMX nonsusceptibility. None of the ST1292 and SLV isolates was isolated prior to 2000, while 7 of the 16 invasive isolates collected in 2008 to 2009 belonged to this cluster. The other large cluster, ST473 and SLVs, also increased over time. Five of the 10 invasive isolates belonging to this group were collected in 2008 to 2009. ST473 and SLVs contrasted with the ST1292 group in their pansusceptibility to the antibiotics tested; only one isolate was resistant to erythromycin.
Carvalho et al. reported their experience with serotype 6C from isolates obtained in 1999, 2006, and 2007. They observed an increase in 6C over time, and 6C represented 69.1% (141/204) of the total serogroup 6 isolates in 2007 (5). We observed that the most dramatic increase in serotype 6C occurred in 2008 to 2009, where serotype 6C virtually replaced 6A. Carvalho and colleagues reported a total of 12 STs among 42 isolates tested. Clonal clusters 1292 and SLVs and 473 and SLVs represented the majority of isolates in their study, as in ours. ST473 was also found among our small sample of serotype 6A isolates and has been reported among serotype 6A isolates in the United States and in Israel (5, 25).
Jacobs et al. found that 35% of 172 serotype 6A isolates, derived from both adults and children, were serotype 6C (12). Five of nine clusters of serotype 6C isolates were related to clonal complexes associated with serotype 6A. The authors suggested that the genetic diversity among serotype 6C isolates points toward an increase in incidence over a considerable period of time. We noted the presence of serotype 6C beginning in 1994 in our population. Several of the STs in our collection have been described previously in association with serotypes 6A and/or 6B. The 6C isolates in our study represented 24 different STs. However, ST1292 and SLVs represented 23/57 (40%) of the isolates.
Isolates belonging to the ST1292 cluster had a high frequency of resistance to TMP-SMX and erythromycin and also had higher median penicillin and ceftriaxone MICs than other 6C isolates. Several U.S. studies report penicillin nonsusceptibility among the 6C isolates obtained from both pediatric and adult patient populations, with a prevalence ranging from 2.8% to 50%, although serotype 6C isolates tend to be more susceptible to antibiotics than serotype 6A isolates (5, 12, 19, 22). Serotype 6C strains have also been associated with antibiotic resistance on other continents (4, 20, 26).
Since the introduction of PCV7, invasive disease caused by serotype 6C has increased while invasive disease caused by serotype 6A has decreased in both adults and children (5, 22). We observed an increase in the numbers of invasive serotype 6C cases per year, with 77% of the invasive isolates collected from 2005 to 2009. The modest numbers limit statistical analysis, and continuous surveillance will be necessary to validate our observation. Another study limitation was the fact that all noninvasive isolates were continuously collected only at Texas Children's Hospital.
Serotype 6C has emerged as an important pathogen secondary to the reduction of PCV7 vaccine serotype strains. Recent studies using sera from PCV7-immunized children showed that PCV7 failed to elicit or induced a low opsonophagocytic activity against most 6C strains, suggesting that it provides little immune protection against 6C (19, 22). However, there are data to indicate that the 6A polysaccharide contained in the 13-valent conjugate vaccine may provide cross-protection against 6C (8). Until immunizations with the 13-valent vaccine have become more widespread, serotype 6C likely will increasingly cause invasive pneumococcal disease in children, and increasing resistance to antibiotics may also occur both as a result of an increase of specific clones and by sharing of resistance markers between strains. Further investigation will better define this new replacement serotype and its propensity to cause disease.
ACKNOWLEDGMENTS
We thank Michael R. Jacobs for kindly providing type strains for the multiplex PCR and Cynthia Bishop at Imperial College, London, for her help with submissions to the MLST database and the assignment of new alleles and sequence types.
This project was supported, in part, by a grant from Pfizer.
Footnotes
Published ahead of print on 30 March 2011.
REFERENCES
- 1. American Academy of Pediatrics Committee on Infectious Diseases 2000. Policy statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics 106:362–366 [DOI] [PubMed] [Google Scholar]
- 2. American Academy of Pediatrics Committee on Infectious Diseases 2010. Recommendations for the prevention of Streptococcus pneumoniae infections in infants and children: use of 13-valent pneumococcal conjugate vaccine (PCV13) and pneumococcal polysaccharide vaccine (PPSV23). Pediatrics 126:186–190 [DOI] [PubMed] [Google Scholar]
- 3. Bentley S. D., et al. 2006. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campos L. C., et al. 2009. Prevalence of Streptococcus pneumoniae serotype 6C among invasive and carriage isolates in metropolitan Salvador, Brazil, from 1996 to 2007. Diagn. Microbiol. Infect. Dis. 65:112–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carvalho M., et al. 2009. PCR-based quantitation and clonal diversity of the current prevalent invasive serogroup 6 pneumococcal serotype, 6C, in the United States in 1999 and 2006 to 2007. J. Clin. Microbiol. 47:554–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casey J. R., Adlowitz D. G., Pichichero M. E. 2010. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 29:304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. CLSI 2010. Performance standards for antimicrobial susceptibility testing; 20th informational supplement, CLSI document M100-S18. Clinical and Laboratory Standards Institute; Wayne, PA [Google Scholar]
- 8. Cooper D., et al. 2010. 13-valent pneumococcal conjugate vaccine elicits strong opsonophagocytic killing (OPA) activity to Streptococcus pneumoniae serotypes 6A, 6B, and 6C abstr. 156, p. 267 Abstr. 7th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD-7), Tel Aviv, Israel [Google Scholar]
- 9. Dagan R., Greenberg D., Jacobs M. R., Philips B. L. 2009. Pneumococcal infections, p. 1288–1342 In Feigin R. D., Cherry J. D., Demmler-Harrison G. J., Kaplan S. L. (ed.), Textbook of pediatric infectious diseases, 6th ed., vol. 1 Saunders Elsevier, Philadelphia, PA [Google Scholar]
- 10. Enright M. C., Spratt B. G. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049–3060 [DOI] [PubMed] [Google Scholar]
- 11. Hare K. M., et al. 2009. “Dodgy 6As”: differentiating pneumococcal serotype 6C from 6A by use of the Quellung reaction. J. Clin. Microbiol. 47:1981–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacobs M. R., et al. 2009. Occurrence, distribution, and origins of Streptococcus pneumoniae serotype 6C, a recently recognized serotype. J. Clin. Microbiol. 47:64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacobs M. R., Dagan R., Bajaksouzian S., Windau A. R., Porat N. 2010. Validation of factor 6d antiserum for serotyping Streptococcus pneumoniae serotype 6C. J. Clin. Microbiol. 48:1456–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jacobs M. R., Good C. E., Bajaksouzian S., Windau A. R. 2008. Emergence of Streptococcus pneumoniae serotypes 19A, 6C, and 22F and serogroup 15 in Cleveland, Ohio, in relation to introduction of the protein-conjugated pneumococcal vaccine. Clin. Infect. Dis. 47:1388–1395 [DOI] [PubMed] [Google Scholar]
- 15. Jin P., et al. 2009. First report of putative Streptococcus pneumoniae serotype 6D among nasopharyngeal isolates from Fijian children. J. Infect. Dis. 200:1375–1380 [DOI] [PubMed] [Google Scholar]
- 16. Kaplan S. L., et al. 2002. Six year multicenter surveillance of invasive pneumococcal infections in children. Pediatr. Infect. Dis. J. 21:141–147 [DOI] [PubMed] [Google Scholar]
- 17. Kaplan S. L., et al. 2004. Decrease of invasive pneumococcal infections in children among 8 children's hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics 113:443–449 [DOI] [PubMed] [Google Scholar]
- 18. Lambertsen L., Kerrn M. B. 2010. Test of a novel Streptococcus pneumoniae serotype 6C type specific polyclonal antiserum (factor antiserum 6d) and characterisation of serotype 6C isolates in Denmark. BMC Infect. Dis. 10:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nahm M. H., Lin J., Finkelstein J. A., Pelton S. I. 2009. Increase in the prevalence of the newly discovered pneumococcal serotype 6C in the nasopharynx after introduction of pneumococcal conjugate vaccine. J. Infect. Dis. 199:320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nunes S., Valente C., Sa-Leao R., de Lencastre H. 2009. Temporal trends and molecular epidemiology of recently described serotype 6C of Streptococcus pneumoniae. J. Clin. Microbiol. 47:472–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Brien K. L., et al. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902 [DOI] [PubMed] [Google Scholar]
- 22. Park I. H., et al. 2008. Differential effects of pneumococcal vaccines against serotypes 6A and 6C. J. Infect. Dis. 198:1818–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park I. H., Park S., Hollingshead S. K., Nahm M. H. 2007. Genetic basis for the new pneumococcal serotype, 6C. Infect. Immun. 75:4482–4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park I. H., et al. 2007. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 45:1225–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Porat N., Amit U., Givon-Lavi N., Leibovitz E., Dagan R. 2010. Increasing importance of multidrug-resistant serotype 6A Streptococcus pneumoniae clones in acute otitis media in southern Israel. Pediatr. Infect. Dis. J. 29:126–130 [DOI] [PubMed] [Google Scholar]
- 26. Satzke C., et al. 2010. Molecular epidemiology of Streptococcus pneumoniae serogroup 6 isolates from Fijian children, including newly identified serotypes 6C and 6D. J. Clin. Microbiol. 48:4298–4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization 2007. Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly. Epidemiol. Rec 82:93–104 [PubMed] [Google Scholar]