Abstract
In this study we compared some common Bartonella culturing methodologies using four diverse species causing human illnesses. Based on a review of the literature, we focused on three major inconsistencies between protocols: base medium, cell coculture, and temperature. Our data showed that Bartonella tamiae demonstrated temperature-dependent growth limitations between common culturing conditions only 2°C apart. Additionally, growth of B. quintana was significantly enhanced by the presence of mammalian cell coculture under mammalian cell culture conditions; however, when the medium was modified to incorporate insect cell culture-based medium, coculturing with mammalian cells was no longer needed. In this study, we were able to overcome these temperature- and cell-dependent limitations and accommodate all of the strains tested by combining mammalian cell culture-based medium with insect cell culture-based medium.
INTRODUCTION
Bartonella is a genus of Gram-negative, facultative intracellular bacteria that have been detected in a plethora of insect and mammalian hosts (5, 14, 19, 25). Numerous Bartonella species have been associated with clinical illnesses ranging from mild skin lesions to more severe manifestations, including persistent fevers, neurological symptoms, and endocarditis (6, 10). These bacteria can be fastidious under current laboratory conditions, and attempts to isolate cells from pure culture of biological specimens are often unsuccessful, despite positive molecular detection (1, 7).
The majority of culturing methods for Bartonella species found in the literature focus on growth requirements for the two species commonly associated with human infection, Bartonella henselae and B. quintana (3, 9, 22, 27, 35). Through a review of the literature on methods for Bartonella isolation from biological samples, we found many differences in the culturing protocols. To summarize, most laboratories use cocultivation with mammalian cells or an axenic, insect cell culture-based medium or plating onto blood-supplemented agar (15, 24). The mammalian cell coculture and insect cell culture-based medium are usually used for enrichment before plating of samples on agar. Of particular interest is the variability between the cell culture systems, as this method is traditionally considered to be the most successful for the initial isolation of Bartonella species (22, 23, 27, 31). We focused our comparison on the protocols of three prominent laboratories that commonly culture Bartonella spp. and found the major differences to be localized to three main variables: (i) medium base (RPMI, medium 199 [M199], or Dulbecco modified Eagle medium [DMEM]), (ii) mammalian cell line (bovine endothelial, human endothelial, primate epithelial), and (iii) culturing temperature (35°C, 37°C).
In 2005, a cell-free, liquid medium called Bartonella alphaproteobacteria growth medium (BAPGM) was developed to detect Bartonella in veterinary and human blood samples (28). It is a modified formulation of liquid medium designed to support insect cells. This medium required sheep blood supplementation; however, upon discovering “Candidatus Bartonella melophagi” in commercial sheep blood (4), some researchers now increase the amount of the blood sample being tested instead of using commercial blood (24). More recently, Riess et al. (32) reported the benefits of another modified insect cell culture-based medium (Schneider's medium) that did not require whole-blood supplementation. This study demonstrated the ability of this medium to support three species of Bartonella (B. henselae, B. quintana, and B. vinsonii). Although this medium was not used to isolate Bartonella from biological samples in this study, we chose to use this formulation instead of BAPGM because whole-blood additive was not necessary.
Our laboratory has a particular interest in the possible association of Bartonella species with febrile illnesses and culture-negative infective endocarditis cases due to ongoing projects in many countries. We selected four representative species of Bartonella that have been associated with human disease and that are also phylogenetically diverse. B. henselae and B. quintana were extensively studied as pathogens accounting for the majority of Bartonella infections; both have been detected in cases of endocarditis (11, 18, 30). B. elizabethae was selected as it was originally isolated from a case of human infective endocarditis in North America (12). Lastly, B. tamiae was recently isolated from human patients in Thailand during a study on febrile illnesses (24).
The main goals of this study were to compare mammalian cell culture-based methodologies with the cell-free, insect cell culture medium-based protocols to determine the best conditions to culture diverse Bartonella species that may be of clinical importance. Based on a review of current protocols in the literature, we focused on three questions: (i) Is there an optimal medium to accommodate diverse species of Bartonella? (ii) Is the presence of mammalian cells necessary for culturing? and (iii) Is there a difference between the commonly used culturing temperatures of 35°C and 37°C?
MATERIALS AND METHODS
Bacterial isolates.
The following strains were used in the current study: B. henselae ATCC 49793, B. elizabethae ATCC 49927, B. quintana ATCC 51694, and B. tamiae ATCC BAA-1343. All strains were used at the lowest known laboratory passage available, estimated to be between 4 and 8 passages.
Cell culture and growth curves.
Growth curves were compared for five medium variations and two temperatures for a total of 10 test conditions (summarized in Table 1). Briefly, the M10 (mammalian cell culture-based) medium and MS10 (mammalian and insect cell culture-based combination) medium were analyzed in both the absence and presence of Vero E6 cells (primate kidney epithelial cells; ATCC CRL-1586). S10 (Schneider's) medium is a modification developed elsewhere from insect cell culture-based medium (32). S10 medium alone was not able to support Vero E6 cells; therefore, this medium was tested only without cells.
Table 1.
Medium formulations with and without VeroE6 cellsa
| Abbreviation | Medium | Vero E6 cells | Supplementation | pH | Reference |
|---|---|---|---|---|---|
| M10-V | M199b | Present | 10% fetal bovine serum, 1 mM sodium pyruvate, 10 mM HEPES, 1 μg/ml of amphotericin Bc | 7.6 | 11 |
| M10 | M199 | 10% fetal bovine serum, 1 mM sodium pyruvate, 10 mM HEPES, 1 μg/ml of amphotericin B | 7.6 | 11 | |
| S10 | Schneider's | 10% fetal bovine serum, 5% sucrose, 1 μg/ml of amphotericin B | 7.1 | 22 | |
| MS10-V | 40% M199b and 40% Schneider's | Present | 10% fetal bovine serum, 1 mM sodium pyruvate, 10 mM HEPES, 1 μg/ml of amphotericin B | 7.4 | This study |
| MS10 | 40% M199b and 40% Schneider's | 10% fetal bovine serum, 1 mM sodium pyruvate, 10 mM HEPES, 1 μg/ml of amphotericin B | 7.4 | This study |
The tests were repeated at both 35°C and 37°C.
With Earle's salts and l-glutamine.
Amphotericin B is recommended when biological specimens are cultured in our laboratory.
Conditions involving the presence of Vero E6 cells were prepared by standard methods (16) 24 h prior to inoculation. Briefly, Vero E6 cells were seeded into 24-well plates at 1 × 105 cells/ml in M10 medium, which consists of M199 (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (Invitrogen, Baltimore, MD), 10 mM sodium pyruvate (Invitrogen), and 10 mM HEPES (Invitrogen). After 24 h, the medium was removed and the cells were rinsed with sterile phosphate-buffered saline (PBS), before being replaced with the medium appropriate for the preinoculated test condition (described below).
Media were inoculated similarly for both cell-free and Vero E6 cell conditions. Frozen stocks of each bacterial isolate were diluted to between 1.8 × 103 and 5.5 × 104 cells/ml and placed into 40 ml of medium for each test condition (Table 1). The inoculated medium was mixed thoroughly by pipetting, before being aliquoted into 24-well plates and placed at either 35°C with 5% CO2 or 37°C with 5% CO2. Each inoculated medium was aliquoted into triplicate wells per time point so that each well was sampled only once. This protocol has been used previously (9) as a method to count total bacterial growth (intra- and extracellular) when conditions using cell culture are compared. At every time point, the bottom of each well was scraped to remove the adherent Vero E6 cells and the medium was mixed by pipetting before removal of a 200-μl sample. This plate was then discarded. The samples were serially diluted in sterile PBS, and dilutions were then spotted in triplicate onto 10% rabbit blood-supplemented brain heart infusion agar plates and quantified (CFU/ml) on the basis of a method described previously (29). The initial inoculated medium was considered time point 0, and the bacteria were quantified at that time point, followed by quantification on days 1 (24 h postinoculation), 3, 5, 7, and 9. Each experiment was conducted in triplicate and repeated at least twice. The growth curve data are presented as medians ± ranges.
Statistical analyses.
The data were analyzed for each strain and between conditions at the time course endpoint of 9 days or at the peak of growth using the Kruskal-Wallis or Wilcoxon rank sum tests with χ2 approximations. The day 9 endpoint was used for the purpose of comparing media and cell presence, as this was most biologically relevant to Bartonella culturing protocols, which usually require incubation for from 7 to 28 days (3, 22, 24). The peak growth of each strain was compared between temperatures at day 9, as it was the most logical point to show temperature-dependent differences because of such a discrepancy in growth at day 9, when the growth either plateaued or dramatically declined. Statistical analyses were conducted using JMP statistical software (SAS Institute, Cary, NC), with results considered significant if P was <0.05.
Immunofluorescence assay for autoagglutination.
It has been observed previously that autoagglutination is a phenotype corresponding to expression of trimeric autotransporter adhesins (TAAs) in B. henselae and B. quintana, which are crucial for host cell adherence (20, 36). Highly passaged laboratory strains have been shown to lose this phenotype (2). In the current study, we examined this phenotype first to confirm that the strains being tested were able to express the TAA proteins and second to qualitatively compare changes in this phenotype under different environmental conditions (according to mammalian cell presence, temperature, and medium formulation). Our protocol is a modification developed elsewhere by Riess et al. (33).
Briefly, the bacteria were prepared by growing bacterial isolates in M10, MS10, or S10 medium at either 35°C with 5% CO2 or 37°C with 5% CO2 in 24-well plates. The bacteria were used undiluted for all strains except B. elizabethae, which was diluted 1:2 in sterile PBS in order to maintain consistent bacterial numbers between strains. After 36 h, 2 μl of culture taken from the bottom of each well was pipetted onto sterilized 10-mm Gold Seal fluorescent-antibody slides (Daigger, Portsmouth, NH) and fixed for 10 min at room temperature with 4% paraformaldehyde. Slides were washed 3 times with PBS containing 0.1% Triton (TX-PBS), rinsed with distilled water, and air dried. Nonspecific binding was blocked using 20 μl of 3% bovine serum albumin (Jackson ImmunoResearch, West Grove, PA) diluted in TX-PBS. After 30 min at room temperature, excess blocking buffer was removed using a pipette and 20 μl of mouse immune serum (ProSci Inc., Poway, CA) diluted 1:32 in sterile PBS was added. After the slides were washed 3 times with TX-PBS, 20 μl fluorescein isothiocyanate (FITC)-labeled, goat anti-mouse IgG conjugate (Kirkegaard & Perry Laboratories, Gaithersburg, MD) diluted 1:150 in sterile PBS was added. Slides were incubated in a moist chamber for 30 min at room temperature, washed 2 times with PBS, rinsed with distilled water, and air dried. Slides were then read using a Zeiss Axioscope2 Plus camera and fluorescence microscope (Thornwood, NY) with ×40 objectives and ×10 oculars. Images were processed using Photoshop CS software (Adobe, San Jose, CA).
RESULTS
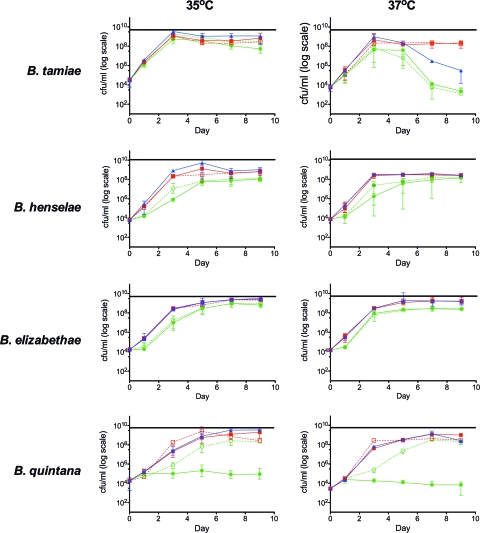
Figure 1 plots the growth curves for each Bartonella species tested under five conditions at 35°C and at 37°C. The first two are the mammalian cell culture-based conditions with and without Vero E6 cells (M10-V medium and M10 medium, respectively), the next one is growth on axenic S10 medium, and the last two use a combination of the mammalian and insect cell culture-based media with and without Vero E6 cells (MS10-V medium and MS10 medium, respectively).
Fig. 1.
Growth curves of four Bartonella species, B. tamiae, B. henselae, B. elizabethae, and B. quintana, measured at two temperatures, 35°C and 37°C. There are five conditions tested for each strain at both temperatures. ●, M10 cell-free medium; ○, M10 medium with Vero E6 cells; ■, MS10 cell-free medium; □ MS10 medium with Vero E6 cells; ▴, S10 cell-free medium.
Visual inspection of the growth curves shows that within our period of observation, most strains reach a peak of growth and then viability either plateaus or declines. The only exception to this trend is B. quintana in M10 medium, where the peak was minimal and the viability did not vary much over the 9-day time course. Due to the logarithmic scale used to display the data, there are some statistical differences that may not be evident without the further analysis.
At 9 days postinoculation, the CFU counts were consistently similar between cultures grown in MS10 and S10 media (χ2 ≤ 2.877, P ≥ 0.089), with two exceptions: when B. tamiae and B. quintana were grown at 37°C, cultures grown in MS10 medium yielded significantly higher CFU counts than those grown in S10 medium (χ2 ≥ 8.366, P ≤ 0.004). Additionally, in each pair-wise comparison and at both 35°C and 37°C, both MS10 medium and S10 medium consistently yielded significantly higher CFU counts than M10 or M10-V medium (χ2 ≥ 8.308, P ≤ 0.004).
To determine if addition of mammalian cells (specifically in this study, Vero E6 cells) affected the Bartonella viability by 9 days postinoculation, we compared CFU counts within media with and without cells (M10-V for Vero cells with M10 medium and MS10-V for Vero cells with MS10 medium). No significant differences between endpoint viabilities were found for any of the strains tested, with the exception of B. quintana, which yielded conflicting results. At both 35°C and 37°C, under the combination medium conditions (MS10 and MS10-V media), the CFU counts were significantly lower when cells were present (χ2 ≥ 8.308, P ≤ 0.004). However, when growth was under the mammalian cell culture-based conditions (M10 and M10-V media), the CFU counts of B. quintana were significantly higher when cells were present (χ2 ≥ 8.308, P ≤ 0.004).
Using the rationale that the growth curves either plateau or decline after they reach the peak, we compared the effects of temperature on peak CFU values. For visual comparison, a horizontal line that represents the peak growth at 35°C for each strain is drawn for comparison with the peak growth at 37°C (Fig. 1). The growth peak was significantly higher at 35°C than at 37°C for all strains in MS10 and S10 media (χ2 ≥ 8.337, P ≤ 0.004), with the exception of the growth peak for B. elizabethae, which yielded no significant difference in peak growth between the two temperatures. We restricted our analysis to these two media, as the growth peaks under the mammalian cell culture-based conditions (M10 and M10-V media) were consistently lower than those under the conditions with S10, MS10, and MS10-V media.
The autoagglutination phenotype was examined for two main reasons: first, to test if the strains used in our experiments were able to express the TAAs associated with this phenotype (36), as it has been shown to be lost after multiple passages in vitro (2). Second, the assay was utilized to investigate temperature- or medium-dependent changes to this phenotype under our current test conditions.
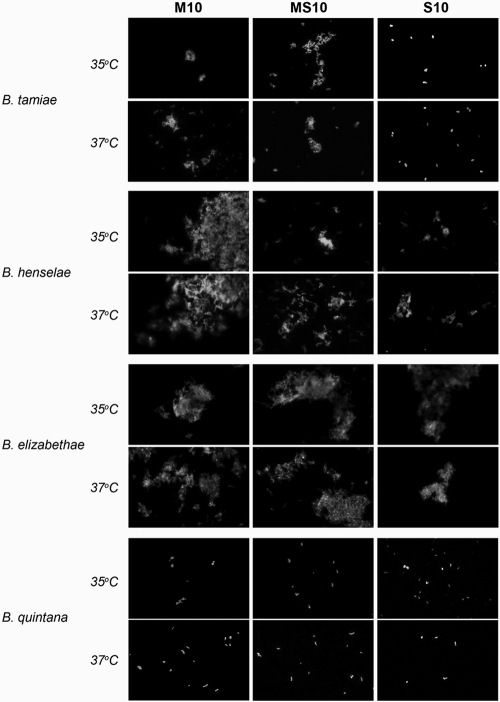
Results of the agglutination assay are summarized in Fig. 2. Agglutination of B. tamiae was noted in M10 and MS10 media; however, in the S10 medium, no agglutination was seen. This change appeared to be only medium dependent, as results were similar between the two temperatures. Agglutination of B. henselae also appeared to be medium dependent and temperature independent. In M10 medium, B. henselae cultures produced very large aggregations. This could explain the large range in viability measured during the growth of B. henselae under the M10 and M10-V medium conditions (Fig. 1B). In the MS10 and S10 media, B. henselae still agglutinated; however, the clumps were smaller in comparison with those in M10 medium. With respect to B. elizabethae, agglutination was seen in all media and at both temperatures. There did not appear to be a difference in the size or quantity of aggregates under any condition, which agrees with the similarities seen between conditions from the quantitative growth curve data for B. elizabethae. Conversely, B. quintana did not agglutinate under any of the conditions tested.
Fig. 2.
Differences in autoagglutination between different temperatures and media after 36 h. Representative immunofluorescent micrographs of each bacterial strain grown in axenic media (M10, MS10, and S10) at both 35°C and 37°C are shown. Images were taken with a ×40 objective before the contrast was adjusted, and the images were cropped using Adobe Photoshop.
DISCUSSION
The data presented in this study showed that there are medium- and temperature-dependent growth differences in vitro between diverse isolates of the Bartonella genus. We compared three culturing techniques, the first using mammalian cell culture-based conditions with and without the presence of Vero E6 cells (M10-V and M10, respectively), the second using insect cell culture-based medium (S10), and the third using a combination of the mammalian and insect cell culture-based medium with and without Vero E6 cells (MS10-V and MS10, respectively).
Our data showed that the combination media, MS10 and MS10-V, were able to support the growth of all four Bartonella spp. well at both 35°C and 37°C. These growth curves were comparable to the curve with S10 medium for all cases except B. tamiae at 37°C, where by the end of the time course at day 9 the viability of the bacteria in S10 medium declined to a significantly lower level than that under the conditions with MS10 and MS10-V media.
Under the conditions with MS10 and MS10-V media, our data suggest that the presence of Vero E6 cells did not significantly affect the growth or sustainability of the bacterial cultures, suggesting that cell culture may not be necessary for culture of these bacterial species. However, due to the high bacterial loads that were reached for these growth curves, it is difficult to interpret this to be true, as monolayers had lifted off at between 5 and 7 days in most cases (data not shown). B. quintana was the only strain for which the presence of mammalian cells in the culture (M10-V medium) was a significant benefit to growth compared to the growth achieved by culture under the same conditions but with cell-free (M10) medium. These results agree with those from previous work suggesting that under mammalian cell culture-based conditions, cell coculture is important for B. quintana growth (3, 22). Recent developments, however, have shown that cell culture is unnecessary when insect cell culture-based medium is used (28, 32). In the near future, our laboratory will be comparing the medium conditions described in this study with biological samples to more accurately test if cell coculture is necessary upon initial isolation of Bartonella species that have not been adapted to laboratory conditions. For this reason, we see the ability of the MS10 medium to sustain mammalian cell culture as a benefit if coculture becomes necessary. Our laboratory also tested the combination MS10 medium with other cell lines and found that it was able to adequately support Huh-7 (human hepatocyte) and THP-1 (human monocyte) cell lines (microscopic observations; data not shown).
Vero E6 cells were chosen for this study as there is evidence in the literature that both B. henselae and B. quintana are able to adhere to different cell lines in vitro, including monocytes, endothelial cells, and epithelial cells from various mammalian hosts (2, 13, 21, 34). Additionally, Vero E6 cells were used to successfully isolate B. tamiae for the first time (24). We acknowledge that endothelial cells are typically chosen for culturing Bartonella species; however, they may not be necessary when the medium composition is changed, as shown in the present work with the use of combination medium or by replacement with insect cell culture-based medium. As vector-borne bacteria, Bartonella species are required to survive in various environments. Clinically, Bartonella species have been shown to cause a variety of clinical manifestations, such as bacteremia, endocarditis, bacillary angiomatosis, and peliosis, where the bacteria interact with multiple cell types within the same host (6, 10). Endothelial cells such as human umbilical vein endothelial cells provide an excellent in vitro model with which to study the pathogenesis of Bartonella, as there is evidence that endothelial cells are invaded in vivo (8, 14); however, for the purpose of a culturing protocol to isolate Bartonella species from biological material, endothelial cells may not be optimal, as many of the cell lines require a higher passaging frequency and extra growth factors, which increase the cost substantially.
The bacterial strains for these experiments were used at the lowest passage possible, as B. henselae has been shown to undergo phase variation after multiple laboratory passages and shows decreased autoagglutination in association with decreased cell adherence (2, 26). Immunofluorescence was used to assess the presence of the autoagglutination phenotype and also to examine if there were changes associated with temperature or medium differences under our test conditions (summarized in Fig. 2). B. quintana was the only strain that did not show agglutination under any condition. We still, however, observed medium- and cell-dependent differences in growth (Fig. 1) which are similar to those found in the literature (22). Specifically, we found that coculture with mammalian cells under mammalian cell culture-based conditions (M10-V medium) was required for growth; however, when insect cell culture-based medium is incorporated (S10 or MS10 medium), B. quintana was able to grow in cell-free medium. Autoagglutination is associated with expression of the TAA proteins (26, 36). Although medium-dependent differences were seen with B. tamiae and B. henselae, it is unclear whether the agglutination phenotype correlates with growth or viability of the culture. It would be interesting to further investigate if there are changes in transcriptional regulation or if genes associated with host adaptability or pathogenicity are lost under these various culture conditions and also over a broader range of temperatures that includes lower temperatures more relevant to a vector host.
There is a discrepancy in the literature concerning the temperature for culturing Bartonella species. The majority of studies that coculture Bartonella species with mammalian cells use 37°C; in contrast, studies that use axenic insect cell culture-based medium and agar use a culture temperature of 35°C (15, 17, 28). Although a 2°C difference may seem minor or trivial, it was interesting to see that there were statistically significant differences in peak growth between the two temperatures for three of the four strains tested (B. elizabethae was the exception, showing no significant difference in peak growth between the two temperatures). Additionally, the drastic divergence in growth between these temperatures for B. tamiae was surprising, as this strain was isolated from a febrile human patient. We are very interested to continue this investigation, as the temperature-dependent growth requirements may provide new insight into the life cycle and biological niche of this microorganism. This information is important to know because these two temperatures are very commonly used in the clinical laboratory setting and may sometimes be used interchangeably. Additionally, because of the minor difference in temperature, incubator settings should be calibrated properly, as the temperatures can fluctuate and may impact the growth of some Bartonella species.
The goals of this study were to compare key components of common Bartonella culturing methodologies, as well as combine them to investigate the best conditions under which to culture diverse Bartonella species of clinical importance. We have shown that by combining the mammalian and insect cell culture-based media (MS10 medium) we were able to achieve peak growth comparable to that achieved with the insect cell culture-based medium alone (S10 medium) at 35°C but that at 37°C the MS10 medium performed significantly better for both B. tamiae and B. quintana. Our data suggest that mammalian cell coculture is not necessary for growth of laboratory-adapted Bartonella strains; however, the combination medium is still able to support mammalian cell culture, which may remain an important variable during initial isolation of Bartonella from biological samples. Our data also show that B. tamiae has temperature-dependent growth requirements that are not adequately met with the mammalian or insect cell culture-based medium alone at 37°C, regardless of mammalian cell coculture.
This is the first study, to our knowledge, that has compared these methodologies using diverse Bartonella spp. These findings are important for clinical microbiologists, as Bartonella species are fastidious organisms and Bartonella is a diverse genus whose members are a growing concern as emerging or reemerging pathogens.
ACKNOWLEDGMENTS
We thank the ASM/CCID Postdoctoral Fellowship Program for funding, N. Zeidner for the use of his immunofluorescence microscope, R. Eisen for her guidance and expertise with the statistical analysis, and also R. Gilmore for his scientific advice and review of the manuscript.
Footnotes
Published ahead of print on 2 February 2011.
REFERENCES
- 1. Bai Y., et al. 2010. Enrichment culture and molecular identification of diverse Bartonella species in stray dogs. Vet. Microbiol. 146:314–319 [DOI] [PubMed] [Google Scholar]
- 2. Batterman H. J., Peek J. A., Loutit J. S., Falkow S., Tompkins L. S. 1995. Bartonella henselae and Bartonella quintana adherence to and entry into cultured human epithelial cells. Infect. Immun. 63:4553–4556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Battisti J. M., Minnick M. F. 2008. Laboratory maintenance of Bartonella quintana, p. 1-1–1-13 In Current protocols in microbiology, unit 3C. John Wiley & Sons, Inc., New York, NY: [DOI] [PubMed] [Google Scholar]
- 4. Bemis D. A., Kania S. A. 2007. Isolation of Bartonella sp. from sheep blood. Emerg. Infect. Dis. 13:1565–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Breitschwerdt E. B., et al. 1995. Endocarditis in a dog due to infection with a novel Bartonella subspecies. J. Clin. Microbiol. 33:154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Breitschwerdt E. B., Maggi R. G., Chomel B. B., Lappin M. R. 2010. Bartonellosis: an emerging infectious disease of zoonotic importance to animals and human beings. J. Vet. Emerg. Crit. Care (San Antonio) 20:8–30 [DOI] [PubMed] [Google Scholar]
- 7. Breitschwerdt E. B., et al. 2007. Bartonella species in blood of immunocompetent persons with animal and arthropod contact. Emerg. Infect. Dis. 13:938–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brouqui P., Lascola B., Roux V., Raoult D. 1999. Chronic Bartonella quintana bacteremia in homeless patients. N. Engl. J. Med. 340:184–189 [DOI] [PubMed] [Google Scholar]
- 9. Chenoweth M. R., Somerville G. A., Krause D. C., O'Reilly K. L., Gherardini F. C. 2004. Growth characteristics of Bartonella henselae in a novel liquid medium: primary isolation, growth-phase-dependent phage induction, and metabolic studies. Appl. Environ. Microbiol. 70:656–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chomel B. B., et al. 2009. Ecological fitness and strategies of adaptation of Bartonella species to their hosts and vectors. Vet. Res. 40:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chomel B. B., et al. 2009. Bartonella endocarditis: a pathology shared by animal reservoirs and patients. Ann. N. Y. Acad. Sci. 1166:120–126 [DOI] [PubMed] [Google Scholar]
- 12. Daly J. S., et al. 1993. Rochalimaea elizabetae sp. nov. isolated from a patient with endocarditis. J. Clin. Microbiol. 31:872–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dehio C., Meyer M., Berger J., Schwarz H., Lanz C. 1997. Interaction of Bartonella henselae with endothelial cells results in bacterial aggregation on the cell surface and the subsequent engulfment and internalisation of the bacterial aggregate by a unique structure, the invasome. J. Cell Sci. 110(Pt 18):2141–2154 [DOI] [PubMed] [Google Scholar]
- 14. Droz S., et al. 1999. Bartonella koehlerae sp. nov., isolated from cats. J. Clin. Microbiol. 37:1117–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duncan A. W., Maggi R. G., Breitschwerdt E. B. 2007. A combined approach for the enhanced detection and isolation of Bartonella species in dog blood samples: pre-enrichment liquid culture followed by PCR and subculture onto agar plates. J. Microbiol. Methods 69:273–281 [DOI] [PubMed] [Google Scholar]
- 16. Freshney R. I. 1992. Animal cell culture: a practical approach, 2nd ed. IRL Press at Oxford University Press, Oxford, England [Google Scholar]
- 17. Gouriet F., Fenollar F., Patrice J. Y., Drancourt M., Raoult D. 2005. Use of shell-vial cell culture assay for isolation of bacteria from clinical specimens: 13 years of experience. J. Clin. Microbiol. 43:4993–5002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jenkins N. E., Ferguson D. J., Alp N. J., Harrison T. G., Bowler I. C. 2009. Urban trench fever presenting as culture-negative endocarditis. QJM 102:63–65 [DOI] [PubMed] [Google Scholar]
- 19. Kabeya H., et al. 2010. Detection of Bartonella tamiae DNA in ectoparasites from rodents in Thailand and their sequence similarity with bacterial cultures from Thai patients. Vector Borne Zoonotic Dis. 10:429–434 [DOI] [PubMed] [Google Scholar]
- 20. Kaiser P. O., et al. 2008. The head of Bartonella adhesin A is crucial for host cell interaction of Bartonella henselae. Cell. Microbiol. 10:2223–2234 [DOI] [PubMed] [Google Scholar]
- 21. Kempf V. A., et al. 2000. Interaction of Bartonella henselae with endothelial cells results in rapid bacterial rRNA synthesis and replication. Cell. Microbiol. 2:431–441 [DOI] [PubMed] [Google Scholar]
- 22. Koehler J. E., Quinn F. D., Berger T. G., LeBoit P. E., Tappero J. W. 1992. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N. Engl. J. Med. 327:1625–1631 [DOI] [PubMed] [Google Scholar]
- 23. Kosoy M., et al. 2010. Identification of Bartonella infections in febrile human patients from Thailand and their potential animal reservoirs. Am. J. Trop. Med. Hyg. 82:1140–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kosoy M., et al. 2008. Bartonella tamiae sp. nov., a newly recognized pathogen isolated from three human patients from Thailand. J. Clin. Microbiol. 46:772–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kosoy M., Murray M., Gilmore R. D., Jr., Bai Y., Gage K. L. 2003. Bartonella strains from ground squirrels are identical to Bartonella washoensis isolated from a human patient. J. Clin. Microbiol. 41:645–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kyme P., Dillon B., Iredell J. 2003. Phase variation in Bartonella henselae. Microbiology 149:621–629 [DOI] [PubMed] [Google Scholar]
- 27. La Scola B., Raoult D. 1999. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J. Clin. Microbiol. 37:1899–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maggi R. G., Duncan A. W., Breitschwerdt E. B. 2005. Novel chemically modified liquid medium that will support the growth of seven Bartonella species. J. Clin. Microbiol. 43:2651–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miles A. A., Misra S. S., Irwin J. O. 1938. The estimation of the bactericidal power of the blood. J. Hyg. (Lond.) 38:732–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Montcriol A., et al. 2009. Fatal myocarditis-associated Bartonella quintana endocarditis: a case report. J. Med. Case Rep. 3:7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morway C., et al. 2008. A longitudinal study of Bartonella infection in populations of woodrats and their fleas. J. Vector Ecol. 33:353–364 [DOI] [PubMed] [Google Scholar]
- 32. Riess T., et al. 2008. Analysis of a novel insect cell culture medium-based growth medium for Bartonella species. Appl. Environ. Microbiol. 74:5224–5227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Riess T., Raddatz G., Linke D., Schafer A., Kempf V. A. 2007. Analysis of Bartonella adhesin A expression reveals differences between various B. henselae strains. Infect. Immun. 75:35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schulte B., et al. 2006. Bartonella quintana variably expressed outer membrane proteins mediate vascular endothelial growth factor secretion but not host cell adherence. Infect. Immun. 74:5003–5013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wong M. T., Thornton D. C., Kennedy R. C., Dolan M. J. 1995. A chemically defined liquid medium that supports primary isolation of Rochalimaea (Bartonella) henselae from blood and tissue specimens. J. Clin. Microbiol. 33:742–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang P., et al. 2004. A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and autoaggregation in Bartonella quintana. Proc. Natl. Acad. Sci. U. S. A. 101:13630–13635 [DOI] [PMC free article] [PubMed] [Google Scholar]