Abstract
Microsporidia are currently considered emerging pathogens responsible for life-threatening infections in organ transplant recipients. Here, we describe the first cases of intestinal microsporidiosis by Enterocytozoon bieneusi genotype D in two non-HIV-infected renal transplant recipients from Spain. Previously reported cases of microsporidiosis in organ transplant recipients have also been reviewed, highlighting the necessity of considering organ transplant recipients a risk group for microsporidiosis. A systematic search for these parasites is recommended in cases of persistent diarrhea and in the differential diagnosis of other syndromes, such as chronic fever of unknown etiology.
INTRODUCTION
Microsporidia are ubiquitous, obligate intracellular opportunistic parasites, recently related to fungi, capable of infecting a wide range of vertebrate and invertebrate hosts (2, 12). Within microsporidia, 8 genera and 14 species have been associated with human infections, among which Enterocytozoon bieneusi and Encephalitozoon intestinalis are the most commonly reported (12). These opportunistic pathogens cause a variety of systemic and nonsystemic diseases, with chronic diarrhea as the most common clinical manifestation, although the spectrum of diseases caused by them is broad and includes eye, respiratory, renal, and central nervous system infections (12).
Most microsporidial infections have been reported to occur in severely immunocompromised individuals, mainly HIV/AIDS patients, but cases in HIV-negative people, including travelers and elderly people, are constantly increasing (11, 27). Additionally, the number of non-HIV-infected patients with other forms of immunosuppression is also increasing. Among these, organ transplant recipients (OTR) have recently been considered a group of patients at risk for these pathogens (22). To date, only 21 cases of microsporidiosis in solid organ transplant (SOT) and bone marrow transplant (BMT) recipients who were HIV negative have been described (4, 13–17, 19, 20, 22, 23, 28–31, 34, 40, 44, 49). In these patients, diarrhea is the most frequent clinical manifestation and E. bieneusi the species mainly involved (Table 1). Moreover, there are three retrospective studies in which microsporidia have been reported to occur in transplant patients (25, 35, 46). Liguory and collaborators studied microsporidial infection in stool specimens from 100 patients obtained over a 6-year period (1994 to 2000), and they found 8 organ transplant recipients (6 renal, 1 liver, and 1 heart-lung) who were positive for E. bieneusi (25). Rabodonirina and collaborators, in a retrospective study carried out in France, found 23 cases of microsporidiosis in transplant patients, including 3 who had already been described in the literature between 1993 and 2001 (35). Recently, ten Hove and collaborators performed a retrospective phylogenetic analysis on isolates of E. bieneusi collected between 2003 and 2004, and they included five kidney transplant recipients that were positive for this microsporidian (46, 47).
Table 1.
Microsporidiosis in transplant recipientsa
| Case (reference[s]) | Type of transplant (no. of patients) | Age (yr) | Gender (no. of patients) | Species/genotype (no. of patients) | Immunosuppressive treatment agent(s) | Clinical manifestation(s) | Laboratory diagnosis method(s) | Treatment/outcome | Country/Publication yr |
|---|---|---|---|---|---|---|---|---|---|
| 1b (25) | Kidney (6); liver (1); heart-lung (1) | E. bieneusi/C (7) and D (1) | NA | Diarrhea | MTS, PCR, PCR-RFLP | NA | France/2001 | ||
| 2b (35) | Kidney (14); liver (5); heart and/or lung (4) | F (7); M (16) | NA | ATG, CS, AZ, MMF, tacrolimus | Asymptomatic, diarrhea, wt loss | PCR, TEM | Albendazole, fumagillin | France/2003 | |
| 3b (46) | Kidney (5) | F (2); M (3) | E. bieneusi/C | NA | NA | PCR | NA | Netherlands/2009 | |
| 4 (40) | Liver | 48 | F | Undetermined | Tacrolimus, prednisone | Intestinal | MTS | Metronidazole/recovery | U.S./1995 |
| 5 (34) | Heart; lung | 48 | M | E. bieneusi | CS, AZ, methylprednisone | Intestinal | MTS, TEM | Metronidazole/recovery | France/1996 |
| 6 (20) | Bone marrow | 27 | F | Undetermined | l-Asparaginasa, vincristin, daunomycine, prednisone, CS | Intestinal, respiratory | TEM | India/1997 | |
| 7 (16) | Kidney | 46 | M | E. bieneusi | AZ, CS, prednisone, MMF | Intestinal | MTS, PCR | Albendazole/recovery | France/1999 |
| 8 (16) | Kidney | 24 | M | E. bieneusi | Thymoglobuline, prednisone, CS, AZ, MMF | Intestinal | MTS, PCR | France/1999 | |
| 9 (17) | Heart | 48 | M | E. bieneusi | CS, AZ, methylprednisone | Intestinal | MTS | U.S./1999 | |
| 10 (29) | Kidney | 38 | F | E. bieneusi | Tacrolimus, prednisone, MMF | Intestinal | MTS, PCR | Albendazole, metronidazole/recovery | France/2000 |
| 11 (41) | Liver | 36 | F | E. bieneusi/C | Tacrolimus | Intestinal | MTS, PCR | Albendazole/ E. bieneusi persistence | Germany/2001 |
| 12 (15) | Liver | 36 | F | E. bieneusi | Tacrolimus | Intestinal | MTS, PCR-RFLP | Albendazole/symptomatic improvement, E. bieneusi persistence | Germany/2001 |
| 13 (23) | Kidney | 39 | M | Encephalitozoon sp. | AZ, CS, Prednisone | Fever, renal impairment | GCS, TEM | Albendazole/recovery | South Africa/2001 |
| 14 (30) | Kidney | 43 | F | E. cuniculi/type III strain | Methylprednisone | Disseminated | GCS, IFAT, TEM, PCR | Albendazole, topical fumagillin (keratitis)/death related to neurological involvement | Canada/2002 |
| 15 (31) | Liver | NA | E. bieneusi | Tacrolimus | NA | MTS, PCR | Fumagillin/recovery | France/2002 | |
| 16 (31) | Kidney | NA | E. bieneusi | Tacrolimus, prednisone | NA | MTS, PCR | Fumagillin/recovery | France/2002 | |
| 17 (14) | Kidney | 42 | M | E. cuniculi | Rapamycin, CS, prednisone | Disseminated | IFAT, TEM | Albendazole, fumagillin/relapse 1 year later | Mexico/2003 |
| 18 (28) | Kidney | 45 | F | E. cuniculi | Steroids | Disseminated | PCR, TEM | Antimicrobial therapy/death | U.S./2003 |
| 19 (33, 45) | Bone marrow | 21 | F | E. cuniculi/type III strain | Thiotepa, CYP, ATG, CS | Pulmonary | MTS, TEM, PCR | NA | U.S./2004-2005 |
| 20 (4) | Pancreas; kidney | 43 | M | Encephalitozoon sp. | Tacrolimus, MMF, prednisone, AZ | Disseminated | TEM | Postmortem diagnosis | U.S./2004 |
| 21 (19) | Cornea | 60 | M | Undetermined | Prednisolone acetate | Keratoconjunctivitis | CW, GS, PCR | Topical ciprofloxacin | India/2006 |
| 22 (22) | Kidney | 64 | M | E. bieneusi | Tacrolimus, MMF | Intestinal | Uvitex-2B, PCR | France/2009 | |
| 23 (22) | Liver | 45 | M | E. bieneusi | CS, everolimus | Intestinal | HE, Uvitex-2B, PCR | France/2009 | |
| 24 (44) | Kidney | 38 | F | E. cuniculi/type IV strain | Thymoglobuline, MMF, CsA | Disseminated | Uvitex-2B, PCR | Albendazole/recovery | France/2010 |
| Current report | Kidney | 66 | M | E. bieneusi /D | Steroids, tacrolimus, MMF | Intestinal | MTS, PCR | Filgrastrim/recovery | Spain |
| Current report | Kidney | 54 | M | E. bieneusi /D | Tacrolimus, MMF | Intestinal | MTS, PCR | Albendazole, metronidazole/recovery | Spain |
F, female; M, male; CS, cyclosporine; AZ, azathioprine; MMF, mycophenolate mofetil; CsA, cyclosporine A; Cyp, cyclophosphamide; ATG, antilymphocyte globulin; MTS, modified trichrome stain; RFLP, restriction fragment length polymorphism; GCS, Gram chromotrope stain; HE, hematoxylin-eosin stain; TEM, transmission electron microscopy; IFA, indirect immunofluorescence assay; PCR, polymerase chain reaction; CW, calcofluor white; GS, Gram stain; NA, not available; ND, not determined.
Retrospective study.
We describe the first findings of intestinal infection caused by E. bieneusi in two renal transplant recipients from Gran Canaria, Spain, and we review the published cases of microsporidiosis in SOT and BMT recipients.
Case Reports
Patient 1.
A 66-year-old male who received a renal transplant in April 2009 was admitted to the nephrology unit of the Hospital Universitario Insular de Gran Canaria (Spain) for severe leucopenia (1,000 cells/mm3) and abundant liquid diarrhea in July of the same year. He had a history of chronic renal failure secondary to a nephroangiosclerosis, arterial hypertension, and a heart attack. Immunosuppressive therapy consisted of steroids, tacrolimus, and mycophenolate mofetil (MMF). This treatment, as well as valganciclovir and septrim, was suspended after admittance to the hospital, due to the suspicion of pharmacologic toxicity. No bacterial or viral pathogens were found in the stool samples. The colonoscopy was normal. Cytomegalovirus (CMV) antigen in colonic mucosa was positive. Calcofluor white (48) and modified trichrome (53) stains showed structures evocative of microsporidial spores in stool samples (Fig. 1). The patient was treated with three doses of filgrastrim, fluid therapy, and diet control. The clinical symptoms disappeared, and the patient was discharged afebrile, with a normal white blood cell count and normal bowel movements.
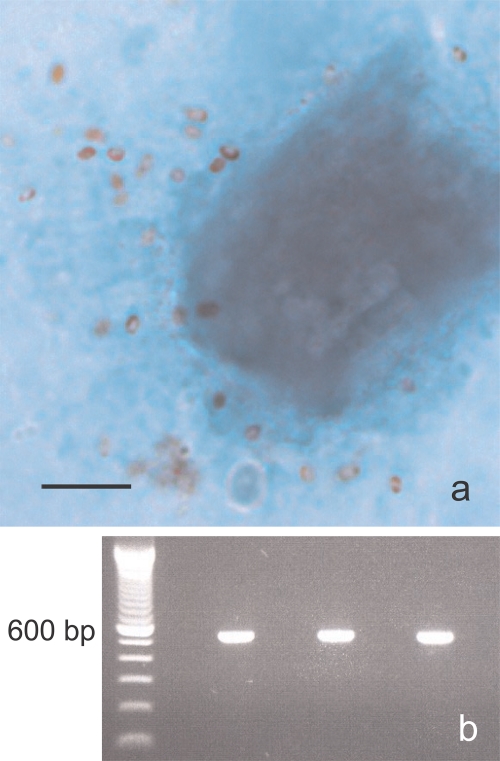
Fig. 1.
(a) Microsporidial spores stained with a modified trichrome stain from patient 1. Bar, 5 μm. (b) PCR amplification of the rDNA coding region containing the 243 bp of the ITS of E. bieneusi. First lane, molecular marker (100-bp ladder); third lane, patient 1; fifth lane, patient 2; seventh lane, positive control; eighth lane, negative control.
Patient 2.
A 54-year-old man with a history of kidney transplant in 1994 was admitted to the nephrology unit of the Hospital Universitario Insular de Gran Canaria for persistent liquid diarrhea on 9 June 2009. His immunosuppression regimen consisted of tacrolimus and MMF. The patient did not improve after MMF suspension and dietary treatment. Laboratory tests showed a slight deterioration of renal function. Cytomegalovirus antigen was negative. Clostridium difficile toxin was detected in fecal samples. Fecal smears showed microsporidial spores stained by calcofluor white (48) and modified trichrome (53) stains. The clinical symptoms disappeared after initiation of fluid therapy, diet control, and metronidazole treatment. Normal bowel habits and renal function were recovered. Two weeks later, the patient showed episodes of diarrhea with epigastric pain, microsporidial spores were observed in stool samples, and results for C. difficile toxin, virus, bacteria, and parasites were negative. Treated with albendazole, he became asymptomatic but continued seeding a smaller amount of microsporidial spores, detected only by PCR.
MATERIALS AND METHODS
Staining methods.
Thin smears from one diarrheic stool sample from patient 1 and three samples from patient 2 were prepared and stained, using calcofluor white stain (48) and Weber's chromotrope-based stain (53).
DNA extraction and purification.
DNA from unpreserved stools was extracted by following the methods described earlier (5). DNA from fecal samples was extracted by bead disruption of spores using a fast-DNA-spin kit, according to the manufacturer's instructions (Bio 101, Carlsbad, CA). PCR inhibitors were removed using a QIAquick PCR kit (Qiagen, Chatsworth, CA).
PCR amplification.
Microsporidial small-subunit-rRNA (SSU-rRNA) coding regions were amplified using the following species-specific primers: EBIEF1/EBIER1 for E. bieneusi (5), SINTF/SINTR for E. intestinalis (6), EHELF/EHELR for Encephalitozoon hellem (50), and ECUNF/ECUNR for Encephalitozoon cuniculi (7). The PCR amplification was done with a GenAmp kit (Perkin-Elmer Cetus, Norwalk, CT) according to the manufacturer's procedures and the conditions for the reaction described previously (8). Purified samples were tested for the presence of PCR inhibitors, as described previously (8). Amplification products were analyzed, electrophoresis was performed with 2% agarose gel, and the samples were visualized by ethidium bromide staining (8).
DNA sequencing analysis.
Genotyping of E. bieneusi was performed by sequence analysis of the internal transcribed spacer (ITS) region of ribosomal DNA (rDNA). For this purpose, primers that amplified a fragment of 536 bp containing the 243 bp of the ITS were designed (primer F, 5′CTTCGGCTCTGAATATCTAT3′, and primer R, 5′GCCACTACTAACGGAATCCTA3′). PCR amplifications were performed with the following cycling conditions: denaturing at 94°C for 30 s, alignment at 55°C for 30 s, and extension at 72°C for 90 s. Each PCR product was sequenced in both directions using a BigDye Terminator sequencing kit with an ABI PrismR 3130 genetic analyzer (Applied Biosystems). The resulting sequences were analyzed by the Bioedit program and compared with reference sequences from GenBank.
RESULTS
Staining methods.
The two patients studied showed structures evocative of microsporidial spores in the sample analyzed from patient 1 and in two samples from patient 2, obtained before albendazole treatment when analyzed by calcofluor white staining (48) and Weber's chromotrope-based staining (53). Spores in the chromotrope-stained smears appeared pinkish red and measured 0.9 to 1.2 μm in length. Many spores exhibited the characteristic posterior vacuole and beltlike stripe in the middle (Fig. 1a). However, in the second stool sample from patient 2, analyzed 2.5 months later, and after albendazole treatment, no microsporidial spores were observed by the staining methods.
PCR.
PCR was performed with unfixed stools from the two patients. Amplification of DNA isolated with specific primers for the most common microsporidia infecting humans showed positive results with E. bieneusi-specific primers (5) in both cases for all samples. However, E. intestinalis-specific PCR (6), E. hellem-specific PCR (50), and E. cuniculi-specific PCR (7) were negative (Fig. 1b) for the two patients studied.
Genotyping.
Genotyping of the E. bieneusi isolates was performed by sequence analysis of the ITS region of rDNA. The sequence analysis of PCR amplified products showed 100% homology with genotype D in both cases (GenBank accession number AF101200.1) (38).
DISCUSSION
Enterocytozoon bieneusi is the most common microsporidian associated with human disease, particularly in severely immunosuppressed individuals with CD4+ counts of <100/mm3 (12). In the presence of HIV infection, it is associated with diarrhea and wasting syndrome, and cellular immunoresponse has been considered essential for the control and elimination of this microsporidian (12). In SOT and BMT recipients, an immunosuppressive therapy is always prescribed, leading to a profound cellular immunodeficiency (22). However, few cases of microsporidiosis have been reported to occur in transplant patients (Table 1). Chronic diarrhea is the main clinical manifestation in most infections and E. bieneusi the most common species encountered in more than half of the cases in OTR, followed by E. cuniculi (Table 1) (22, 35). This agrees with the observations for the two patients in our study, with persistent diarrhea with E. bieneusi detected in stool samples by PCR and a modified trichrome stain. However, microsporidiosis occurred in reported cases from 19 days up to 7 years after transplantation (22). In patient 1 in our study, it appeared 3 months later, but in patient 2, it appeared 15 years after transplantation.
In both patients, we detected other microorganisms that have been associated with gastrointestinal symptoms in transplant recipients, including diarrhea. Patient 1 was positive for CMV antigen in colonic mucosa, which is a common finding, since CMV infection is one of the major infectious complications in transplant recipients with nonsystemic symptoms that include fever, diarrhea, myalgias, malaise, and, in severe cases, hepatitis, pneumonia, and colitis (18). However, the gastrointestinal tract is one of the least common sites of CMV disease. Taking into account that this patient showed no other symptoms besides diarrhea and received prophylaxis with an antiviral, we believe that E. bieneusi would play an important role in the diarrhea observed. It should be noted that in one of the renal transplant recipients reviewed, the authors suggest that CMV infection may further enhance the susceptibility to microsporidial infection (30). Patient 2 showed a test positive for Clostridium difficile, which is a significant pathogen leading to diarrhea and colitis in transplant recipients (32). However, the majority of C. difficile infections concern patients in the early posttransplant period, with a prior long-lasting treatment with antibiotics, which was not the case for patient 2. Nevertheless, the patient was treated with metronidazole, a first-line therapy for C. difficile (21), and the results for the C. difficile test were negative from then on.
The patients' outcomes were as follows. Patient 1 showed a clinical course similar to that described in previously reported cases for SOT recipients (31), in which suspension of the immunosuppressive treatment led to recovery. Therefore, we suggest that restored immune balance after MMF withdrawal and filgrastrim treatment allowed the recovery of the patient. Patient 2 was capable of resolving the symptoms only after a sequential therapy with metronidazole and albendazole: metronidazole treatment initially allowed the elimination of C. difficile, and afterwards, albendazole treatment allowed a patent decrease in microsporidial spore seeding and the resolution of diarrheal symptoms. To date, no curative therapy for E. bieneusi infection exists. Metronidazole, which is indicated for C. difficile treatment (21), has occasionally been reported to cause transient improvement of the symptoms of microsporidiosis (17, 41, 54). However, albendazole, which is effective against microsporidia other than E. bieneusi, seems to alleviate diarrhea in E. bieneusi-infected patients without clearing the infection, but with a notable decrease in spore seeding (8, 41, 51, 52).
Both patients received immunosuppressive therapy with tacrolimus and MMF at the time of diagnosis. Other immunosuppressive therapies described for OTR diagnosed with microsporidiosis included cyclosporine, prednisone, azathioprine, rapamycin, antilymphocyte globulin, or methotrexate (22, 35). It has been suggested that the lack of gamma interferon (IFN-γ) resulting from the Th cell depletion induced by MMF may be responsible, at least in part, for the onset of microsporidiosis (16) and the triggering of the intestinal symptomatology by the parasite. Since patient 1 recovered from symptoms after treatment suspension, it is very likely that this recovery was associated with the immune reconstitution balance. The same situation was described in previously published reports of microsporidiosis in renal transplant recipients (16). For patient 2, discontinuation of the immunosuppressive therapy helped by metronidazole and albendazole treatment improved the patient's health conditions, including normal bowel movements. The same outcome has been described to occur in two of the four OTR with E. bieneusi infection who were treated with albendazole (34, 41). However, in the other two cases, a complete clearance of spores was achieved (16, 29), suggesting that the discontinuation of the immunosuppressive therapy was probably what mainly improved the patients' health conditions (16, 29, 41).
In reference to the presence of microsporidia in Spain, they have mainly been reported to occur in HIV/AIDS patients (8–10) but also in HIV-negative individuals, including travelers (26), elderly people (27), and the immunocompetent population (1). In most of these studies, E. bieneusi was the microsporidian responsible for clinical symptoms. However, to date, no cases of microsporidiosis in OTR have been described. This report recognizes the implication of microsporidia in OTR pathology for the first time in Spain.
In most cases, microsporidia detected in OTR were characterized only to the species level (22, 35). Encephalitozoon cuniculi is the best-characterized microsporidian in OTR isolates at the genotype level (30, 33, 44). In three of five E. cuniculi infections described to occur in SOT and BMT recipients, the genotype was investigated; two of these infections showed ITS-related genotype III, known as the “dog strain” (30), and for the third infection, a new genotype, genotype IV, was recently described (44). In reference to the genetic variation among isolates of E. bieneusi (18, 47), analyses of ribosomal DNA internal transcribed spacer (ITS) sequences have identified more than 70 genotypes of E. bieneusi (18, 47). Some of these genotypes have been recognized as host specific, while others have been found to infect humans and animals, supporting the likelihood of zoonotic transmission (18, 47). In our 2 patients, genotype D was identified. This genotype belongs to group 1, which includes numerous genotypes from various origins: humans, both HIV-positive and negative, but also domestic and wild animals. Genotype D is widespread in nature (47). This genotype was first found in humans in Germany and afterwards in other countries of America, Asia, and Africa. It was also found in numerous diverse animals (swine, cattle, macaque, muskrat, raccoon, beaver, fox, dog, and falcon) (47). The type D genotype was commonly reported to occur in HIV-positive patients in Thailand (24) and Peru (42) and in two isolated cases in Europe (36, 37), and it was recently isolated from 3 HIV-negative individuals in Cameroon (3), which confirms the wide spread of this genotype. Genotype D represents 15% of isolates from four species of wildlife animals in North America (43) and 26% of isolates found in cats in Colombia (39), supporting a zoonotic route of transmission for this strain.
Information about molecular epidemiological data for E. bieneusi isolates from transplant recipients is limited. Several studies have described the predominance of genotype C in this population (25, 41, 46). Liguory and collaborators (25) genotyped 100 E. bieneusi isolates from both HIV- and non-HIV-infected patients, including eight transplant recipients. In the transplant patients, they found genotype II (genotype C) in seven of these patients and genotype IV (genotype D) in one (25). They also described that the distribution of genotypes was significantly different among HIV-infected patients compared to that among non-HIV-infected patients, and they suggested differences in the epidemiology of the infection according to HIV infection status or differences in the virulence of the microsporidial strain (25).
Sing and collaborators also found human genotype C in a liver transplant recipient, based on analysis of the ribosomal DNA (rDNA) internal transcribed spacer (ITS) sequence (41). ten Hove and colleagues performed a molecular characterization of E. bieneusi isolates from immunosuppressed and immunocompetent patient groups in Malawi and the Netherlands (46). They identified 16 genotypes, 9 of which had not previously been described. Genotypes B, K, and D were most prevalent among HIV patients, whereas genotype C was identified in five isolates from kidney transplant recipients and was not seen in any of the other groups of patients (46).
In contrast to the microsporidiosis caused by E. bieneusi, which is generally confined to the digestive tract, as shown in reported cases (15–17, 29, 31, 34) and in the 2 cases described here, Encephalitozoon infections were frequently disseminated in OTR (4, 14, 30, 44, 45). In these cases, microsporidia were most frequently identified in urine samples but were also isolated from various tissues or body fluids, including those from stools, sputum, conjunctival scraping, brain, and kidney biopsy specimens. The most commonly reported clinical symptoms in disseminated microsporidiosis in OTR were keratoconjunctivitis, fever, abdominal pain, and respiratory symptoms (cough and thoracic pain) (44). Diagnosis of microsporidiosis in these patients was conducted mainly by trichrome staining and PCR. Most of the cases were described to occur in Europe, but there were also seven in America, two in India, and one in Africa. The antiparasitic treatment used included albendazole, metronidazole, and fumagillin, independent of the microsporidian species found. In most cases of infection by E. bieneusi, the recovery was related to immune reconstitution and/or immunosuppressive therapy suspension (22).
In conclusion, transplant recipients undergoing immunosuppressive therapy should be considered a risk group for acquisition of microsporidiosis. Therefore, in all countries (including those in which microsporidia have not yet been recognized), microsporidia should be considered in cases of persistent diarrhea and also in the differential diagnosis of other syndromes, such as chronic fever of unknown etiology, after more-common causes of diarrheal disease are ruled out. The search should be performed not only in stool samples but also, at least, in urine samples. A molecular characterization of the parasite isolates should be considered to convey information about the frequency and distribution of microsporidial species and genotypes in this group of patients.
ACKNOWLEDGMENTS
We thank Sergio Llorens for his excellent technical support. We are indebted to Anne Deane for helpful revision of the manuscript.
This work was supported by a grant from Fundación San Pablo-CEU (USP-PC03/08) and by grant PI061593 from Instituto de Salud Carlos III (FISS). Ana Luz Galvan was supported in Spain by an overseas fellowship from Colciencias (Antioquía University, Colombia).
There is no conflict of interest in this study.
Footnotes
Published ahead of print on 16 February 2011.
REFERENCES
- 1. Abreu-Acosta N., et al. 2005. Enterocytozoon bieneusi (microsporidia) in clinical samples from immunocompetent individuals in Tenerife, Canary Islands, Spain. Trans. R. Soc. Trop. Med. Hyg. 99:848–855 [DOI] [PubMed] [Google Scholar]
- 2. Adl S. M., et al. 2005. The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol. 52:399–451 [DOI] [PubMed] [Google Scholar]
- 3. Breton J., et al. 2007. New highly divergent rRNA sequence among biodiverse genotypes of Enterocytozoon bieneusi strains isolated from humans in Gabon and Cameroon. J. Clin. Microbiol. 45:2580–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carlson J. R., et al. 2004. Disseminated microsporidiosis in a pancreas/kidney transplant recipient. Arch. Pathol. Lab. Med. 128:e41–e43 [DOI] [PubMed] [Google Scholar]
- 5. da Silva A. J., et al. 1996. Sensitive PCR diagnosis of Infections by Enterocytozoon bieneusi (microsporidia) using primers based on the region coding for small-subunit rRNA. J. Clin. Microbiol. 34:986–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. da Silva A. J., et al. 1997. Detection of Septata intestinalis (microsporidia) Cali et al. 1993 using polymerase chain reaction primers targeting the small submit subunit ribosomal RNA coding region. Mol. Diagn. 2:47–52 [DOI] [PubMed] [Google Scholar]
- 7. De Groote M. A., et al. 1995. Polymerase chain reaction and culture confirmation of disseminated Encephalitozoon cuniculi in a patient with AIDS: successful therapy with albendazole. J. Infect. Dis. 171:1375–1378 [DOI] [PubMed] [Google Scholar]
- 8. del Aguila C., et al. 1997. Identification of Enterocytozoon bieneusi spores in respiratory samples from an AIDS patient with a 2-year history of intestinal microsporidiosis. J. Clin. Microbiol. 35:1862–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. del Aguila C., et al. 2001. In vitro culture, ultrastructure, antigenic, and molecular characterization of Encephalitozoon cuniculi isolated from urine and sputum samples from a Spanish patient with AIDS. J. Clin. Microbiol. 39:1105–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. del Aguila C., et al. 1997. Microsporidiosis in HIV-positive children in Madrid (Spain). J. Eukaryot. Microbiol. 44:84S–85S [DOI] [PubMed] [Google Scholar]
- 11. Didier E. S., et al. 2004. Epidemiology of microsporidiosis: sources and modes of transmission. Vet. Parasitol. 126:145–166 [DOI] [PubMed] [Google Scholar]
- 12. Didier E. S., Weiss L. M. 2006. Microsporidiosis: current status. Curr. Opin. Infect. Dis. 19:485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fogla R., Padmanabhan P., Therese K. L., Biswas J., Madhavan H. N. 2005. Chronic microsporidial stromal keratitis in an immunocompetent, non-contact lens wearer. Indian J. Ophthalmol. 53:123–125 [DOI] [PubMed] [Google Scholar]
- 14. Gamboa-Dominguez A., et al. 2003. Disseminated encephalitozoon cuniculi infection in a Mexican kidney transplant recipient. Transplantation 75:1898–1900 [DOI] [PubMed] [Google Scholar]
- 15. Goetz M., Eichenlaub S., Pape G. R., Hoffmann R. M. 2001. Chronic diarrhea as a result of intestinal microsposidiosis in a liver transplant recipient. Transplantation 71:334–337 [DOI] [PubMed] [Google Scholar]
- 16. Guerard A., et al. 1999. Intestinal microsporidiosis occurring in two renal transplant recipients treated with mycophenolate mofetil. Transplantation 68:699–707 [DOI] [PubMed] [Google Scholar]
- 17. Gumbo T., Hobbs R. E., Carlyn C., Hall G., Isada C. M. 1999. Microsporidia infection in transplant patients. Transplantation 67:482–484 [DOI] [PubMed] [Google Scholar]
- 18. Henriques-Gil N., Haro M., Izquierdo F., Fenoy S., del Aguila C. 2010. Phylogenetic approach to the variability of the microsporidian Enterocytozoon bieneusi and its implications for inter- and intrahost transmission. Appl. Environ. Microbiol. 76:3333–3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kakrania R., Joseph J., Vaddavalli P. K., Gangopadhyay N., Sharma S. 2006. Microsporidia keratoconjunctivitis in a corneal graft. Eye (Lond.) 20:1314–1315 [DOI] [PubMed] [Google Scholar]
- 20. Kelkar R., et al. 1997. Pulmonary microsporidial infection in a patient with CML undergoing allogeneic marrow transplant. Bone Marrow Transplant. 19:179–182 [DOI] [PubMed] [Google Scholar]
- 21. Kutty P. K., et al. Risk factors for and estimated incidence of community-associated Clostridium difficile infection, North Carolina, U. S. A. Emerg. Infect. Dis. 16:197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lanternier F., et al. 2009. Microsporidiosis in solid organ transplant recipients: two Enterocytozoon bieneusi cases and review. Transpl. Infect. Dis. 11:83–88 [DOI] [PubMed] [Google Scholar]
- 23. Latib M. A., Pascoe M. D., Duffield M. S., Kahn D. 2001. Microsporidiosis in the graft of a renal transplant recipient. Transpl. Int. 14:274–277 [DOI] [PubMed] [Google Scholar]
- 24. Leelayoova S., et al. 2006. Identification of genotypes of Enterocytozoon bieneusi from stool samples from human immunodeficiency virus-infected patients in Thailand. J. Clin. Microbiol. 44:3001–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liguory O., Sarfati C., Derouin F., Molina J. M. 2001. Evidence of different Enterocytozoon bieneusi genotypes in patients with and without human immunodeficiency virus infection. J. Clin. Microbiol. 39:2672–2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lopez-Velez R., et al. 1999. Microsporidiosis in travelers with diarrhea from the tropics. J. Travel Med. 6:223–227 [DOI] [PubMed] [Google Scholar]
- 27. Lores B., et al. 2002. Intestinal microsporidiosis due to Enterocytozoon bieneusi in elderly human immunodeficiency virus-negative patients from Vigo, Spain. Clin. Infect. Dis. 34:918–921 [DOI] [PubMed] [Google Scholar]
- 28. Mahmood M. N., Keohane M. E., Burd E. M. 2003. Pathologic quiz case: a 45-year-old renal transplant recipient with persistent fever. Arch. Pathol. Lab. Med. 127:e224–e226 [DOI] [PubMed] [Google Scholar]
- 29. Metge S., et al. 2000. A case of Enterocytozoon bieneusi infection in an HIV-negative renal transplant recipient. Eur. J. Clin. Microbiol. Infect. Dis. 19:221–223 [DOI] [PubMed] [Google Scholar]
- 30. Mohindra A. R., et al. 2002. Disseminated microsporidiosis in a renal transplant recipient. Transpl. Infect. Dis. 4:102–107 [DOI] [PubMed] [Google Scholar]
- 31. Molina J. M., et al. 2002. Fumagillin treatment of intestinal microsporidiosis. N. Engl. J. Med. 346:1963–1969 [DOI] [PubMed] [Google Scholar]
- 32. Niemczyk M., et al. 2005. Infections caused by clostridium difficile in kidney or liver graft recipients. Ann. Transplant. 10:70–74 [PubMed] [Google Scholar]
- 33. Orenstein J. M., et al. 2005. Fatal pulmonary microsporidiosis due to encephalitozoon cuniculi following allogeneic bone marrow transplantation for acute myelogenous leukemia. Ultrastruct. Pathol. 29:269–276 [DOI] [PubMed] [Google Scholar]
- 34. Rabodonirina M., et al. 1996. Enterocytozoon bieneusi as a cause of chronic diarrhea in a heart-lung transplant recipient who was seronegative for human immunodeficiency virus. Clin. Infect. Dis. 23:114–117 [DOI] [PubMed] [Google Scholar]
- 35. Rabodonirina M., Cotte L., Radenne S., Besada E., Trepo C. 2003. Microsporidiosis and transplantation: a retrospective study of 23 cases. J. Eukaryot. Microbiol. 50(Suppl.):583. [DOI] [PubMed] [Google Scholar]
- 36. Rinder H., Katzwinkel-Wladarsch S., Thomschke A., Loscher T. 1998. Strain differentiation in microsporidia. Tokai J. Exp. Clin. Med. 23:433–437 [PubMed] [Google Scholar]
- 37. Sadler F., et al. 2002. Genotyping of Enterocytozoon bieneusi in AIDS patients from the north west of England. J. Infect. 44:39–42 [DOI] [PubMed] [Google Scholar]
- 38. Santin M., Fayer R. 2009. Enterocytozoon bieneusi genotype nomenclature based on the internal transcribed spacer sequence: a consensus. J. Eukaryot. Microbiol. 56:34–38 [DOI] [PubMed] [Google Scholar]
- 39. Santin M., Trout J. M., Vecino J. A., Dubey J. P., Fayer R. 2006. Cryptosporidium, Giardia and Enterocytozoon bieneusi in cats from Bogota (Colombia) and genotyping of isolates. Vet. Parasitol. 141:334–339 [DOI] [PubMed] [Google Scholar]
- 40. Sax P. E., Rich J. D., Pieciak W. S., Trnka Y. M. 1995. Intestinal microsporidiosis occurring in a liver transplant recipient. Transplantation 60:617–618 [DOI] [PubMed] [Google Scholar]
- 41. Sing A., Tybus K., Heesemann J., Mathis A. 2001. Molecular diagnosis of an Enterocytozoon bieneusi human genotype C infection in a moderately immunosuppressed human immunodeficiency virus-seronegative liver-transplant recipient with severe chronic diarrhea. J. Clin. Microbiol. 39:2371–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sulaiman I. M., et al. 2003. A molecular biologic study of Enterocytozoon bieneusi in HIV-infected patients in Lima, Peru. J. Eukaryot. Microbiol. 50(Suppl.):591–596 [DOI] [PubMed] [Google Scholar]
- 43. Sulaiman I. M., et al. 2003. Molecular characterization of microsporidia indicates that wild mammals Harbor host-adapted Enterocytozoon spp. as well as human-pathogenic Enterocytozoon bieneusi. Appl. Environ. Microbiol. 69:4495–4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Talabani H., et al. 2010. Disseminated infection with a new genovar of Encephalitozoon cuniculi in a renal transplant recipient. J. Clin. Microbiol. 48:2651–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Teachey D. T., et al. 2004. Pulmonary infection with microsporidia after allogeneic bone marrow transplantation. Bone Marrow Transplant. 33:299–302 [DOI] [PubMed] [Google Scholar]
- 46. ten Hove R. J., et al. 2009. Characterization of genotypes of Enterocytozoon bieneusi in immunosuppressed and immunocompetent patient groups. J. Eukaryot. Microbiol. 56:388–393 [DOI] [PubMed] [Google Scholar]
- 47. Thellier M., Breton J. 2008. Enterocytozoon bieneusi in human and animals, focus on laboratory identification and molecular epidemiology. Parasite 15:349–358 [DOI] [PubMed] [Google Scholar]
- 48. van Gool T., et al. 1993. Diagnosis of intestinal and disseminated microsporidial infections in patients with HIV by a new rapid fluorescence technique. J. Clin. Pathol. 46:694–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vemuganti G. K., et al. 2005. Is microsporidial keratitis an emerging cause of stromal keratitis? A case series study. BMC Ophthalmol. 5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Visvesvara G. S., et al. 1994. Polyclonal and monoclonal antibody and PCR-amplified small-subunit rRNA identification of a microsporidian, Encephalitozoon hellem, isolated from an AIDS patient with disseminated infection. J. Clin. Microbiol. 32:2760–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wanke C. A., DeGirolami P., Federman M. 1996. Enterocytozoon bieneusi infection and diarrheal disease in patients who were not infected with human immunodeficiency virus: case report and review. Clin. Infect. Dis. 23:816–818 [DOI] [PubMed] [Google Scholar]
- 52. Weber R., Bryan R. T. 1994. Microsporidial infections in immunodeficient and immunocompetent patients. Clin. Infect. Dis. 19:517–521 [DOI] [PubMed] [Google Scholar]
- 53. Weber R., et al. 1992. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. The Enteric Opportunistic Infections Working Group. N. Engl. J. Med. 326:161–166 [DOI] [PubMed] [Google Scholar]
- 54. Weber R., Bryan R. T., Schwartz D. A., Owen R. L. 1994. Human microsporidial infections. Clin. Microbiol. Rev. 7:426–461 [DOI] [PMC free article] [PubMed] [Google Scholar]