Abstract
The ViroSeq HIV-1 genotyping system is used in many African countries for drug resistance testing. In this study, we used a panel of diverse HIV-1 group M isolates circulating in Cameroon to show that the performance of this assay can be altered by the sequence variation of non-B HIV-1 strains that predominate in African settings.
Human immunodeficiency virus (HIV) resistance testing is an important monitoring tool for the management of antiretroviral-treated patients and is largely used in industrialized countries to select the appropriate regimen when initiating or switching antiretroviral treatments (ART) (5). In developing countries, because of financial issues, the prohibitive cost of the assay, the lack of adequately trained personnel, and laboratory infrastructures, access to resistance testing is still greatly limited and is not even recommended by the World Health Organization (WHO) for the routine management of patients (14). However, with the ongoing scale-up of ART in these countries since 2000, the demand for drug resistance testing is increasing. A limited number of tests are performed for routine patient management, and the majority of tests are performed for research purposes and resistance surveillance studies recommended by the WHO (1).
Drug resistance testing can be performed using either phenotypic or genotypic assays. Generally, sequence-based genotyping assays, also known as population-based genotyping assays, is preferred to phenotypic assays for the routine management of patients because of their reduced cost and required infrastructures and because they are less time-consuming. Sequence-based HIV-1 genotyping assays detect resistance mutations in the protease (PR), reverse transcriptase (RT), and integrase (I) genes by comparing the gene sequences of the investigated virus with those of a wild-type reference HIV-1 subtype B strain. Currently, two Food and Drug Administration (FDA)-approved genotyping methods are commercially available, the ViroSeq HIV-1 genotyping system, version 2.0 (Celera Diagnostics, Alameda, CA) (4), and the Trugene HIV-1 genotyping kit for drug resistance (Siemens Healthcare Diagnostics, Deerfield, IL) (6).
Like most of the biological tools used to manage HIV infection, e.g., diagnostic tests, viral load assays, etc., sequence-based genotyping assays are subjected to subtype-specific constraints, especially when dealing with HIV-1 non-B viruses, which predominate in sub-Saharan Africa (2, 11). The ViroSeq HIV-1 genotyping system is FDA approved for analysis of subtype B HIV-1 only, but it is widely commercialized and used in sub-Saharan Africa, where non-B HIV-1 variants are largely found. However, only a few studies have evaluated the performance of the ViroSeq system in the context of non-B HIV-1 variants, and these studies reported limitations of ViroSeq in correctly amplifying and sequencing some non-B variants, such as subtype C, which predominates worldwide and in South African areas (4, 9, 13). Here we report on the performance of the ViroSeq genotyping system in Cameroon, a country with a very high genetic diversity of HIV-1, where all known groups of HIV-1 (M, N, O, and P) and group M subtypes, as well as numerous recombinant forms, cocirculate.
As part of ongoing clinical trials conducted in Cameroon, we tested 145 samples for antiretroviral drug resistance using the ViroSeq HIV-1 genotyping system, version 2.0. Samples were from patients receiving ART in different clinics in the area of Yaoundé, the capital city; genotyping was recommended for those with viral loads above 1,000 RNA copies/ml, and all patients eligible for a drug resistance genotyping were included in chronological order in the current substudy. In addition, a subset of samples from patients at baseline was also tested. The trials were all approved by the Cameroonian Ethics Committee, and resistance testing was included in these studies. The resistance tests were conducted locally, at the CREMER/IMPM/IRD Virology Laboratory, which is also the National WHO Reference Laboratory for HIV drug resistance surveillance. The ViroSeq assay was performed as recommended by the manufacturer's instructions with 0.5 ml plasma. All samples except three had a viral load of >4 log10 copies/ml. The other three samples had a viral load between 1,000 and 2,000 copies/ml but were also tested, since our routine experience showed that the ViroSeq assay generally amplified such samples correctly. The ViroSeq kit contains all the necessary reagents for RNA extraction, reverse transcription, PCR amplification, PCR product purification, and sequencing reactions. A final PCR product of 1.8 kb is expected, and seven sequencing primers (A, B, C, D, F, G, and H) are provided to sequence both DNA strands (Fig. 1), primer D being provided as a substitute for A in case the latter fails (4). In cases where the ViroSeq assay did not provide adequate amplification or sequencing result, a genotyping assay developed in-house was performed (12). This “homemade” system used outer primers G25REV (5′-GCAAGAGTTTTGGCTGAAGCAATGAG-3′) and IN3 (5′-TCTATBCCATCTAAAAATAGTACTTTCCTGATTCC-3′), inner primers AV150 (5′-GTGGAAAGGAAGGACACCAAATGAAAG-3′) and polM4 (5′-CTATTAGCTGCCCCATCTACATA-3′), and five additional primers for sequencing: polM0 (5′-TCCCTCAGATCACTCTTTGGCA-3′), polM1 (5′-GTTAAACAATGGCCATTGACAGA-3′), polM4(5′-CTATTAGCTGCCCCATCTACATA-3′), polM8 (5′-CTGTATATCATTGACAGTCCAG-3′), and polM9 (5′-ATTGAACTTCCCAGAAGTCTTGAGTT-3′). The sequences obtained were assembled and analyzed using the ViroSeq HIV-1 genotyping system software, version 2.8 (Celera Diagnostics, Alameda, CA), and/or SeqMan II software (DNASTAR, Madison, WI).
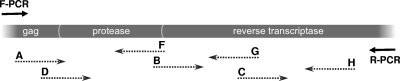
Fig. 1.
Schematic representation of the amplification and sequencing strategies of the ViroSeq HIV-1 genotyping system. Forward and reverse primers are respectively represented by F-PCR and R-PCR. The positions and orientations of the sequencing primers (A, B, C, D, F, G, and H) are shown with respect to the protease and the reverse transcriptase regions.
Subtypes or circulating recombinant forms (CRFs) were determined by constructing phylogenetic trees and performing recombination analyses using ClustalX 2.0 (10) and Simplot 3.5.1 (8), respectively. To determine whether the genetic diversity of HIV-1 strains circulating in Cameroon can play a role in the performance of the ViroSeq assay, we estimated the performance of each of the assay's sequencing primers according to viral subtypes in our sample panel. In addition, we estimated the binding position of these primers within the gag-pol region based on the position where the sequence generated by the primer started. We did that because ViroSeq primers are not publicly accessible for such analyses. We therefore studied the variability of sequences in these areas where the primers are expected to bind, using the online tool SeqPublish from Los Alamos (http://www.hiv.lanl.gov) and HIV-1 subtype B HXB2 (accession no. K03455) as the reference sequence in the alignment. Also, we included in the alignment a set of HIV-1 subtypes B sequences from GenBank (http://www.hiv.lanl.gov) and the sequences from our sample panel.
Of the 145 samples tested, 144 were successfully amplified using the ViroSeq assay, resulting in a good amplification rate of 99.3%. The sample that failed to be amplified had a viral load of 4.27 log10 copies/ml and was successfully amplified by the in-house assay. Subsequent analyses showed that this sample was a subtype D HIV-1 strain. Thus, all samples but one were correctly PCR amplified and taken to the sequencing phase. Phylogenetic analyses of the viral pol region showed that all the amplified samples were HIV-1 group M viruses, and although the recombinant form CRF02_AG (n = 89) predominated (61.8%), numerous other subtypes and CRFs were also found: A/A1 (n = 3), D (n = 5), F2 (n = 7), G (n = 5), CRF01_AE (n = 1), CRF09_cpx (n = 2), CRF11_cpx (n = 6), CRF22_01A1 (n = 3), CRF25_cpx (n = 1), CRF36_cpx (n = 1), CRF37_cpx (n = 2), complex recombinant (n = 12), and unclassified (n = 7).
Using this panel of HIV-1 subtypes and CRFs, representing the genetic diversity of HIV-1 group M viruses circulating in Cameroon, we investigated the performance of the sequencing primers of the ViroSeq system. We categorized the results of sequencing as heterogeneous signal, negative, and positive. The heterogeneous signal referred to primers that generated sequences with two or more overlapping chromatograms, partially or on the full sequence length, with no chance of distinguishing any existing mutation. As they could not be used, heterogeneous signals were considered primer failure. The primers that completely failed were designated negative, and primers were considered positive when the sequencing was successful. As shown in Table 1, the primer with the highest failure rate was primer D, which showed a failure rate between 0% and 100% depending on HIV-1 subtype. This primer showed a 100% failure for subtypes A/A1 (n = 3), F2 (n = 7), CRF09_cpx (n = 2), and CRF37_cpx (n = 2) and for the only CRF01_AE sequence. In addition, this primer failed with up to 42% of the predominant variant CRF02_AG samples. Overall, the failure rate of primer D was 52.6%, meaning that it failed to sequence more than half of the samples tested. Primer H had the second highest failure rate. This primer showed a 100% failure rate with CRF25_cpx (n = 1) and CRF37_cpx (n = 2) samples and a failure rate of up to 31.5% with the predominant variant in Cameroon, CRF02_AG. The overall failure rate of primer H was 24.3%. The last two primers with the highest failure rates were primers A and F, which showed overall failure rates of 12.5% and 11.8%, respectively. Subtypes and CRFs with which these primers failed more frequently were F2, CRF02_AG, CRF11_cpx, CRF22_01A1, and unclassified isolates for primer A and A/A1, F2, CRF11_cpx, CRF25_cpx, and unclassified isolates for primer F (Table 1). We observed very low failure rates for primer B (0.7%), primer C (1.4%), and primer G (0.7%).
Table 1.
Performance of ViroSeq primers for each subtype and CRF
| HIV-1 subtype or CRF | na | % of samples with result with primerb |
% for which >1 primer failed | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A |
B |
C |
D |
F |
G |
H |
|||||||||||||||||
| HS | Neg | Pos | HS | Neg | Pos | HS | Neg | Pos | HS | Neg | Pos | HS | Neg | Pos | HS | Neg | Pos | HS | Neg | Pos | |||
| A/A1 | 3 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 | 33.3 | 33.3 | 33.3 | 0.0 | 0.0 | 100.0 | 0.0 | 33.3 | 66.7 | 66.7 |
| D | 5 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 20.0 | 80.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 |
| F2 | 7 | 0.0 | 28.6 | 71.4 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 | 0.0 | 14.3 | 85.7 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 28.6 |
| G | 5 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 60.0 | 40.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 |
| CRF01_AE | 1 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 |
| CRF02_AG | 89 | 0.0 | 12.4 | 87.6 | 0.0 | 0.0 | 100.0 | 0.0 | 1.2 | 98.8 | 0.0 | 41.7 | 58.3 | 4.5 | 6.7 | 88.8 | 0.0 | 0.0 | 100.0 | 3.4 | 28.1 | 68.5 | 21.3 |
| CRF09_cpx | 2 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 |
| CRF11_cpx | 6 | 0.0 | 33.3 | 66.7 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 50.0 | 50.0 | 0.0 | 16.7 | 83.3 | 0.0 | 16.7 | 83.3 | 0.0 | 16.7 | 83.3 | 16.7 |
| CRF22_01A1 | 3 | 0.0 | 33.3 | 66.7 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 |
| CRF25_cpx | 1 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 | 100.0 |
| CRF36_cpx | 1 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 |
| CRF37_cpx | 2 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 | 100.0 |
| Recombinant | 12 | 8.3 | 0.0 | 91.7 | 0.0 | 8.3 | 91.7 | 0.0 | 0.0 | 100.0 | 0.0 | 91.7 | 8.3 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 8.3 | 91.7 | 16.7 |
| Unclassified | 7 | 0.0 | 14.3 | 85.7 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 71.4 | 28.6 | 0.0 | 28.6 | 71.4 | 0.0 | 0.0 | 100.0 | 0.0 | 14.3 | 85.7 | 28.6 |
| Total | 144 | 0.7 | 11.8 | 87.5 | 0.0 | 0.7 | 99.3 | 0.0 | 1.4 | 98.6 | 0.0 | 52.6 | 47.4 | 3.5 | 8.3 | 88.2 | 0.0 | 0.7 | 99.3 | 2.8 | 21.5 | 75.7 | 20.1 |
Total number of samples that were sequenced using the ViroSeq primers.
HS, heterogeneous signal; Neg, negative; Pos, positive.
To elucidate whether sequence variation can influence the performance of the ViroSeq assay, we compared the sequences of subtypes B viruses, since the assay is optimized for this subtype, with the sequences that we generated. We did the analyses for the three primers which showed the highest failure rates: A, D, and H. We excluded subtypes for which we had fewer than three sequences and complex recombinant strains, since they were not clearly related to one particular subtype. Sequences that did not cover the investigated region adequately were also excluded, and finally, we arbitrarily removed some sequences in subtypes where more than 50 sequences were available, in order to display usable figures. These analyses provided interesting results: (i) in the areas where primers A, D, and H were developed and expected to bind, the polymorphism of HIV-1 non-B subtypes was higher than that observed in the B strains (Fig. 2, 3, and 4); (ii) within each non-B subtype represented (CRF02_AG, D, F2, and CRF11_cpx), no particular polymorphism pattern was associated with failure or success, and indeed, we observed the same degree of polymorphism between sequences that failed and those that worked, although for some cases the number of failing sequences was too limited to allow an objective analysis; (iii) finally, we observed no particular polymorphism pattern or mutations between the represented non-B subtypes that could help predict the success or failure of the primers investigated (A, D, and H).
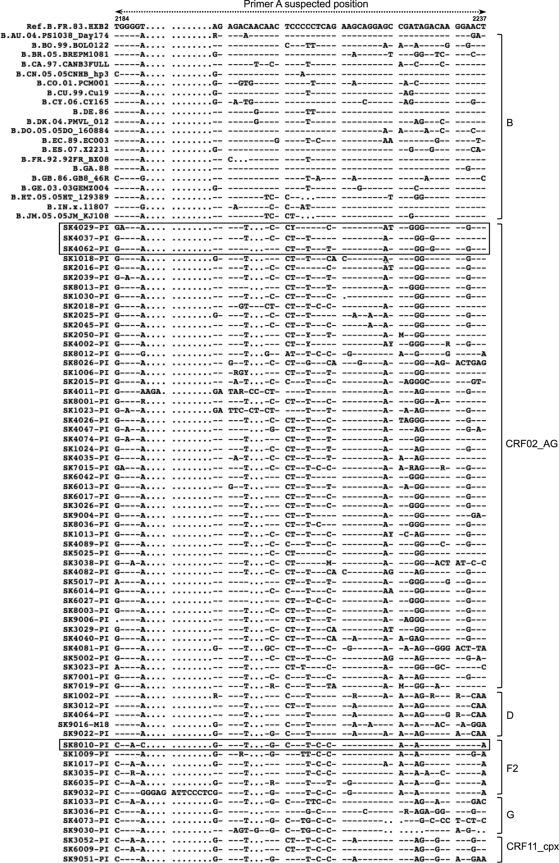
Fig. 2.
Comparative alignment of HIV-1 subtype B and non-B strains in the gag-pol region where primer A is expected to bind. Based on the reference subtype B sequence HXB2, the region spans nucleotides 2184 to 2237. Subtypes are shown on the right, and sequences that failed within each subtype are boxed.
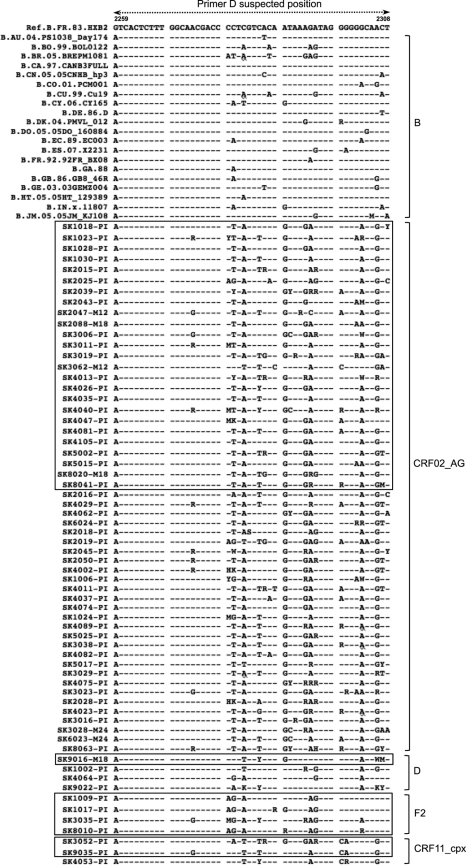
Fig. 3.
Comparative alignment of HIV-1 subtype B and non-B strains in the gag-pol region where primer D is expected to bind. Based on the reference subtype B sequence HXB2, the region spans nucleotides 2259 to 2308. Subtypes are shown on the right, and sequences that failed within each subtype are boxed.
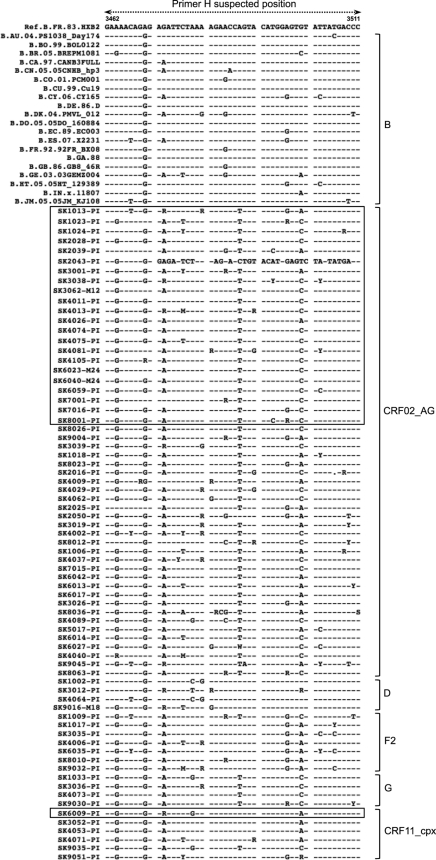
Fig. 4.
Comparative alignment of HIV-1 subtype B and non-B strains in the pol region where primer H is expected to bind. Based on the reference subtype B sequence HXB2, the region spans nucleotides 3462 to 3511. Subtypes are shown on the right, and sequences that failed within each subtype are boxed.
Adequate identification of drug resistance mutations requires successful sequencing of both forward and reverse sequences to confirm mixture peaks and exclude possible bias in the form of noise peaks that can result from the use of a unique sequence (7). For this reason, we also evaluated the overall sequencing efficiency of the ViroSeq assay by determining the sequencing failure rate, defined as the inability of the assay to generate a full set of acceptable sequences that cover the regions investigated in the protease (amino acids 1 to 99) and RT (amino acids 1 to 335) genes in both directions. Sequencing failures mainly involved the 5′ end of the protease and the 3′ end of the RT genes because of the high failure rate of primers A, D, F, and H, which covered these regions. Sequencing failures involving primer A were observed for 12.5% of samples, including subtypes F2, CRF02_AG, CRF11_cpx, and CRF22_cpx and recombinant and unclassified isolates. Primer D was included in the ViroSeq kit to overcome issues with primer A, but this primer has the highest failure rate (Table 1), and more than 50% of samples failing with primer A also failed with primer D, consistent with the results of previous studies reporting the high failure rate of this primer on non-B HIV-1 isolates (9). When primer H failed, the result was an RT sequence covered only at the 3′ end by a forward sequence (primer C) at positions of key mutations, resulting in resistance to nucleoside reverse transcriptase inhibitors (NRTI) (Q151, M184, L210, T215, and K219) and non-NRTI resistance mutations (V179, Y181, Y188, G190, P225, F227, M230, P236, and K238). Such sequencing failures involving primer H were observed for 24.3% of samples tested and, more importantly, for up to 31.5% of isolates of CRF02_AG, which represents the major HIV-1 variant in Cameroon. Previous studies showed this high failure rate of the ViroSeq assay because of primer H failure with a limited number of CRF02-AG isolates (9) and associated the failure with a high number of mismatches between primer H and CRF02_AG strains (4). Here, we did not identify specific polymorphisms in areas where primers A, D, and H are expected to bind in non-B sequences, but our results clearly showed the considerable degree of polymorphism that exists in non-B isolates in the areas for which these primers are designed, compared to B strains. Our hypothesis is that this high rate of polymorphism of non-B strains reduces the ability of sequencing primers designed based on B viruses to bind, reducing their sensitivity. It is clear that without the primer sequences, no conclusion can be drawn about the presence of specific mismatches that can prevent primer success.
The ViroSeq HIV-1 genotyping system was optimized for use on subtype B strains (3) and is currently widely commercialized and used in African settings where non-B HIV-1 strains predominate. The present observations in Cameroon, a country where almost all cocirculating strains are non-B HIV-1 variants, showed the impact of non-B HIV-1 genetic divergence on the performance of this assay. Because of the high failure rate of some primers, repeated tests are more frequent, and that affects the overall assay cost and delay of result delivery. In addition, having a validated “in-house” method or alternative primers designed on the basis of the local HIV genetic diversity as backup solutions is nearly mandatory to overcome sequencing failures by the ViroSeq assay. This report stresses the need for biological assays that perform robustly on non-B HIV-1 strains, which represent up to 90% of HIV-1 variants circulating worldwide.
Nucleotide sequence accession numbers.
Nucleotide sequences of the HIV-1 isolates characterized in this study are available in GenBank under accession numbers HQ864323 to HQ864467.
Acknowledgments
This work was supported by the Institut de Recherche pour le Développement (IRD) and the National Agency for AIDS Research, France (ANRS).
We thank all the people who directly or indirectly contributed to the successful completion of this study.
We declare that we have no competing interests.
Footnotes
Published ahead of print on 26 January 2011.
REFERENCES
- 1. Bennett D. E., Myatt M., Bertagnolio S., Sutherland D., Gilks C. F. 2008. Recommendations for surveillance of transmitted HIV drug resistance in countries scaling up antiretroviral treatment. Antivir. Ther. 13(Suppl. 2):25–36 [PubMed] [Google Scholar]
- 2. Bile E. C., et al. 2005. Performance of drug-resistance genotypic assays among HIV-1 infected patients with predominantly CRF02_AG strains of HIV-1 in Abidjan, Cote d'Ivoire. J. Clin. Virol. 32:60–66 [DOI] [PubMed] [Google Scholar]
- 3. Eshleman S. H., et al. 2005. Sensitivity and specificity of the ViroSeq human immunodeficiency virus type 1 (HIV-1) genotyping system for detection of HIV-1 drug resistance mutations by use of an ABI PRISM 3100 genetic analyzer. J. Clin. Microbiol. 43:813–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eshleman S. H., et al. 2004. Performance of the Celera Diagnostics ViroSeq HIV-1 genotyping system for sequence-based analysis of diverse human immunodeficiency virus type 1 strains. J. Clin. Microbiol. 42:2711–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The EuroGuidelines Group for HIV Resistance 2001. Clinical and laboratory guidelines for the use of HIV-1 drug resistance testing as part of treatment management: recommendations for the European setting. AIDS 15:309–320 [DOI] [PubMed] [Google Scholar]
- 6. Grant R. M., et al. 2003. Accuracy of the TRUGENE HIV-1 genotyping kit. J. Clin. Microbiol. 41:1586–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang D. D., Eshleman S. H., Brambilla D. J., Palumbo P. E., Bremer J. W. 2003. Evaluation of the editing process in human immunodeficiency virus type 1 genotyping. J. Clin. Microbiol. 41:3265–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lole K. S., et al. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73:152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maes B., et al. 2004. Performance of ViroSeq HIV-1 genotyping system in routine practice at a Belgian clinical laboratory. J. Virol. Methods 119:45–49 [DOI] [PubMed] [Google Scholar]
- 10. Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tong C. Y., et al. 2005. Genotyping of B and non-B subtypes of human immunodeficiency virus type 1. J. Clin. Microbiol. 43:4623–4627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vergne L., et al. 2000. Genetic diversity of protease and reverse transcriptase sequences in non-subtype-B human immunodeficiency virus type 1 strains: evidence of many minor drug resistance mutations in treatment-naive patients. J. Clin. Microbiol. 38:3919–3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wallis C. L., et al. 2010. Affordable in-house antiretroviral drug resistance assay with good performance in non-subtype B HIV-1. J. Virol. Methods 163:505–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. WHO 2009. Rapid advice. Antiretroviral therapy for HIV infection in adults and adolescents. http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf Accessed 13 October 2010