Abstract
I report that a 75-year-old man with severe atherosclerosis experienced two episodes of bacteremia with Streptococcus pyogenes of type emm87. Recurrent sepsis with S. pyogenes is extremely rare, and a foot ulcer was the suspected point of entry. The patient did not develop opsonizing antibodies to the isolate.
CASE REPORT
The patient, a 75-year-old man, presented in March 2010 at our hospital with a 24-hour history of disorientation and fever. He was a smoker and had diabetes mellitus type II, postapoplectic epilepsy, and claudicatio intermittens. He has had a mechanical aortic valve since 1989, suffered a cerebral infarction in 2002, and was subjected to coronary artery bypass grafting in 2003. Upon examination, a temperature of 38.1°C and tachycardia were noted, but other vital signs were normal. Neurological examination revealed left-sided finger-nose dysmetria. A tender redness around the left big toe was noted. The white blood cell count (WBC) was 9.8 × 109 cells/liter, and the C-reactive protein (CRP) level was 7 mg/liter. A computed tomography (CT) scan of the brain showed no signs of bleeding or ischemia, though several older lesions were seen. The chest X ray was unremarkable. The patient received empirical treatment with cefotaxime. Both sets of blood cultures (BacT/Alert; bioMérieux, Durham, NC) grew group A streptococci (GAS), as did the culture from the infected toe, and the treatment was changed to penicillin G. In addition, the culture from the toe grew Staphylococcus aureus, and the urine grew Escherichia coli. Transesophageal echocardiography did not show signs of prosthesis endocarditis, and the patient improved over the following days, with less disorientation and fever. A maximum CRP level of 49 mg/liter was noted. The bacteremia was judged to be secondary to the wound on the toe, and treatment with penicillin G (3 g three times a day [t.i.d.]) was continued for 6 days, followed by 14 days of clindamycin (300 mg t.i.d.).
In April 2010, the patient suffered a cerebral infarction, and in May 2010, he reappeared in the emergency room. He had experienced an epileptic seizure and was febrile. He was lucid but not entirely oriented. Tachycardia and an elevated breathing frequency were noted, and his temperature was 40.1°C. The tip of the left big toe was necrotic, but it was not overtly infected. Otherwise, the physical examination was unremarkable. The WBC was 17 × 109 cells/liter, and the CRP level was 19 mg/liter. The chest X ray was normal. Treatment with cefotaxime was instituted, and the patient became apyrexic within 24 h. Both blood cultures were positive for GAS, as was a culture of a small wound on the left foot. Treatment was changed to penicillin G, and a new transesophageal echocardiography could not reveal signs of endocarditis. Intravenous treatment was given for 7 days, followed by penicillin V for 14 days.
In July 2010, the patient experienced increased pains in both of his feet. Upon examination, the feet were cold, and no pulses could be felt. The wound showed increased signs of inflammation, and wound cultures again grew GAS. The patient was treated with clindamycin for 14 days. Since it was obvious that the patient had critical ischemia of both feet, an endovascular revascularization procedure was tried in August but resulted in massive embolization to the periphery. A conservative approach was undertaken since the patient refused amputation. The patient left the hospital for a nursing home in September, and his condition deteriorated gradually. The last few days, he was stuporous, and 24 h before passing away, the patient became febrile. An autopsy was not performed.
Invasive infections with GAS cause considerable morbidity and mortality worldwide (3), and the majority of GAS belong to the genus Streptococcus pyogenes. Though recurrent skin and throat infections are typical for GAS, recurrent invasive disease infection has to my knowledge been reported only two times previously (1, 14). In one of the two cases the causative GAS was found to be Streptococcus equisimilis (1), commonly carrying a group C or G carbohydrate antigen, and in the other case, an intravenous drug abuser had recurrent septic arthritis and bacteremia with a GAS isolate not further classified (14). In contrast to the lack of reports of recurrent bacteremia with S. pyogenes, reports of beta-hemolytic streptococci of groups C and G, which share many features with S. pyogenes, causing recurrent bacteremia are numerous (4, 10, 13, 16, 17).
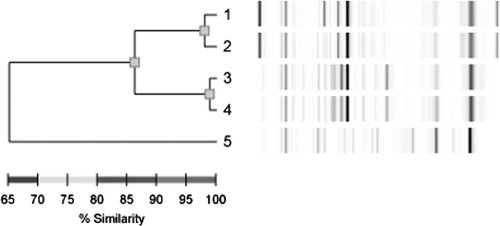
The isolates from the two episodes of bacteremic infection were classified as GAS by their typical appearance by Gram staining and on blood agar, as well as by latex agglutination, according to standard laboratory procedures. T typing, as described in reference 11, identified both isolates as T28. Both isolates were subjected to sequencing of the 16S rRNA as described previously (15a), and the sequences were identical to each other (833 bp were identical). A BLAST search revealed that the sequences were very similar to published 16S sequences from Streptococcus pyogenes (832/833 bp were identical). This confirms that the isolates were indeed of the genus S. pyogenes. Most isolates of the T28 serotype are M type 28 (11, 15), and therefore, PCR with primers hybridizing with the emm28 gene was performed as described previously (7), with the exception of the reverse primer, which was changed to 5′-GTAAAGAATGGGTTAGCTGC-3′. From both isolates, a fragment of 1 kb was amplified, and partial sequencing of the 5′-end part of the PCR product revealed that it was identical between the two isolates. A BLAST search showed that the amplified product was very similar (283/284 bases were identical) to sequences of emm87. Repetitive sequence-based PCR (rep-PCR) with the DiversiLab system (version 3.4; bioMérieux) was performed with the two isolates from the patient, along with two recent clinical isolates of the T28 serotype and an isolate of the T1 type, according to the instructions from the manufacturer. The results are shown in Fig. 1. The two isolates from the patient are highly similar, supporting them belonging to the same clone. Unfortunately, the GAS isolates from the wounds were not available for typing. S. pyogenes of type emm87 is not uncommon in invasive infections and is often associated with soft tissue infections like cellulitis (11). Isolates of type emm87 are also common in noninvasive disease (8) and most often display T type 28 (11). Nothing is known about potential virulence mechanisms of this particular M type.
Fig. 1.
Results from the rep-PCR analysis of two recent T28 blood isolates (1 and 2), two blood isolates from the patient (3 and 4), and a recent clinical T1 isolate (5).
The lack of recurrent bacteremic infections with S. pyogenes is likely explained by specific opsonizing antibodies formed against the M protein during infection (6, 9, 12). The M protein is a virulence determinant of GAS, mediating survival in fresh human blood, and it is used for serotype determination. Type-specific immunity against S. pyogenes depends on antibodies toward the hypervariable amino-terminal part of M proteins (5), but repeated infections can also yield protective antibodies directed to conserved epitopes of the M protein (2). The S. pyogenes strain isolated from the patient was tested for growth in nonimmune human blood essentially as described previously (9a). After a 2-h incubation, 3 to 12 times the initial number of bacteria was present in fresh heparinized blood from two healthy donors (n = 3). If type-specific opsonizing antibodies were present in the patients' sera, they would have opsonized the isolate in the nonimmune blood (9a). The isolate grew in blood samples obtained from the same donors with the addition of convalescence serum from the patient to the same extent as it did without the patient serum (50 μl [drawn at the visit in July] to 250 μl blood and 50 μl diluted bacterial culture). As a control, the AP1 strain of the M1 serotype was shown to multiply in the blood samples obtained from both donors, whereas it did not grow when serum known to contain anti-M1 antibodies was added. The apparent lack of opsonizing antibodies was surprising since initial analyses had shown that the isolate was unable to grow in fresh blood drawn from the patient (also from July). Importantly, the patient was not undergoing treatment with antibiotics at the time of sampling. Since the patient died shortly after these experiments, they could not be repeated. Thus, it seems that the blood of the patient had some protective factors that were not type-specific opsonizing antibodies. The overall immunoglobulin levels of the patient were normal, as determined by routine diagnostic procedures, indicating that there was not a global impairment of antibody production but rather a putative lack of a specific subset of antibodies. The complement function was normal.
The lack of development of opsonizing antibodies could perhaps help to explain the seemingly unique event of a recurrent bacteremic S. pyogenes infection. The infected necrotic toe had diminished local defense mechanisms due to the ischemia and was probably the site for bacterial entry. Despite the many similarities between streptococci of groups A, C, and G, the propensity to cause recurrent disease seems to differ significantly. More work is needed to provide a molecular explanation to this phenomenon.
Acknowledgments
The Swedish Government Funds for Clinical Research (ALF) financed this work.
Ann Cathrine Peterson is acknowledged for important advice, and I thank Lisbeth Elfström for help with the rep-PCR. The patient gave his informed consent to the writing of this report. The regional ethical board approved of the sampling procedures used (2008/657).
Footnotes
Published ahead of print on 23 February 2011.
REFERENCES
- 1. Bert F., Lambert-Zechovsky N. 1997. Analysis of a case of recurrent bacteremia due to group A Streptococcus equisimilis by pulsed-field gel electrophoresis. Infection 25:250–251 [DOI] [PubMed] [Google Scholar]
- 2. Brandt E. R., et al. 1996. Opsonic human antibodies from an endemic population specific for a conserved epitope on the M protein of group A streptococci. Immunology 89:331–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carapetis J. R., Steer A. C., Mulholland E. K., Weber M. 2005. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5:685–694 [DOI] [PubMed] [Google Scholar]
- 4. Cohen-Poradosu R., et al. 2004. Group G streptococcal bacteremia in Jerusalem. Emerg. Infect. Dis. 10:1455–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dale J. B., Seyer J. M., Beachey E. H. 1983. Type-specific immunogenicity of a chemically synthesized peptide fragment of type 5 streptococcal M protein. J. Exp. Med. 158:1727–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hirst G. K., Lancefield R. C. 1939. Antigenic properties of the type-specific substance derived from group a hemolytic streptococci. J. Exp. Med. 69:425–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kahn F., Linder A., Petersson A. C., Christensson B., Rasmussen M. 2010. Axillary abscess complicated by venous thrombosis: identification of Streptococcus pyogenes by 16S PCR. J. Clin. Microbiol. 48:3435–3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kittang B. R., Langeland N., Mylvaganam H. 2008. Distribution of emm types and subtypes among noninvasive group A, C and G streptococcal isolates in western Norway. APMIS 116:457–464 [DOI] [PubMed] [Google Scholar]
- 9. Lancefield R. C. 1928. The antigenic complex of Streptococcus haemolyticus. I. Demonstration of a type-specific substance in extracts of Streptococcus haemolyticus. J. Exp. Med. 47:91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a. Lancefield R. C. 1957. Differentiation of group A streptococci with a common R antigen into three serological types, with special reference to the bactericidal test. J. Exp. Med. 106:525–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liao C. H., Liu L. C., Huang Y. T., Teng L. J., Hsueh P. R. 2008. Bacteremia caused by group G streptococci, Taiwan. Emerg. Infect. Dis. 14:837–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luca-Harari B., et al. 2009. Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J. Clin. Microbiol. 47:1155–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lyons C., Ward H. K. 1935. Studies on the hemolytic streptococcus of human origin. II. Observations on the protective mechanism against the virulent variants. J. Exp. Med. 61:531–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rantala S., Vuopio-Varkila J., Vuento R., Huhtala H., Syrjanen J. 2009. Clinical presentations and epidemiology of beta-haemolytic streptococcal bacteraemia: a population-based study. Clin. Microbiol. Infect. 15:286–288 [DOI] [PubMed] [Google Scholar]
- 14. Santos J., et al. 1994. Bacteremia and recurrent arthritis caused by Streptococcus pyogenes in a heroin addict with AIDS. Enferm. Infecc. Microbiol. Clin. 12:390–392 (In Spanish.) [PubMed] [Google Scholar]
- 15. Siljander T., et al. 2006. emm typing of invasive T28 group A streptococci, 1995–2004, Finland. J. Med. Microbiol. 55:1701–1706 [DOI] [PubMed] [Google Scholar]
- 15a. Sonesson A., et al. 2004. An immunosuppressed patient with systemic vasculitis suffering from a cerebral abscess due to Nocardia farcinica identified with 16S rRNA universal PCR. Nephrol. Dial. Transplant. 19:2896–2900 [DOI] [PubMed] [Google Scholar]
- 16. Sylvetsky N., Raveh D., Schlesinger Y., Rudensky B., Yinnon A. M. 2002. Bacteremia due to beta-hemolytic Streptococcus group G: increasing incidence and clinical characteristics of patients. Am. J. Med. 112:622–626 [DOI] [PubMed] [Google Scholar]
- 17. Tee W. S., Lieu P. K., Ngan C. C. 2002. Epidemiology of beta-haemolytic group G streptococcal bacteraemia in Singapore (1996 to 1998). Ann. Acad. Med. Singapore 31:86–91 [PubMed] [Google Scholar]