Abstract
Gordonia species are aerobic actinomycetes recently recognized as causing human disease, often in the setting of intravascular catheter-related infections. We describe a case of Gordonia bronchialis bacteremia and pleural space infection in the absence of an indwelling intravascular catheter and review the breadth of reported infections with this emerging pathogen.
CASE REPORT
A 52-year-old woman with a history of Hodgkin's lymphoma, prior splenectomy, and breast cancer was experiencing recurrent pleural effusions over several months prior to admission and presented with bloody drainage from an indwelling pleural catheter. The patient had been diagnosed with Hodgkin's lymphoma 35 years earlier and was treated with radiation therapy to the neck, chest, and abdomen at that time. She underwent a splenectomy for a splenic artery aneurysm 14 years prior to presentation. Five years prior to presentation, she had cardiac surgery for radiation-induced valvular disease, with placement of a St. Jude's mechanical prosthetic valve at the aortic position and repair of mitral and tricuspid valves with biological prosthetic material; a cardiac pacemaker for a heart block was placed. Four years prior to presentation, she had undergone bilateral mastectomies for breast cancer and was subsequently treated with tamoxifen and then anastrozole. Six months prior to presentation, she developed bilateral pleural effusions. The pleural fluid sampled on two occasions was consistent with exudative effusions, with slight lymphocyte predominance and negative Gram stains and cultures, including mycobacterial culture; cytology for malignant cells was negative. Based on these studies, the etiology of the pleural disease was unclear but was thought to be a late complication of her radiation therapy 35 years previously. Bilateral indwelling pleural catheters were placed, and the left-sided catheter was later removed when drainage ceased. In the weeks prior to the most recent presentation, the patient initiated systemic anticoagulation therapy for atrial fibrillation. She subsequently developed bloody drainage from the remaining right-sided indwelling pleural catheter, at which time she was admitted for further evaluation. She did not experience fever, chills, night sweats, cough, or other new symptoms.
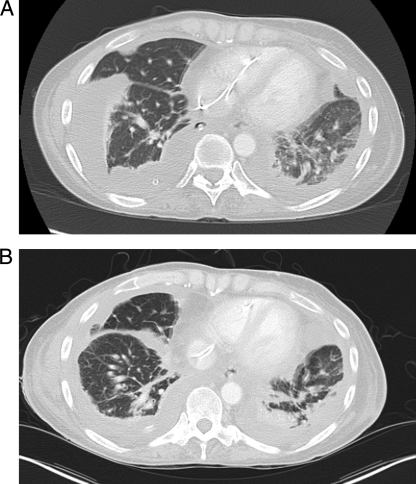
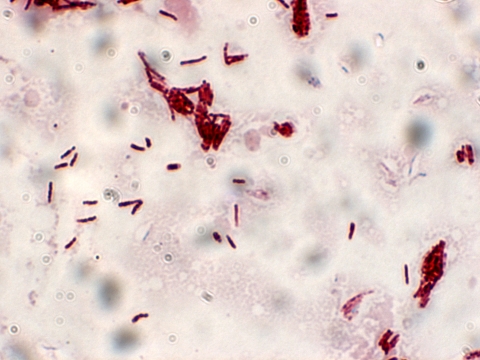
At the time of admission, the patient was afebrile. Blood cultures were drawn, and empirical therapy with vancomycin and ceftazidime was initiated. Anticoagulation therapy was discontinued. She subsequently developed rapid respiratory deterioration. On the fourth hospital day, video-assisted thorascopic surgery (VATS) was performed for drainage of a right-sided loculated pleural effusion, decortication, and placement of a right-sided chest tube (Fig. 1). She subsequently improved clinically, remaining afebrile on empirical antibacterial therapy. However, after 4 days of incubation, the aerobic blood culture bottle drawn on the day of admission grew Gram-positive rods, which also stained weakly acid fast (Fig. 2). All further blood cultures after initiation of antibiotics were without growth.
Fig. 1.
Chest computed tomography at the time of admission (A) and after surgical drainage and decortication of the right-sided bloody effusion (B).
Fig. 2.
Modified acid-fast staining of the pathogen from blood cultures at the time of admission.
The organism was preliminarily identified as an aerobic actinomycete, possibly a Nocardia sp. or Rhodococcus sp. based on observed morphology, and was referred to the Anaerobe Research Laboratory for further characterization by long-chain fatty acid analysis using the MIDI system (Microbial Identification Systems, Newark, DE) and the clinical identification library, which includes long-chain fatty acid profile data for Gordonia species. Antibacterial therapy was changed from vancomycin and ceftazidime to intravenous trimethoprim-sulfamethoxazole and imipenem-cilastatin. Transthoracic and transesophageal echocardiography demonstrated no vegetations or other abnormalities of the cardiac valves, including the mechanical prosthetic aortic valve or the pacemaker. The patient was subsequently transitioned to oral trimethoprim-sulfamethoxazole therapy at a dose equivalent to 12 mg/kg body weight/day and discharged to a rehabilitation facility, with final identification of the pathogen still pending.
Primary pleuropulmonary infection was suspected as the underlying cause of the bacteremia, so further laboratory studies of both the blood isolate and the available pleural specimens were performed. Pleural tissue biopsied at the time of placement of the indwelling pleural catheter 3 months prior to this presentation was examined with tissue Gram and acid-fast staining, and no organisms were identified. Pleural tissue from the surgical drainage of the right-sided bloody pleural effusion on the fourth hospital day of this presentation later grew Gram-positive rods in culture. Both the blood isolate and the pleural isolate were sent to the Actinomycete Reference Laboratory of the Centers for Disease Control and Prevention (CDC), where both isolates were identified as Gordonia bronchialis by 16S rRNA gene (1,495-bp) sequencing analysis (16). Both patient isolates were 100% similar to G. bronchialis type strain ATCC 25592. Using established procedures (22), with gyrB gene (approximately 1,245-bp) sequencing, both patient isolates were 100% similar to each other and 93% related to the type strain of G. bronchialis. There were no differences between the two patient isolates in the utilization of carbohydrates and hydrolysis of various substrates, using methods previously described (6). Antimicrobial drug susceptibility patterns and breakpoints, as determined by the use of published NCCLS standards (5), of G. bronchialis at the CDC laboratory revealed that the organism had intermediate resistance to clarithromycin but was susceptible to all other antibiotics tested: amoxicillin-clavulanate, amikacin, ceftriaxone, ciprofloxacin, linezolid, minocycline, trimethoprim-sulfamethoxazole, tigecycline, and vancomycin. The patient was subsequently transitioned to oral ciprofloxacin and minocycline therapy based on the low MIC results observed from susceptibility testing. She completed a 3-month course of antibiotic therapy and has recovered.
The Gordonia (previously Gordona) genus was first differentiated from other aerobic actinomycetes in 1971 (24). Microbiologic diagnosis of Gordonia species is difficult, often resulting in incorrect identification as other actinomycetes or mycobacteria, as initially occurred in this case. However, increasing use of 16S rRNA sequencing has significantly improved organism identification. In recent years, there have been reports of a variety of infections due to several of the Gordonia spp., such that Gordonia spp. now comprise a significant minority of the aerobic actinomycetes isolated in human diseases. A study in Thailand found that of 171 aerobic actinomycete isolates sent to the National Institutes of Health for identification between 1996 and 2003, approximately 56% were Nocardia spp., 12% Mycobacteria spp., 11% Streptomyces spp., 8% Rhodococcus spp., 6% Gordonia spp., 0.6% Tsukamurella spp., and 0.6% Corynebacterium spp. (18). Since the Gordonia genus was named, there have been 29 different species identified. Several types of infections caused by these Gordonia spp. have been described in the literature, including sternal wound infections (20), ventriculitis with an underlying ventricular shunt (2), otitis externa (10), bronchitis (10), skin and soft tissue infections (1, 13), arthritis associated with a biological absorbable bone/joint screw (11), recurrent breast abscess (26), granulomatous mastitis following nipple piercing (27), keratitis/conjunctivitis (12), endocarditis related to an underlying central venous catheter (15, 25), and bacteremia (both catheter related and without an indwelling catheter) (4, 23).
Gordonia bronchialis, the species isolated in this case, is the type species of Gordonia, first identified from samples of soil and sputum obtained from patients with pulmonary disease (cavitary tuberculosis and bronchiectasis) (24). In our review of the literature, we identified 11 other reported cases of G. bronchialis infections in humans (Table 1), 7 of which were sternal surgical site infections during an outbreak in a single hospital, which was traced to an operating room nurse (20). Of the remaining four cases, one was a case of ventriculitis related to a ventricular shunt infection in a neonate, one was a case of recurrent breast abscess, and two were cases of bacteremia (2, 26). Of the two cases of bacteremia, neither was thought to be due to an indwelling central venous catheter (CVC), which is unusual for Gordonia bacteremia. One case of bacteremia occurred in a patient with nonketotic hyperosmolar coma due to diabetes, and the bacteremia was of unclear significance (3). The other was similar to the case presented here in that the patient had underlying chronic pleuropulmonary disease with a sequestrated lung and developed bacteremia without a current indwelling CVC (23). In that case, the patient was treated with surgical drainage of abscesses, intravenous vancomycin, and ceftriaxone for more than 2 months, followed by oral amoxicillin-clavulanate for 6 weeks, and made a full recovery.
Table 1.
Summary of clinical reports of infections with Gordonia bronchialis
| Type(s) of infection | No. of cases | Patient age(s) | Underlying condition(s) | Yr of publication (reference) |
|---|---|---|---|---|
| Bacteremia and pleural infection | 1 | 52 yr | Lymphoma, splenectomy, breast cancer, and pleural effusions | 2011 (this case report) |
| Bacteremia | 1 | 67 yr | Diabetes and nonketotic hyperosmolar coma | 2009 (3) |
| Intraventricular shunt | 1 | 45 days | Premature neonate | 2007 (2) |
| Recurrent breast abscess | 1 | 43 yr | Pituitary adenoma | 2005 (26) |
| Bacteremia | 1 | 58 yr | Sequestrated lung and diabetes | 2004 (23) |
| Sternal wound (hospital outbreak traced to operating room nurse) | 7 | 51–68 yr | Surgery | 1991 (20) |
Gordonia bronchialis is the identified species in only a minority of human infections due to Gordonia spp. and rarely a cause of bacteremia. In our review of the literature, we identified 30 other reported cases of Gordonia bloodstream infections, with only 2 of these due to G. bronchialis (Table 2). Of the 30 cases of bacteremia, 22 were related to indwelling central venous catheters (CVCs), most were due to Gordonia sputi or Gordonia terrae, and most occurred in patients with an underlying malignancy or other immunocompromised states.
Table 2.
Reported bloodstream infections with Gordonia species
| Gordonia species | Type(s) of infection | No. of cases | Patient age (yr) | Underlying condition(s)a | Yr of publication (reference) |
|---|---|---|---|---|---|
| G. bronchialis | Bacteremia and pleural infection | 1 | 52 | Lymphoma, splenectomy, breast cancer, and pleural effusions | 2011 (this case report) |
| G. polyisoprenivorans | Bacteremia due to CVC and pneumonia | 1 | 17 | Leukemia | 2010 (9, 14) |
| G. sputi | Bacteremia due to CVC | 3 | 43 | SLE and pulmonary hypertension | 2009 (19) |
| 46 | HIV, hepatitis C, pulmonary hypertension | ||||
| 78 | Mesenteric ischemia and TPN | ||||
| Bacteremia and a likely contaminant | 1 | 60 | Pneumonia and pleural effusion | ||
| G. bronchialis | Bacteremia | 1 | 67 | Diabetes and nonketotic hyperosmolar coma | 2009 (3) |
| G. terrae | Bacteremia due to CVC | 1 | 58 | Leukemia | 2009 (13) |
| Bacteremia | 2 | 23 | Leukemia and BMT | 2009 (13) | |
| 75 | COPD and atrial fibrillation | ||||
| G. sputi | Bacteremia due to CVC | 1 | 14 | Short bowel syndrome and TPN | 2009 (13) |
| Bacteremia | 1 | 49 | Gastric cancer and diabetes | 2009 (13) | |
| Bacteremia due to CVC | 1 | 69 | Laryngeal cancer | 2009 (13) | |
| G. terrae | Bacteremia due to CVC | 1 | 24 | Sepsis, abuse of methandienone, dyspnea | 2007 (8) |
| Bacteremia due to CVC | 3 | 3 | Wilms' tumor | 2007 (2) | |
| 3 | Leukemia | ||||
| 5 | LACH syndrome and hypogammaglobulinemia | ||||
| G. otitidis | Bacteremia due to CVC | 1 | 11 | Periodic fever syndrome and bowel necrosis | 2007 (2) |
| G. polyisoprenivorans | Catheter-related bacteremia and native valve endocarditis | 1 | 78 | Osler-Weber-Rendu and myelodysplastic syndromes | 2006 (25) |
| G. terrae | Bacteremia and acute cholecystitis | 1 | 61 | Hepatitis C | 2006 (7) |
| G. bronchialis | Bacteremia | 1 | 58 | Sequestrated lung and diabetes | 2004 (23) |
| G. polyisoprenivorans | Bacteremia due to CVC | 1 | 26 | Leukemia and BMT | 2004 (12) |
| G. terrae | Bacteremia due to CVC | 5 | 28 | Leukemia and splenectomy | 2003 (17) |
| 44 | Brain tumor | ||||
| 54 | Leukemia | ||||
| 46 | Unknown primary cancer, metastatic to liver | ||||
| 60 | Thyroid cancer with metastasis | ||||
| Gordonia sp. most closely resembling G. sputi | Catheter-related bacteremia and native valve endocarditis | 1 | 31 | Splenectomy, hemoglobinopathy, and cirrhosis | 2000 (15) |
| G. sputi | Bacteremia due to cutaneous lesions | 1 | 34 | Metastatic melanoma and IL-2 treatment | 1996 (21) |
| G. terrae | Bacteremia due to CVC | 1 | 43 | Chronic intestinal pseudo-obstruction syndrome and TPN | 1992 (4) |
| Unidentified Gordonia sp. | Bacteremia due to CVC | 1 | 65 | Breast and ovarian cancer and TPN | 1992 (4) |
Abbreviations: CVC, central venous catheter; SLE, systemic lupus erythematosus; HIV, human immunodeficiency virus; TPN, total parenteral nutrition; BMT, bone marrow transplant; COPD, chronic obstructive pulmonary disease; LACH, leukoencephalopathy, arthritis, colitis, and hypogammaglobulinemia; IL-2, interleukin-2.
Our case represents a rare instance of Gordonia bacteremia in the absence of a current indwelling intravascular catheter. In our case, the source of the bacteremia may have been a primary pleuropulmonary infection, although inoculation via intravascular catheters during prior hospital admissions cannot be excluded. A pleuropulmonary infection may have been chronic and the original cause of the patient's pleural effusions, which developed 6 months prior to her presentation with bacteremia. The patient had reported gardening regularly during the preceding summer in the weeks and months prior to the development of her pleural effusions, indicating possible soil inhalation exposure to G. bronchialis. Alternatively, it is possible that the G. bronchialis infection was a recent infection related to the indwelling pleural catheter. Our patient's history of hematologic malignancy, radiation therapy, and splenectomy may have predisposed her to this rare infection.
There are no standardized recommendations for treatment of infections due to Gordonia spp. Available data suggest that Gordonia spp., in contrast to some other actinomycetes, such as Rhodococcus spp., are generally susceptible to many antimicrobial drugs. Susceptibility testing at reference laboratories has been useful to guide the choice of antibiotic therapy in the reported cases of human infection with Gordonia spp. In the previous reported cases of Gordonia bacteremia, numerous different antibiotics were used, either alone or in a variety of combinations, including vancomycin (often in combination with an expanded-spectrum cephalosporin or carbapenem or with rifampin in some pediatric patients), linezolid, amoxicillin or amoxicillin-clavulanate (often as a subsequent oral treatment after completion of intravenous therapy), trimethoprim-sulfamethoxazole, ceftriaxone, ceftazidime, piperacillin-tazobactam, ticarcillin-clavulanate, imipenem-cilastatin, meropenem, ciprofloxacin, levofloxacin, amikacin or gentamicin (in combination with a cephalosporin or carbapenem), azithromycin, and clindamycin. The duration of treatment also varied from case to case, with many patients completing between 6 and 12 weeks of antibiotic therapy. For catheter-associated Gordonia infections, catheter removal is recommended for infections in children, but there are no formal guidelines for management of catheters in adults. In the majority of the cases described above, the catheters were removed as a part of treatment of the Gordonia infection. The prognosis for Gordonia bloodstream infections is variable, depending on the underlying conditions of the host, the clinical factors related to the infection, and the time to diagnosis.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences from G. bronchialis X0216 (81-10) and X0217 (82-10) were deposited in GenBank under accession numbers HQ316181 and HQ316182, respectively. The gyrB gene sequences of X0216 (81-10) and X0217 (82-10) were deposited in GenBank under accession numbers HM352642 and HM352643, respectively.
Footnotes
Published ahead of print on 26 January 2011.
REFERENCES
- 1. Bakker X. R., Spauwen P. H., Dolmans W. M. 2004. Mycetoma of the hand caused by Gordona terrae: a case report. J. Hand Surg. Br. 29:188–190 [DOI] [PubMed] [Google Scholar]
- 2. Blaschke A. J., et al. 2007. Gordonia species: emerging pathogens in pediatric patients that are identified by 16S ribosomal RNA gene sequencing. Clin. Infect. Dis. 45:483–486 [DOI] [PubMed] [Google Scholar]
- 3. Brust J. C., Whittier S., Scully B. E., McGregor C. C., Yin M. T. 2009. Five cases of bacteraemia due to Gordonia species. J. Med. Microbiol. 58:1376–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buchman A. L., McNeil M. M., Brown J. M., Lasker B. A., Ament M. E. 1992. Central venous catheter sepsis caused by unusual Gordona (Rhodococcus) species: identification with a digoxigenin-labeled rDNA probe. Clin. Infect. Dis. 15:694–697 [DOI] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute/NCCLS 2003. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes. Approved standard M24-A. National Committee for Clinical Laboratory Standards, Wayne, PA: [PubMed] [Google Scholar]
- 6. Conville P. S., Witebsky F. G. 2007. Nocardia, Rhodococcus, Gordonia, Actinomadura, Streptomyces, and other aerobic actinomycetes, p. 515–542 In Murray P. R., Baron E. J., Jorgensen J. H., Landry M. L., Pfaller M. A. (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC [Google Scholar]
- 7. Gil-Sande E., et al. 2006. Etiological misidentification by routine biochemical tests of bacteremia caused by Gordonia terrae infection in the course of an episode of acute cholecystitis. J. Clin. Microbiol. 44:2645–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grisold A. J., et al. 2007. Isolation of Gordonia terrae from a patient with catheter-related bacteraemia. J. Med. Microbiol. 56:1687–1688 [DOI] [PubMed] [Google Scholar]
- 9. Gupta M., Prasad D., Khara H. S., Alcid D. 2010. A rubber-degrading organism growing from a human body. Int. J. Infect. Dis. 14:75–76 [DOI] [PubMed] [Google Scholar]
- 10. Iida S., et al. 2005. Gordonia otitidis sp. nov., isolated from a patient with external otitis. Int. J. Syst. Evol. Microbiol. 55:1871–1876 doi:10.1099/ijs.0.63282-0 [DOI] [PubMed] [Google Scholar]
- 11. Jannat-Khah D. P., et al. 2009. Gordonia araii infection associated with an orthopedic device and review of the literature on medical device-associated Gordonia infections. J. Clin. Microbiol. 47:499–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kempf V. A. J., et al. 2004. Gordonia polyisoprenivorans septicemia in a bone marrow transplant patient. Eur. J. Clin. Microbiol. Infect. Dis. 23:226–228 [DOI] [PubMed] [Google Scholar]
- 13. Lai C. C., et al. 14 October 2009, posting date Infections caused by Gordonia species at a medical center in Taiwan, 1997 to 2008. Clin. Microbiol. Infect. doi:10.1111/j.1469-0691.2009.03085.x [DOI] [PubMed] [Google Scholar]
- 14. Langer A. J., et al. 2010. Investigation of an apparent outbreak of Rhodococcus equi bacteremia. Diagn. Microbiol. Infect. Dis. 67:95–100 [DOI] [PubMed] [Google Scholar]
- 15. Lesens O., et al. 2000. Bacteremia and endocarditis caused by a Gordonia species in a patient with a central venous catheter. Emerg. Infect. Dis. 6:382–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morey R. E., et al. 2006. Species-specific identification of Leptospiraceae by 16S rRNA gene sequencing. J. Clin. Microbiol. 44:3510–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pham A. S., De I., Rolston K. V., Tarrand J. J., Han X. Y. 2003. Catheter-related bacteremia caused by the nocardiform actinomycete Gordonia terrae. Clin. Infect. Dis. 36:524–527 [DOI] [PubMed] [Google Scholar]
- 18. Poonwan N., et al. 2005. Characterization of clinical isolates of pathogenic Nocardia strains and related actinomycetes in Thailand from 1996 to 2003. Mycopathologia 159:361–368 doi:10.1007/s11046-005-1045-7 [DOI] [PubMed] [Google Scholar]
- 19. Renvoise A., Harle J. R., Raoult D., Roux V. 2009. Gordonia sputi bacteremia. Emerg. Infect. Dis. 15:1535–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richet H. M., et al. 1991. A cluster of Rhodococcus (Gordona) Bronchialis sternal-wound infections after coronary-artery bypass surgery. N. Engl. J. Med. 324:104–109 [DOI] [PubMed] [Google Scholar]
- 21. Riegel P., et al. 1996. Bacteremia due to Gordonia sputi in an immunocompromised patient. J. Clin. Microbiol. 8:2045–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shen F. T., et al. 2006. Phylogenetic analysis of members of the metabolically diverse genus Gordonia based on proteins encoding the gyrB gene. Res. Microbiol. 157:367–375 doi:10.1016/j.resmic.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 23. Sng L. H., et al. 2004. Bacteremia caused by Gordonia bronchialis in a patient with sequestrated lung. J. Clin. Microbiol. 42:2870–2871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsukamura M. 1971. Proposal of a new genus, Gordona, for slightly acid-fast organisms occurring in sputa of patients with pulmonary disease and in soil. J. Gen. Microbiol. 68:15–26 [DOI] [PubMed] [Google Scholar]
- 25. Verma P., et al. 2006. Native valve endocarditis due to Gordonia polyisoprenivorans: case report and review of literature of bloodstream infections caused by Gordonia species. J. Clin. Microbiol. 44:1905–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Werno A. M., Anderson T. P., Chambers S. T., Laird H. M., Murdoch D. R. 2005. Recurrent breast abscess caused by Gordonia bronchialis in an immunocompetent patient. J. Clin. Microbiol. 43:3009–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zardawi I. M., Jones F., Clark D. A., Holland J. 2004. Gordonia terrae-induced suppurative granulomatous mastitis following nipple piercing. Pathology 36:275–278 doi:10.1080/00313020410001692639 [DOI] [PubMed] [Google Scholar]