Abstract
Pneumococcal phase variation of 37 middle ear and 31 nasopharyngeal isolates obtained from children with acute otitis media was examined in the absence of intervening culture. The fraction of the opaque colonies was significantly higher in middle ear isolates than in nasopharyngeal isolates. The difference is probably the result of the pneumococci adapting to differential selective environments.
Streptococcus pneumoniae first colonizes the mucosal surface of the human nasopharynx and can then infect the middle ear cavity via the Eustachian tubes to cause acute otitis media (AOM). In 1994, Weiser et al. reported spontaneous, reversible intrastrain phase variation of pneumococci in colony opacity on transparent agar surfaces (15). Transparent variants associated with low levels of capsular polysaccharide are able to most efficiently colonize the nasopharynx in animal models of pneumococcal carriage (8). In contrast, opaque variants associated with greater amounts of capsular polysaccharide colonize poorly in animals and provide greater resistance to clearance by phagocytes but at the same time decrease the adherence of opaque variants to host cells (2, 4). Thus, the intrastrain phase shifts of pneumococci are hypothesized to be adaptations to different stages in the pathogenesis of infections (1, 8, 10, 13, 14). However, the phase shifts have never been clearly demonstrated with human samples. A study in 2001 examined 19 “minimally passaged” paired nasal and blood isolates, but no statistical association was observed between the phase and body isolation site (P = 0.51) (12). The present study was designed to investigate the morphological phase of pneumococci by plating them on transparent agar immediately upon their isolation from the nasopharynx and middle ear of children with AOM. By avoiding any growth between isolation and plating, we hoped to minimize the effects of bacterial growth in vitro on bacterial phase. The study focused on samples from young children because of their high rates of pneumococcal carriage and AOM.
Middle ear and nasopharyngeal cultures were collected from all children between 5 and 70 months of age who presented at our clinic with AOM from 2008 to 2010 and who had not previously been treated with antibiotics. No subsequent cultures were collected for the study from any patients at later clinic visits. The swabs used to collect cultures were stored on transport agar with charcoal media (Eiken Chemical Co., Tokyo, Japan) at room temperature and transported to the laboratory for processing, and within 18 h of collection, they were plated on tryptic soy agar (TSA) plates for phase determination (with no intervening culture).
Samples were obtained from 42 children, 25 males and 17 females, with a median age of 21.5 months. The children were all unrelated to each other, and only one culture from the nasopharynx and one from each ear were included in the study for a single patient, when available from the patient. All together, from the 42 children, 37 middle ear and 31 nasopharyngeal cultures were examined. Eighteen patients had paired cultures in the study that were isolated on the same day, one culture from their nasopharyngeal secretions (NPSs) and the second and/or third culture from their middle ear fluids (MEFs). A total of 6 of 18 patients had cultures from all three sites. For the other 12 of 18 patients, there was an NPS culture and a culture from one ear only. There were an additional 13 patients for whom there were nasopharyngeal cultures alone, 9 patients for whom there were single MEF cultures, and 2 patients for whom there was a MEF culture from each ear. The study was approved by the institutional review board of the ethical committee of Wakayama Medical University, and informed consent for the use of the cultures was obtained from the parent or guardian of each patient.
Within 18 h of collection, each culture swab was suspended in 150 μl of sterile phosphate-buffered saline (PBS). Serial dilutions of each sample were plated on tryptic soy plates solidified with 1% agar containing 5,000 U/ml of catalase (Worthington Biochemical, Freehold, NJ) to allow for direct evaluation of individual colony morphology while limiting any growth of bacteria between extraction of the culture from the patient and the time it was plated on TSA (1, 15). Transparent and opaque bacteria identified macroscopically on the TSA plates and classified by phase were subsequently confirmed to be pneumococci by streaking representative colonies on blood agar and examining them for colony morphology, alpha hemolysis, and sensitivity to optochin. Of those that appeared to be pneumococci, representative colonies were examined by Gram staining, bile solubility testing, and PCR for the presence of the autolysin gene (lytA). The decimal fraction of total colonies that were opaque was determined for each isolate. For each isolate, at least 20 distinguishable pneumococcal colonies on TSA plates were evaluated to determine the fraction of opaque colonies. The restriction fragment patterns of genomic DNAs purified from the restreaks of one colony of each phase type isolated from each body site were analyzed by pulsed-field gel electrophoresis (PFGE) as described elsewhere (9). Statistical analysis was performed with Prism 4 (GraphPad Software, Inc.) to compare the fractions of opaque colonies. P values of <0.05 were considered statistically significant.
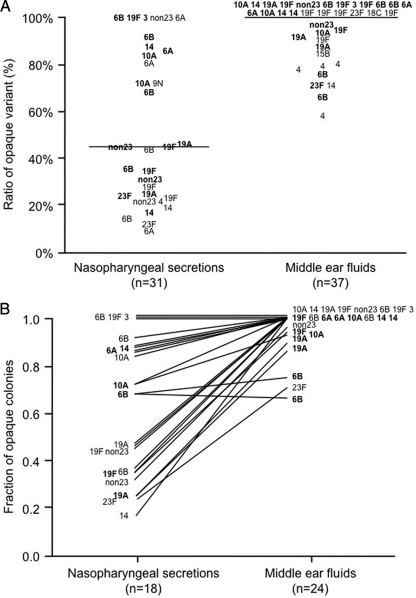
The fractions of opaque colonies in MEF cultures ranged from 0.58 to 1.00 (median, 1.00; 25% percentile, 0.87; and 75% percentile, 1.00). In 59% (22/37) of the MEF cultures, all of the pneumococcal colonies were opaque. In contrast, the fractions of opaque variants in the NPS cultures covered a wide range, from 0.09 to 1.00 (median, 0.45; 25% percentile, 0.23; and 75% percentile, 0.86). Only 16% (5/31) of the NPS cultures were exclusively composed of opaque variants. The prevalence of opaque variants in MEFs was significantly higher than that in NPSs (P < 0.01) (Fig. 1A).
Fig. 1.
Fractions of the opaque variant in pneumococcal isolates obtained from nasopharyngeal secretions and middle ear fluids. (A) Each number designates a serotype of an isolate from an individual patient. From 18 patients, cultures were obtained from both the middle ear and the nasopharynx (in boldface). The horizontal bar shows the median fraction of variants that are opaque. This difference has a P value of 0.0007 by the Mann-Whitney two-sample rank test. (B) Numbers show the serotypes of isolates from the 18 cases from which pneumococci were recovered simultaneously from MEFs and NPSs from the same patient. From 6 of these 18 patients, cultures from both the left and right ears (in boldface) were obtained. Lines connect the results obtained with cultures from NPSs and MEFs from the same patient. The trend to have a higher fraction of opaque colonies from MEF cultures than from NPS cultures was significant at P values of <0.0001 by the Wilcoxon matched-pairs signed-rank test.
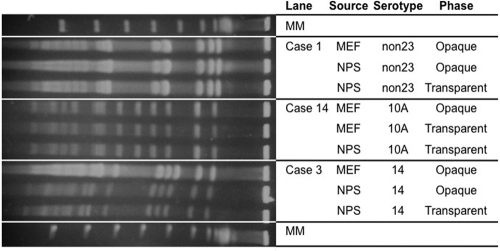
Among the 18 sets of paired samples, the fractions of opaque or transparent variants in the MEF cultures and the NPS cultures differed in the same fashion as the larger data set, with the tendency being toward exclusively opaque variants in the MEFs and mostly transparent variants in the NPSs (Fig. 1B). The fraction of opaque variants in the paired group of MEFs (range, 1.00 to 0.66; median, 1.00; 25% percentile, 0.94; and 75% percentile, 1.00) was significantly (by paired sign rank test; P < 0.001) higher than that in the NPSs (range, 1.00 to 0.17; median, 0.57; 25% percentile, 0.33; and 75% percentile, 0.89). The isolates collected on the same day from MEFs and NPSs of the 18 patients were found in each case to be identical to each other in the same patient, as determined by PFGE (Fig. 2). Although strains of different capsular types had varied percentages of opaque and transparent variants, there was no statistically significant correlation between capsular serotypes and pneumococcal phase variations.
Fig. 2.
PFGE patterns of SmaI digests of genomic DNA from paired nasopharyngeal and middle ear isolates of S. pneumoniae. PFGE patterns of paired nasopharyngeal and middle ear isolates collected from three representative patients are shown. The three different isolates examined from each of these three patients appeared identical by PFGE analysis, but each patient clearly had a different strain. In each case, the NPS and MEF isolates from each of the 15 remaining patients were also found to be identical, but differences between the isolates of the different patients were observed (data not shown). MM is molecular size marker (48.5-kb lambda DNA ladder). The non23 strain is the serotype not including the 23-valent pneumococcal capsular polysaccharide vaccine.
Previous studies in animal models have shown that transparent variants are released when nasal tissues are washed with physiological salt solutions, while the majority of S. pneumoniae isolates recovered from previously washed homogenized nasal tissue, from blood specimens, or from lung aspirates were in the opaque phase (1, 12). In chinchillas pretreated with live influenza virus, inoculation with opaque pneumococci caused more severe middle ear infections than did inoculation with transparent pneumococci (10).
Relatively little is understood about the means by which pneumococci become established in the human nasopharynx and then make the transition to the middle ear (10, 12). Laboratory cultures of most of the previously obtained clinical isolates of pneumococci consist of heterogeneous populations of transparent and opaque variants, and the ability of pneumococci to switch back and forth between transparent and opaque variants is thought to represent a viable strategy for adaptation to the different local environments during the course of disease pathogenesis (13–15).
This study is the first to report on the phase variation of pneumococci obtained from MEFs and NPSs from children with AOM. The differences in the distribution of phase variants between the NPS and MEF isolates may result from differential selection within the two environments. The ability to adhere may have greater importance within the nasopharyngeal environment, thus allowing a selection toward the greater frequency of transparent variants. The ability to evade opsonophagocytosis may be a much stronger requirement of the middle ear, especially as it enters into an inflammatory state during AOM. This requirement to evade opsonophagocytosis would impose the selection of the more highly encapsulated opaque variants within the middle ear environment, as is proposed for the blood and other invasive sites (11). Li-Korotky et al. and Weiser et al. suggested that environments with lower oxygen contents, such as the middle ear space of AOM patients, promote selection for the more virulent opaque variant (7, 12). Opaque variants are more efficient at survival and multiplication in the middle ear cavity of the chinchilla (10).
Changes in the amount of cell wall teichoic acid are a second phenotype associated with the described phase variation. In this case, the transparent-phase variants are observed to have more cell-associated teichoic acid than the opaque-phase variants (5). Phosphocholine (PC) is a major determinant of teichoic acid. Since teichoic acid is a major component of the cell surface, the differences in the amount of phosphocholine (PC) that distinguish phase variations could alter the surface localization of pneumococcal proteins anchored to the PC. Evidence that expression of choline binding proteins varies in association with phase variation has been reported (4, 6, 12). The difference in surface-localized PC may also directly affect the bacterial adherence to some epithelial cells that occurs through phosphocholine (2, 3).
Overall, the distributions of phase variants that we observed are consistent with the proposed hypothesis that the transparent variant may be better adapted to adhere in the normal microenvironments of the nasopharynx and, consequently, may be a key player in the initial step of pathogenesis of pneumococcal AOM. In this study, we did not find any correlation between tympanic membrane findings or clinical outcomes of AOM and phase variation (data not shown). This may not be surprising, however, since the disease outcome may be more related to which virulence genes the bacterium is able to express in its tissue-specific phases than to the absolute percentage of bacteria in a particular phase at each site. Further investigation will be required to completely evaluate the roles of the phase variations in the clinical features of AOM.
Acknowledgments
We greatly thank Janice King (Department of Microbiology, University of Alabama at Birmingham) for her technical assistance in helping us learn to determine phase variation. We also greatly thank Yuki Tatsumi (Department of Otolaryngology—Head and Neck Surgery, Wakayama Medical University, Wakayama, Japan), Gen Sugita (Department of Otolaryngology—Head and Neck Surgery, Wakayama Medical University, Wakayama, Japan), and Rinya Sugita (Sugita ENT Clinic, Chiba, Japan) for their assistance in conducting the study.
This work was supported by national grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology (grant 21791638), NIH grant AI021458 (to D.E.B. and S.K.H.), and the National Research Council of Korea grant WCU (to D.E.B.). We have no conflicts of interest.
Footnotes
Published ahead of print on 23 February 2011.
REFERENCES
- 1. Briles D. E., Novak L., Hotomi M., van Ginkel F. W., King J. 2005. Nasal colonization with Streptococcus pneumoniae includes subpopulations of surface and invasive pneumococci. Infect. Immun. 73:6945–6951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cundell D. R., Weiser J. N., Shen J. J., Young A., Tuomanen E. I. 1995. Relationship between colonial morphology and adherence of Streptococcus pneumoniae. Infect. Immun. 63:757–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cundell D. R., Gerard N. P., Gerard C., Idanpaan-Heikkila I., Tuomanen E. I. 1995. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377:435–438 [DOI] [PubMed] [Google Scholar]
- 4. Kim J. O., et al. 1999. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect. Immun. 67:2327–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim J. O., Weiser J. N. 1998. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. 177:368–377 [DOI] [PubMed] [Google Scholar]
- 6. Li-Korotky H. S., Lo C. Y., Zeng F. R., Lo D., Banks J. M. 2009. Interaction of phase variation, host and pressure/gas composition: pneumococcal gene expression of PsaA, SpxB, Ply and LytA in simulated middle ear environments. Int. J. Pediatr. Otorhinolaryngol. 73:1417–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li-Korotky H. S., et al. 2008. Interaction of pneumococcal phase variation and middle ear pressure/gas composition: an in vitro model of simulated otitis media. Microb. Pathog. 45:201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nelson A. L., et al. 2007. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect. Immun. 75:83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shimada J., et al. 2002. Household transmission of Streptococcus pneumoniae among siblings with acute otitis media. J. Clin. Microbiol. 40:1851–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tong H. H., Weiser J. N., James M. A., DeMaria T. F. 2001. Effect of influenza A virus infection on nasopharyngeal colonization and otitis media induced by transparent or opaque phenotype variants of Streptococcus pneumoniae in the chinchilla model. Infect. Immun. 69:602–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weiser J. N. 2010. The pneumococcus: why a commensal misbehaves. J. Mol. Med. 88:97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weiser J. N., et al. 2001. Changes in availability of oxygen accentuate differences in capsular polysaccharide expression by phenotypic variants and clinical isolates of Streptococcus pneumoniae. Infect. Immun. 69:5430–5439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weiser J. N., Markiewicz Z., Tuomanen E. I., Wani J. H. 1996. Relationship between phase variation in colony morphology, intrastrain variation in cell wall physiology, and nasopharyngeal colonization by Streptococcus pneumoniae. Infect. Immun. 64:2240–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weiser J. N., Kapoor M. 1999. Effect of intrastrain variation in the amount of capsular polysaccharide on genetic transformation of Streptococcus pneumoniae: implications for virulence studies of encapsulated strains. Infect. Immun. 67:3690–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weiser J. N., Austrian R., Sreenivasan P. K., Masure H. R. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 62:2582–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]