Abstract
The aim of this study was to develop a highly sensitive and specific one-step multiplex reverse transcriptase PCR assay for the simultaneous and differential detection of Rift Valley Fever virus (RVFV), bluetongue virus (BTV), rinderpest virus (RPV), and Peste des petits ruminants virus (PPRV). These viruses cause mucosal lesions in cattle, sheep, and goats, and they are difficult to differentiate from one another based solely on their clinical presentation in suspected disease cases. In this study, we developed a multiplex reverse transcriptase PCR to detect these viruses using a novel dual-priming oligonucleotide (DPO). The DPO contains two separate priming regions joined by a polydeoxyinosine linker, which blocks extension of nonspecifically primed templates and consistently allows high PCR specificity even under less-than-optimal PCR conditions. A total of 19 DPO primers were designed to detect and discriminate between RVFV, BTV, RPV, and PPRV by the generation of 205-, 440-, 115-, and 243-bp cDNA products, respectively. The multiplex reverse transcriptase PCR described here enables the early diagnosis of these four viruses and may also be useful as part of a testing regime for cattle, sheep, or goats exhibiting similar clinical signs, including mucosal lesions.
INTRODUCTION
Rift Valley Fever virus (RVFV), bluetongue virus (BTV), rinderpest virus (RPV), and Peste des petits ruminants virus (PPRV) induce diseases (primarily in cattle, sheep, and goats) that have considerable economic impact on domestic livestock production. Due to the potential for very serious and rapid spread, the diseases caused by these four viruses are classified by OIE, the World Organization for Animal Health, as notifiable diseases (formerly List A). During outbreaks, affected countries are banned from trading livestock and livestock products, which can have major impact in the international trade of animals or animal products and also have serious socio-economic or public health consequences (22).
Rift Valley Fever is an important viral zoonosis in Africa that affects animals and humans. In cattle and sheep, the disease may cause sudden abortion, and mortality rates close to 100% are observed in newborns (1, 9). Many infected humans become severely ill, and serious hemorrhagic fevers often lead to death (9). In nonfatal cases, complications can also progressively develop, resulting in blindness and various signs of encephalitis, including paralysis of one or more limbs. BTV can cause a severe clinical disease, with mortality rates exceeding 10% in sheep (12, 18), whereas the infection is mostly asymptomatic or mild in cattle, goats, and wild ruminants (2). Lesions detected in postmortem examinations of BTV-affected sheep include hyperemia, hemorrhages, erosion and ulceration of the mucosa of the gastrointestinal tract (oral cavity, esophagus, and forestomachs), and lymph node edema and hemorrhaging (17). Rinderpest is caused by a morbillivirus belonging to the family Paramyxoviridae, and it affects cloven-hoofed animals. Rinderpest is highly infectious in cattle and classically affects all individuals in contact with a diseased animal within a short period of time (24). PPRV also belongs to the Paramyxoviridae family, and the disease is responsible for significant losses in sheep and goat productivity in regions where it is endemic, which include Oman, Jordan, Turkey, Bangladesh, India, China, and several African countries. Peste des petits ruminants is also known as goat plague, kata, abu nini, pseudo-rinderpest, and pneumonia-enteritis complex. As in rinderpest, there is usually severe erosive necrotic stomatitis and enterocolitis. PPRV-infected animals show tiny grayish necrotic spots on the inner aspect of the lips and cheeks, and especially on the dorsal surface of the tongue (27).
RVFV, BTV, RPV, and PPRV infections all have similar clinical presentations, and specific diagnosis therefore relies almost entirely on laboratory investigation. In areas where these viruses are absent, distinguishing among RVFV, BTV, RPV, and PPRV is critical for detecting the introduction of these important infectious diseases. To prevent the spread of these diseases, rapid virus detection during the viremic phase of the illness is necessary to provide timely clinical treatment and etiological differentiation. In addition, rapid diagnosis of these diseases in disease-free countries is also needed to allow veterinary health authorities time to effectively counteract their spread.
Recently, virus-specific PCR-based assays have been developed as an alternative to virus isolation for the diagnosis of individual diseases. Nucleic acid detection techniques (e.g., PCR methods) allow rapid and sensitive detection of some of the most serious viral infections, including RVFV, BTV, RPV, and PPRV. However, the use of multiplex reverse transcriptase PCR (RT-PCR) to detect RVFV, BTV, RPV, and PPRV infection has not been reported, although they all cause similar mucosal lesions and are not easy to differentiate from one another based solely on their clinical presentation in suspected cases. The simultaneous detection of infectious agents using single-tube multiplex RT-PCR is an especially convenient diagnostic method with cost- and time-saving benefits. Recently, it was shown that a dual-priming oligonucleotide (DPO) containing two separate priming regions joined by a polydeoxyinosine linker can increase specificity in such assays. The DPO blocks extension of nonspecifically primed templates and consistently produces highly specific PCR amplification even under suboptimal PCR conditions (6, 11, 14, 23, 33).
In the present study, we used this approach to develop and validate a more rapid molecular diagnostic method for the simultaneous detection and differentiation of RVFV, BTV, RPV, and PPRV using a conventional RT-PCR format in a single-tube multiplex platform with a DPO system.
MATERIALS AND METHODS
Primer design and viruses.
Bioinformatic analysis of the published sequences of RVFV, BTV, RPV, and PPRV revealed conserved regions not targeted by previously reported primers. These conserved viral genome regions were chosen as the best candidates for the generation of specific primers. A total of 19 primers of tripartite structure with a polydeoxyinosine linker between the 3′ and 5′ target core sequence were designed within these regions for multiplex RT-PCR to detect RVFV, BTV, RPV, and PPRV based on the generation of 205-, 440-, 115-, and 243-bp cDNA products, respectively (Table 1). These sets of primers were derived from conserved regions of the VP3 gene of RVFV and BTV and from the F gene of RPV and PPRV. Various related viruses in the same family as the viruses targeted in the present study were included in order to evaluate the dynamic range of this assay because the targets were chosen in regions fully conserved between strains. These viruses, which cause mucosal lesions in animals or humans, were included together with mosquito-borne viruses in specificity assays to evaluate the primer sets. All of the viruses used here for this specificity study are listed in Table 2.
Table 1.
Sequences of oligonucleotide primers for the dual priming oligonucleotide system used in this study
| Virus | Target gene | Primer sequence (5′–3′)a | Primer concn (μmol/liter) | Product size (bp) |
|---|---|---|---|---|
| RVFV | NSs | F-1: CCTGGCCTCTTGGAGAACIIIIICTGGCTTTCTT | 3.732 | 205 |
| F-2: CCTGGCCTCTTGGAGAACIIIIICTGGCCTTCTT | 7.464 | |||
| R-1: GCACAGGTCAATCCCTCTGAIIIIIGCCTCAGTC | 3.732 | |||
| R-2: GCACAGGTCAATCCCTCTGAIIIIIGCCTCGGTC | 7.464 | |||
| BTV | VP3 | F-1: GGAACAGGATATAATGGTTGGGIIIIIATTGATGTCG | 7.464 | 440 |
| F-2: GGAACAGGATATAATGGTTGGGIIIIIATYGATGTTG | 7.464 | |||
| F-3: GGAACAGGATATAATGGATGGGCIIIIITAGAYGTTGA | 1.866 | |||
| F-4: CGGGATACAACGGATGGGCIIIIITHGATGTTGA | 3.732 | |||
| F-5: AACGGGGTAYAACGGTTGGGIIIIIATTGATGTTG | 3.732 | |||
| R-1: TCTCATTTCTRTGCGTAGGTTCAAIIIIICTGTTGAAAG | 3.732 | |||
| R-2: TCTCATTCCTATGCGTTGGTTCAAIIIIICTGTTGAAAG | 3.732 | |||
| R-3: CGATGCGTTGGCTCAAAYGACCIIIIIAAGGCAAACC | 7.464 | |||
| R-4: GATGCGTTGGCTCRAACGACCIIIIIAAGGCGAACC | 7.464 | |||
| RPV | Fusion | F-1: TGGCTGGTGCAGCTCTCGIIIIIGCAACCGCAG | 3.732 | 115 |
| R-2: TGTTTCCAGACTTGCCTRAAGACIIIIIATAGCCTGGG | 3.732 | |||
| PPRV | Fusion | F-1: TTTGCTGGARCTGTTCTGGCCIIIIIAGCACYTGGAG | 1.866 | 243 |
| F-2: TTTGTYGGAGCTGTTCTGGCCIIIIIAGCACTTGGAG | 1.866 | |||
| R-1: GCATGACATTCTATGRACAGAAGGGAIIIIITCATTGTTGA | 3.732 | |||
| R-2: ACATGACATTCTATGAACAGAGGGGAIIIIITCATTGTTGA | 3.732 |
Abbreviations: RVFV, Rift Valley Fever virus; BTV, bluetongue virus; RPV, rinderpest virus; PPRV, Peste des petits ruminants virus. Mixed-base code: Y = pyrimidine (C or T); H = A, C, or T (not G); R = purine (A or G).
Table 2.
Information of viruses used in this study
| Species | Host range | Relevancea | Source (reference)b |
|---|---|---|---|
| Aino virus | Cattle, sheep | AF | NVRQS (16) |
| Akabane virus | Cattle, sheep, goats | AF | NVRQS (15) |
| Rift Valley fever virus (RVFV) | Humans, cattle, sheep | AMF | OBP |
| Bluetongue virus (BTV) | Cattle, sheep, goats | AMF | IAH |
| Bovine viral diarrhea virus (BVDV) | Cattle | M | NVRQS (4) |
| Canine distemper virus (CDV) | Dogs, ferrets | F | IVK |
| Chuzan virus | Cattle | AF | NVRQS (16) |
| Eastern equine encephalitis virus (EEEV) | Humans, equids, birds | A | BI |
| Epizootic hemorrhagic disease virus serotype 1 (EHDV-1) | Deer, cattle | AMF | ATCC |
| Epizootic hemorrhagic disease virus serotype 2 (EHDV-2) | Deer, cattle | AMF | ATCC, NVRQS (29) |
| Foot-and-mouth disease virus (FMDV) | Cattle, pigs, sheep, goats | M | NVRQS (21) |
| Japanese encephalitis virus (JEV) | Humans, horses, pigs | A | NVRQS (32) |
| Peste des petits ruminants virus (PPRV) | Sheep, goats | MF | CIRAD-EMVT |
| Rinderpest virus (RPV) | Cattle | MF | NVRQS (5, 19, 20) |
| Swine vesicular disease virus (SVDV) | Pigs | M | ID-DLO |
| Venezuelan equine encephalitis virus (VEEV) | Humans, equids, birds | A | BI |
| Vesicular stomatitis virus (VSV) | Horses, cattle, pigs | AM | ADRI |
| Western equine encephalitis virus (WEEV) | Humans, equids, birds | A | BI, NVRQS |
| West Nile virus (WNV) | Humans, horses, birds | A | ATCC (30, 31) |
Letters are used in a range of combinations to indicate the relevance of the control viruses. A, arthropod-borne viruses; M, viruses affecting mucosal tissues, which are difficult to differentiate from one another based on their clinical presentation and require differentiation in suspected disease cases; F, viruses within the same family as the viruses targeted in this study.
Material source abbreviations: NVRQS, National Veterinary Research and Quarantine Service, Anyang, Republic of Korea; OBP, Onderstepoort Biological Products, Onderstepoort, South Africa; ATCC, American Type Culture Collection, Manassas, VA; IVK, Intervet Korea, Seoul, Republic of Korea; CIRAD-EMVT, Département d'Elevage et de Médecine Vétérinaire, Paris, France; IL-DLO, Institute for Animal Science and Health, Lelystad, Netherlands; IAH, Institute for Animal Health, Pirbright, United Kingdom; LPL, Long Pocket laboratories, Brisbane, Queensland, Australia; ADRI, Animal Disease Research Institute, Coalhurst, Alberta, Canada. BI, RNAs extracted from the Cephalovac VEWT vaccine (Boehringer Ingelheim Vetmedica, St. Joseph, MO).
Optimization and performance evaluation.
To optimize the reaction conditions, preliminary assays were performed to test different concentrations of each primer set in the multiplex RT-PCR. We mimicked positive clinical tissue samples by preparing whole blood and oral mucosal tissues spiked with a known titer of each target virus because neither virally infected whole blood nor mucosal samples were available. Negative controls of whole blood, oral mucosal homogenate, and viruses were also prepared using the same tissue samples. Whole blood was harvested from live cattle, sheep, and goats with no suspected symptoms. Oral mucosal tissues were collected from asymptomatic carcasses of cattle, sheep, and goats. Each assay was performed in parallel with the corresponding uniplex and multiplex RT-PCR for each virus. Finally, the combination of primer concentrations that yielded the best results for the target viruses were selected and are shown in Table 1. Various amplification tests using the 19 primers were performed to determine the best type of DNA polymerase and to establish the optimal reaction protocol for the multiplex RT-PCR assay. Experiments were performed in triplicate with known virus titers to determine the detection threshold for each virus.
RNA extraction and RT-PCR.
Total nucleic acids were extracted from 400 μl of whole blood and from 10% oral mucosal tissue homogenate supernatant spiked with RVFV, BTV, RPV, and PPRV. Automated extraction was performed using a BioRobot M48 workstation apparatus (Qiagen, GmbH, Hilden, Germany) with a MagAttract Virus Mini M48 kit (Qiagen). Nucleic acids were recovered in 50 μl of elution buffer. Eluted RNA was stored at −70°C until use, and 10-fold serial dilutions were prepared with the same diluent. The multiplex RT-PCR was performed using a one-step RT-PCR kit (Qiagen). The reactions were prepared in a volume of 25 μl and contained 2 μl of RNA, 1× buffer [Tris-Cl, KCl, (NH4)2SO4], 2.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphates, 0.4 μM concentrations of each of the specific primers (for uniplex RT-PCR) or the primer mixture (for multiplex RT-PCR), 5 U of RNase inhibitor (Intron Biotechnology, Korea), and 1 μl of enzyme mix (Omniscript and Sensiscript RTs, HotStartTaq DNA polymerase; Qiagen). Reverse transcription-amplification was accomplished in one step with the following optimized incubation program: 30 min at 50°C; 15 min at 95°C; 40 cycles of 94°C for 30 s, 61°C for 90 s, and 72°C for 50 s; and 1 min at 72°C. RT-PCR amplifications were performed using an Eppendorf MasterCycler gradient thermal cycler (Eppendorf, Germany). RT-PCR amplification products (5 μl) were analyzed by gel electrophoresis on a 3% agarose gel containing 0.5 μg of ethidium bromide/ml.
Determination of intra and interassay reproducibility.
Spiked samples were prepared with different concentrations of the respective virus by diluting virus-containing cell supernatant in whole blood or 10% oral mucosal tissue homogenate. The lowest virus titer with a 100% detection rate was considered the detection threshold. Samples were extracted and amplified three times in the same run to evaluate intra-experimental reproducibility and in eight different runs to evaluate interassay reproducibility.
RESULTS
Specificity.
The uniplex and multiplex RT-PCR for RVFV, BTV, RPV, and PPRV were confirmed to be specific; the one-step multiplex RT-PCR assay produced amplification products of the expected sizes, and they were clearly distinguishable by agarose gel electrophoresis. The 19 selected primers amplified 205-, 440-, 115-, and 243-bp PCR products from tissue samples containing RVFV, BTV, RPV, and PPRV, respectively, and products were not amplified from whole blood or oral mucosal homogenate collected from asymptomatic animals and spiked with negative control virus samples as seen in Table 3. The ability of the assay to detect single and multiple infections is shown in Fig. 1. The amplicons were sufficiently separated from one another to distinguish them based on their sizes.
Table 3.
Results on viruses used in this study using RT-PCR method with the oligonucleotide primers for the dual priming oligonucleotide system
| Family | Genus | Species | Straina | Resultb |
|
|---|---|---|---|---|---|
| Expected | Observed | ||||
| Bunyaviridae | Bunyavirus | Aino virus | KSA9910 | X | X |
| Orthobunyavirus | Akabane virus | 93FMX | X | X | |
| Phlebovirus | RVFV | Smithburn | O | O | |
| Flaviviridae | Flavivirus | JEV | Anyang300 | X | X |
| WNV | NY385-99 | X | X | ||
| WNV | B956 | X | X | ||
| Pestivirus | BVDV | KD26-1 | X | X | |
| BVDV | 95002 | X | X | ||
| Paramyxoviridae | Morbillivirus | CDV | Onderstepoort | X | X |
| RPV | L | O | O | ||
| RPV | LA | O | O | ||
| RPV | LATC | O | O | ||
| PPRV | Nigeria 75/1 | O | O | ||
| Picornaviridae | Aphthovirus | FMDV | O/SKR/2002 | X | X |
| Enterovirus | SVDV | UK27/72 | X | X | |
| Reoviridae | Orbivirus | BTV | RSArrr/01 (serotype 1) | O | O |
| BTV | RSArrr/02 (serotype 2) | O | O | ||
| BTV | RSArrr/03 (serotype 3) | O | O | ||
| BTV | RSArrr/04 (serotype 4) | O | O | ||
| BTV | RSArrr/05 (serotype 5) | O | O | ||
| BTV | RSArrr/06 (serotype 6) | O | O | ||
| BTV | RSArrr/07 (serotype 7) | O | O | ||
| BTV | RSArrr/08 (serotype 8) | O | O | ||
| BTV | RSArrr/09 (serotype 9) | O | O | ||
| BTV | RSArrr/10 (serotype 10) | O | O | ||
| BTV | RSArrr/11 (serotype 11) | O | O | ||
| BTV | RSArrr/12 (serotype 12) | O | O | ||
| BTV | RSArrr/13 (serotype 13) | O | O | ||
| BTV | RSArrr/14 (serotype 14) | O | O | ||
| BTV | RSArrr/15 (serotype 15) | O | O | ||
| BTV | RSArrr/16 (serotype 16) | O | O | ||
| BTV | RSArrr/17 (serotype 17) | O | O | ||
| BTV | RSArrr/18 (serotype 18) | O | O | ||
| BTV | RSArrr/19 (serotype 19) | O | O | ||
| BTV | RSArrr/20 (serotype 20) | O | O | ||
| BTV | RSArrr/21 (serotype 21) | O | O | ||
| BTV | RSArrr/22 (serotype 22) | O | O | ||
| BTV | RSArrr/23 (serotype 23) | O | O | ||
| BTV | RSArrr/24 (serotype 24) | O | O | ||
| BTV | MAY1987/01(serotype 1) | O | O | ||
| BTV | IND2003/01(serotype 2) | O | O | ||
| BTV | ISA1991/01(serotype 16) | O | O | ||
| BTV | IND1998/01(serotype 23) | O | O | ||
| BTV | IND2001/01(serotype 1) | O | O | ||
| BTV | Station (serotype 10) | O | O | ||
| BTV | 6245S (serotype 11) | O | O | ||
| BTV | 67-41B (serotype 13) | O | O | ||
| BTV | CSIRO 19 | O | O | ||
| Chuzan virus | YoungAm | X | X | ||
| EHDV-1 | NewJersey | X | X | ||
| EHDV-2 | Alberta | X | X | ||
| EHDV-2 | Ibaraki | X | X | ||
| Rhabdoviridae | Vesiculovirus | VSV | Indiana | X | X |
| Togaviridae | Alphavirus | WEEV | Unidentified | X | X |
| EEEV | Unidentified* | X | X | ||
| WEEV | Unidentified* | X | X | ||
| VEEV | Unidentified* | X | X | ||
*, RNAs extracted from the Cephalovac VEWT vaccine (Boehringer Ingelheim Vetmedica, St. Joseph, MO).
O, the multiplex RT-PCR used in this study produced amplicons of exactly the expected sizes, and they were clearly distinguishable by agarose gel electrophoresis; X, no band was observed.
Fig. 1.
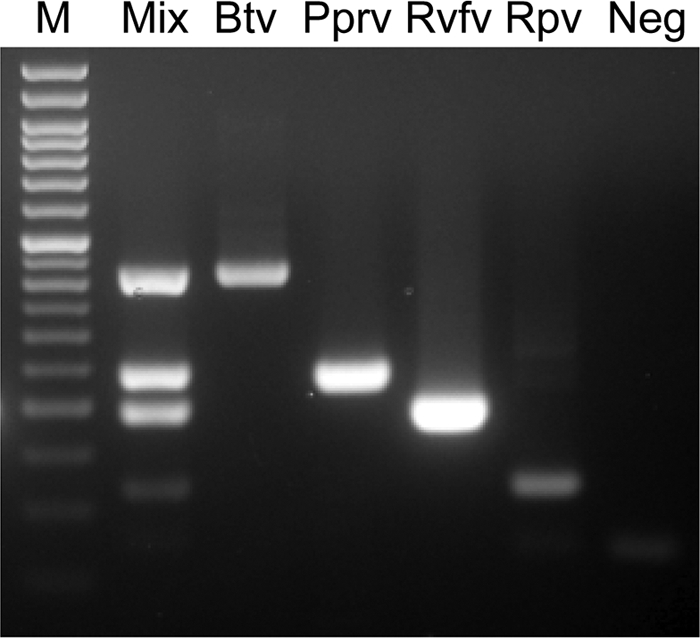
Multiplex RT-PCR amplification for Rift Valley Fever virus (RVFV), bluetongue virus (BTV), rinderpest virus (RPV), and Peste des petits ruminants virus (PPRV) in 10% oral mucosal homogenate. M, 50-bp DNA molecular weight marker (50-bp DNA ladder; Gene Direx); lane Mix, four viruses in a single-tube; lane Btv, BTV strain IND2003/01(serotype 2); lane Pprv, PPRV strain Nigeria 75/1; lane Rvfv, RVFV strain Smithburn; lane Rpv, RPV strain LATC; lane Neg, 10% oral mucosal homogenate collected from asymptomatic cattle.
To test whether the amplified PCR fragments corresponded to the expected virus, the PCR products were run on a gel, and the bands were excised and sequenced. Sequencing data confirmed amplification of the expected products, indicating that the assay was completely specific for RVFV, BTV, RPV, and PPRV. To test whether the uniplex and multiplex RT-PCR assays were specific, we tested whether the expected products were amplified from each virus correctly according to the virus present in both the whole blood and 10% oral mucosal homogenate samples. The results indicated that the assay was specific for these viruses in the clinical samples and that the reactivity was not significantly reduced due to the presence of inhibitors in the tissue samples. None of the negative control viruses that cause mucosal lesions in animals or humans was amplified in the reaction, nor were any of the negative control mosquito-borne viruses. Nor were PCR products observed from any of the related viruses in the same family as the viruses targeted in the present study. All of the 24 serotypes and field isolates of BTV included in the present study were amplified in the multiplex RT-PCR assay.
Sensitivity.
To evaluate the sensitivity of this method, three separate multiplex RT-PCR experiments were performed on serial 10-fold dilutions of whole blood and 10% oral mucosal homogenate suspensions containing a known titer of each target virus. In whole blood, RVFV, BTV, RPV, and PPRV were detected at a minimum titer of 101.1 TCID50/ml (50% tissue culture infectious dose per milliliter), 101.2 TCID50/ml, 10−1.2 TCID50/ml, and 100 TCID50/ml, respectively. Experiments comparing the sensitivity of the uniplex RT-PCR and the multiplex RT-PCR indicated that the multiplex assay was 10-fold less sensitive for RVFV, RPV, and PPRV in oral mucosal homogenate suspensions than in whole blood, whereas the sensitivity for BTV was similar in both the whole blood and 10% oral mucosal homogenate suspension reactions. To test whether the detection limit of the multiplex RT-PCR assay for an individual target is similar to that of the assay when more than one target is present in the sample, we tested single samples of whole blood and 10% oral mucosal tissue homogenate simultaneously spiked with RVFV, BTV, RPV, and PPRV. When four targets were present in the same sample, the assay was 10-fold less sensitive for all four viruses in both the whole blood and 10% oral mucosal homogenate suspension reactions than when single targets were present in tissue sample.
Intra- and interassay reproducibility.
Different dilutions of the reference solutions were used as controls to assess the precision and reproducibility of the assay. The coefficient of variation was determined based on the values obtained from 10 replicates (intra-assay variation) and the values obtained in different experiments (interassay variation). The intra-assay coefficient of variation ranged from 6 to 8% for RVFV, from 5 to 11% for BTV, from 4 to 7% for RPV, and from 2 to 6% for PPRV. In one-step single-tube multiplex RT-PCRs, the interassay coefficient of variation ranged from 4 to 9% for RVFV, RPV, and PPRV and from 3 to 12% for BTV. This analysis was conducted in triplicate in eight independent experiments.
DISCUSSION
RVFV, BTV, RPV, and PPRV all cause mucosal lesions in ruminants, and these four viruses are difficult to differentiate from one another based solely on their clinical presentation, although it is necessary to confirm suspected cases as soon as possible. Early identification of the pathogen will help prevent its spread and is also of particular interest because these four viruses can produce symptoms similar to other viruses that affect animal mucosal tissue. Molecular techniques are more rapid and sensitive than culture-based techniques for detecting viruses and do not require a BioSafety Level 3 laboratory. To our knowledge, the RT-PCR assay described in the present study is the first one-step single-tube multiplex RT-PCR assay developed that allows simultaneous detection of RVFV, BTV, RPV, and PPRV. In addition to diagnosis, the method described here may be useful for epidemiological surveillance, as well as for monitoring outbreaks. This method is also cost-effective, since RVFV, BTV, RPV, and PPRV can be detected in a single assay from a single extract. This multiplex RT-PCR might also be useful for identifying viruses in mosquitoes infected with RVFV or BTV in areas where targeted arboviruses circulate.
This study shows that RVFV, BTV, RPV, and PPRV can be detected using a single sample of whole blood or oral mucosal tissue homogenate through a novel multiplex RT-PCR assay, enabling the early diagnosis of these viruses. This is of particular interest because these viruses can produce similar symptoms and mucosal conditions, and thus there is a risk of overlooking exotic RVFV, BTV, RPV, and PPRV cases in areas where one or more of these viruses is endemic. To date, no RVFV, RPV, or PPRV outbreaks have been reported in the Far East. Recent increases in travel enhance the chances that arboviral diseases such as RVFV and BTV may emerge or re-emerge in tropical regions, as demonstrated by the recent Chikungunya outbreak in India (25), the emergence of dengue in Hawaii (8), and the extensive West Nile fever outbreak in the United States (3). In addition, nontropical areas are also at risk due to climate change (13), as demonstrated by West Nile virus infection cases in Europe and the Mediterranean basin (34) and sporadic Chikungunya cases in Italy (10). RVFV, BTV, RPV, and PPRV are highly infectious diseases in cattle, sheep, and goats, and classically, these viruses can spread within a short period of time (24). Because these viral infections are responsible for significant losses in cattle, sheep, and goat productivity, which can have a serious economic impact, these diseases are classified as notifiable diseases by the OIE. To prevent these outbreaks, there is a need for methods that can rapidly detect viruses in suspected cases and determine the presence of viruses in carrier animals. Epidemiological surveillance is essential for outbreak monitoring and disease control, and it should ideally involve diagnostic tools such as the multiplex assays described herein.
Regarding sensitivity results, the multiplex RT-PCR had sensitivity that was 10-fold lower than that of the corresponding uniplex RT-PCR in oral mucosal homogenate suspension than in whole blood. However, when testing a large number of samples, such as during an outbreak situation or a nationwide surveillance, not only would this multiplex RT-PCR method be more suitable for use as a screening test but, because the majority of the samples received would be whole blood, the lower sensitivity in mucosal homogenate suspensions compared to uniplex PCR would also be less of a factor in such applications. Although inhibition was not detected by spiking blood or tissue samples with known quantities of virus in the present study, the use of a multiplex one-step RT-PCR assay combined with internal control system, e.g., MS2 bacteriophage control recently published, could be a supplemental method to minimize false-negative results induced by inhibition because the possibility of potential inhibition could not be excluded.
In summary, the rapid, specific, and sensitive multiplex one-step RT-PCR assay using the DPO system for detecting RVFV, BTV, RPV, and PPRV described here allows for the detection and differentiation of these four viruses in a single extract in a single run. This novel, single-tube multiplex PCR may thus allow significant cost-savings compared to standard uniplex assays. Importantly, this assay may also be useful in epidemiological surveillance and as part of a diagnostic regime for testing cattle, sheep, and goats with similar clinical signs, including mucosal lesions.
ACKNOWLEDGMENTS
We thank Kyung-Jin Choi, Jin-A Yoon, Hyung-Seok Lee, Hee-Soo Park, Mi-Ran Choi, and Jin-Hwa Lee for all of their help with the experimental work.
This investigation was financially supported by a grant from the National Veterinary Research and Quarantine Service, Ministry for Food, Agriculture, Forestry and Fisheries, the Republic of Korea (grant 6235-320-210-13).
Footnotes
Published ahead of print on 9 February 2011.
REFERENCES
- 1. Abd el-Rahim I. H., Abd el-Hakim U., Hussein M. 1999. An epizootic of Rift Valley fever in Egypt in 1997. Rev. Sci. Technol. 18:741–748 [DOI] [PubMed] [Google Scholar]
- 2. Anderson G. A., Stott J. L., Gershwin L. J., Osburn B. I. 1985. Subclinical and clinical bluetongue disease in cattle: clinical, pathological and pathogenic considerations. Prog. Clin. Biol. Res. 178:103–107 [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention 2008. West Nile Virus—statistics, surveillance, and control. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 4. Cho I.-S. 2001. Studies on serological and genetical characteristics of bovine viral diarrhea viruses isolated in Korea. Konkuk University, Seoul, South Korea [Google Scholar]
- 5. Choi K.-S., Kwon C.-H., Choi C.-U., Lee J.-G., Kang Y.-B. 1998. Biological properties of attenuated rinderpest virus (LATC strain) adapted in Vero cell. RDA J. Vet. Sci. 40:61–70 [Google Scholar]
- 6. Chun J. Y., et al. 2007. Dual priming oligonucleotide system for the multiplex detection of respiratory viruses and SNP genotyping of CYP2C19 gene. Nucleic Acids Res. 35:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dreier J., Stormer M., Kleesiek K. 2005. Use of bacteriophage MS2 as an internal control in viral reverse transcription-PCR assays. J. Clin. Microbiol. 43:4551–4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Effler P. V., et al. 2005. Dengue fever, Hawaii, 2001–2002. Emerg. Infect. Dis. 11:742–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elliott R. M. 1996. The Bunyaviridae, the viruses. Plenum Press, Inc., New York, NY [Google Scholar]
- 10. Enserink M. 2007. Infectious diseases. Chikungunya: no longer a third world disease. Science 318:1860–1861 [DOI] [PubMed] [Google Scholar]
- 11. Kim S. R., Ki C. S., Lee N. Y. 2009. Rapid detection and identification of 12 respiratory viruses using a dual priming oligonucleotide system-based multiplex PCR assay. J. Virol. Methods 156:111–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koumbati M., Mangana O., Nomikou K., Mellor P. S., Papadopoulos O. 1999. Duration of bluetongue viraemia and serological responses in experimentally infected European breeds of sheep and goats. Vet. Microbiol. 64:277–285 [DOI] [PubMed] [Google Scholar]
- 13. Kovats R. S. 2000. El Nino and human health. Bull. World Health Organ. 78:1127–1135 [PMC free article] [PubMed] [Google Scholar]
- 14. Lee C. S., et al. 2008. One-step multiplex RT-PCR for detection and subtyping of swine influenza H1, H3, N1, N2 viruses in clinical samples using a dual priming oligonucleotide (DPO) system. J. Virol. Methods 151:30–34 [DOI] [PubMed] [Google Scholar]
- 15. Lim S. I., Kweon C. H., Tark D. S., Kim S. H., Yang D. K. 2007. Sero-survey on Aino, Akabane, Chuzan, bovine ephemeral fever and Japanese encephalitis virus of cattle and swine in Korea. J. Vet. Sci. 8:45–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lim S. I., Kweon C. H., Yang D. K., Tark D. S., Kweon J. H. 2005. Apoptosis in Vero cells infected with Akabane, Aino, and Chuzan virus. J. Vet. Sci. 6:251–254 [PubMed] [Google Scholar]
- 17. Maclachlan N. J., Drew C. P., Darpel K. E., Worwa G. 2009. The pathology and pathogenesis of bluetongue. J. Comp. Pathol. 141:1–16 [DOI] [PubMed] [Google Scholar]
- 18. Mellor P. S. 1990. The replication of bluetongue virus in Culicoides vectors. Curr. Top. Microbiol. Immunol. 162:143–161 [DOI] [PubMed] [Google Scholar]
- 19. Nakamura J., Kishi S., Kiuchi J., Reisinger R. 1955. An investigation of antibody response in cattle vaccinated with the rabbit-passaged LA rinderpest virus in Korea. Am. J. Vet. Res. 16:71–75 [PubMed] [Google Scholar]
- 20. Nakamura J., Motohashi T., Kishi S. 1958. Propagation of the lapinized-avianized strain of rinderpest virus in the culture of chicken embryo tissue. Am. J. Vet. Res. 19:174–180 [PubMed] [Google Scholar]
- 21. Oem J. K., et al. 2005. Identification and antigenic site analysis of foot-and-mouth disease virus from pigs and cattle in Korea. J. Vet. Sci. 6:117–124 [PubMed] [Google Scholar]
- 22. Office International des Epizooties 2008. OIE terrestrial manual 2008: manual of diagnostic tests and vaccines for terrestrial animals. Office International des Epizooties, Paris, France: [PubMed] [Google Scholar]
- 23. Park Y., et al. 2008. Evaluation of multiplex PCR assay using dual priming oligonucleotide system for detection mutation in the Duchenne muscular dystrophy gene. Korean J. Lab. Med. 28:386–391 (In Korean.) [DOI] [PubMed] [Google Scholar]
- 24. Plowright W. 1968. Rinderpest virus. Monogr. Virol. 3:25–110 [Google Scholar]
- 25. Ravi V. 2006. Re-emergence of Chikungunya virus in India. Indian J. Med. Microbiol. 24:83–84 [DOI] [PubMed] [Google Scholar]
- 26. Rolfe K. J., et al. 2007. An internally controlled, one-step, real-time RT-PCR assay for norovirus detection and genogrouping. J. Clin. Virol. 39:318–321 [DOI] [PubMed] [Google Scholar]
- 27. Scott G. 1990. Peste des petits ruminants, p. 355-361, In Virus infections of ruminants, vol. 3 Elsevier, Amsterdam, Netherlands [Google Scholar]
- 28. Selvaraju S. B., Selvarangan R. 2010. Evaluation of three influenza A and B real-time reverse transcription-PCR assays and a new 2009 H1N1 assay for detection of influenza viruses. J. Clin. Microbiol. 48:3870–3875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shin Y.-K., et al. 2009. Monitoring of five bovine arboviral diseases transmitted by arthropos vectors in Korea. J. Bacteriol. Virol. 39:353–362 [Google Scholar]
- 30. Smithburn K., Hughes T., Burke A., Paul J. 1940. Neurotrophic virus isolated from blood of native of Uganda. Am. J. Trop. Med. Hyg. 20:471–492 [Google Scholar]
- 31. Steele K. E., et al. 2000. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Vet. Pathol. 37:208–224 [DOI] [PubMed] [Google Scholar]
- 32. Yang D. K., et al. 2005. Immunogenicity of baculovirus expressed recombinant proteins of Japanese encephalitis virus in mice. J. Vet. Sci. 6:125–133 [PubMed] [Google Scholar]
- 33. Yoo S. J., Kuak E. Y., Shin B. M. 2007. Detection of 12 respiratory viruses with two-set multiplex reverse transcriptase-PCR assay using a dual priming oligonucleotide system. Korean J. Lab. Med. 27:420–427 [DOI] [PubMed] [Google Scholar]
- 34. Zeller H. G., Schuffenecker I. 2004. West Nile virus: an overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. Eur. J. Clin. Microbiol. Infect. Dis. 23:147–156 [DOI] [PubMed] [Google Scholar]


