Abstract
Community respiratory viruses (CRVs) are commonly associated with seasonal infections. They have been associated with higher morbidity and mortality among children, elderly individuals, and immunosuppressed patients. In April 2009, the circulation of a new influenza A virus (FLUA H1N1v) was responsible for the first influenza pandemic of this century. We report the clinical and epidemiological profiles of inpatients infected with CRVs or with FLUA H1N1v at a tertiary care hospital in southern Brazil. In addition, we used these profiles to evaluate survivor and nonsurvivor patients infected with FLUA H1N1v. Multiplex reverse transcription-PCR (RT-PCR) and real time RT-PCR were used to detect viruses in inpatients with respiratory infections. Record data from all patients were reviewed. A total of 171 patients were examined over a period of 16 weeks. Of these, 39% were positive for FLUA H1N1v, 36% were positive for CRVs, and 25% were negative. For the FLUA H1N1v- and CRV-infected patients, epidemiological data regarding median age (30 and 1.5 years), myalgia (44% and 13%), need for mechanical ventilation (44% and 9%), and mortality (35% and 9%) were statistically different. In a multivariate analysis comparing survivor and nonsurvivor patients infected with influenza A virus H1N1, median age and creatine phosphokinase levels were significantly associated with a severe outcome. Seasonal respiratory infections are a continuing concern. Our results highlight the importance of studies on the prevalence and severity of these infections and that investments in programs of clinical and laboratory monitoring are essential to detect the appearance of new infective agents.
INTRODUCTION
Community-acquired respiratory viruses (CRVs), including respiratory syncytial virus (RSV), influenza A virus (FLUA), influenza B virus (FLUB), adenovirus (AdV), rhinovirus (RHV), coronavirus (CoV), parainfluenza virus (PIV), human metapneumovirus (hMPV) (26), coronaviruses (HK1 and NL63) (10, 28), and bocavirus (1), are important causes of morbidity and mortality in pediatric, elderly, and immunosuppressed patients.
In adults, respiratory viruses are among the leading causative agents of community-acquired pneumonia (6, 9). However, because laboratory diagnostic studies are performed mainly to investigate inpatients, the prevalence of respiratory viruses in the general population remains largely unknown (18). On the other hand, emerging and reemerging respiratory viruses have been a subject of concern because of the risk of rapid spread, high fatality rates, and the difficulty of control with chemotherapy. In addition, these viruses can compromise people regardless of their age and immunity status and the presence of risk factors. Therefore, the early detection of these pathogens in the community is of utmost importance.
On 15 April 2009, the description of a novel swine-origin influenza A H1N1 virus (FLUA H1N1v) in California, followed by the identification of the same virus in other countries, led to the declaration of an influenza pandemic by the WHO. In Brazil, this alert triggered the implementation of measures to contain the epidemic and a strengthening of the surveillance program. The first case was confirmed at epidemiological week (EW) 27 (May 2009). The period of highest incidence was EW 31, followed by a 99% reduction of case reports by WE 47 (December 2009). The rate of incidence in the whole country was 14.5/100,000 inhabitants. However, incidences of 66.2/100,000 and 9.7/100,000 inhabitants were observed in the southern and southeastern regions, respectively. A total of 5.8% of confirmed cases ended in death, and the mortality rate was about 0.85/100,000 inhabitants. The highest rates of mortality were also detected in the southern (2.32/100,000) and southeastern (1.02/100,000) regions (2).
In the state of Paraná in southern Brazil, 110,720 respiratory infection cases were reported between the first confirmed case of FLUA H1N1v in May 2009 and January 2010. From these, a total of 53,578 (48%) cases of influenza A H1N1 virus infections were confirmed, with the mortality rate being 0.5% (290 deaths). The Teaching Hospital of the Federal University of Paraná was charged with the handling of the suspected cases of influenza A H1N1 virus infection. In this article, we report the results of characterization of the viral infection and the clinical outcomes of hospitalized patients with suspected pandemic influenza virus or other CRV infection. We also correlated the clinical and epidemiological data with the survival rate of FLUA H1N1v-positive patients during the first wave of this epidemic in the Southern Hemisphere.
MATERIALS AND METHODS
Patients.
Specimens collected from the respiratory tract, nasopharyngeal aspirates, or bronchoalveolar lavage (BAL) fluid of all inpatients presenting with acute respiratory infection (ARI) were analyzed. Samples were collected for the detection of the CRVs and also to investigate FLUA H1N1v. All cases were reported by completing a specific notification form. Records, including each patient's medical history, epidemiological data, laboratory findings, and clinical outcome, were reviewed for patients with respiratory virus detection. The study was approved by the Institutional Ethics Review Board of the Hospital de Clínicas/UFPR (IRB-2160.055/2010-03).
Pandemic influenza A H1N1 virus detection.
The detection and characterization of the virus were performed by using real-time reverse transcription-PCR (rtRT-PCR) according to the CDC protocol (27). Viral RNA was extracted using a nucliSENS easyMAG kit (bioMérieux, Marcy l'Etoile, France), in accordance with the manufacturer's instructions.
Community-acquired respiratory virus detection.
CRVs were detected using a multiplex RT-PCR technique. The viral genome was extracted using a High Pure viral RNA kit (Roche Inc., Mannheim, Germany), in accordance with the manufacturer's instructions. First-strand cDNA synthesis was achieved using random primers and an Improm-II reverse transcription system (Promega Inc., Madison, WI). The resulting cDNA was then subjected to PCR by using a Seeplex RV12 ACE detection kit (Seegene Inc., South Korea), in accordance with the manufacturer's protocol. This multiplex PCR technology enables the simultaneous detection of multiple viruses: AdV, CoV types 229E/NL63 and OC43/HKU1, hMPV, PIV type 1 (PIV-1), PIV-2, PIV-3, FLUA, FLUB, RSV type A (RSV-A), RSV-B, and human rhinovirus types A and B (HRV-A/B).
Statistical analysis.
Data were compiled using JMP software (version 5.2.1) and analyzed using GraphPad Prism software (version 5.03). Fisher's exact test or the χ2 test was used to assess differences between groups, and the Mann-Whitney test was used for continuous variables, as appropriate (univariate analysis). Results for continuous data are expressed as medians ± interquartile ranges. The difference between the medians of onset of illness was calculated by a nonparametric test (Kruskal-Wallis). Univariate analysis was performed to identify factors affecting the risk of death. A multivariable regression technique was applied using all statistically significant variables identified during univariate analysis (P > 0.02). Time-to-event analyses (discharge or death) were performed using the Kaplan-Meier method. This was used to give survival estimates over time for patients receiving antiviral therapy within the first 3 days of onset of illness. All P values are two-tailed, and a P value of <0.05 was considered significant.
RESULTS
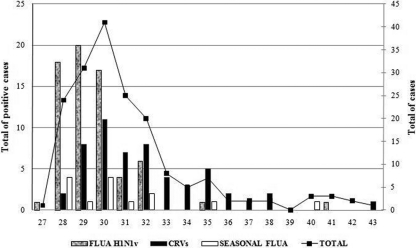
Between EWs 27 and 43 (May to December 2009), a total of 171 patients were hospitalized with influenza-like symptoms in the school hospital. Respiratory viruses were detected in 129 (75%) of the cases, and 67 (39%) were diagnosed with FLUA H1N1v infection and 62 (36%) were diagnosed with CRV infection. Of the 171 patients, 15 (9%) had seasonal influenza A virus infection (Fig. 1A). In addition to seasonal FLUA, the most frequent CRVs were RSV-A/B (8%) and HRV-A/B (8%). Viral coinfections were more common among CRV cases (8%) than FLUA H1N1v cases (1%) (Fig. 1B) (Table 1).
Fig. 1.
Pandemic influenza A virus, seasonal influenza A virus, and CRV cases recorded by epidemiological week, 2009.
Table 1.
Viral laboratory results for analyzed samples from inpatients in a tertiary care hospital, May to December 2009a
| Virus | No. (%) of patients | Viral coinfection (no. of patients) |
|---|---|---|
| Negative | 42 (25) | |
| Positive | 129 (75) | |
| FLUA H1N1v | 67 (39) | RSV-A (1), RSV-B (1) |
| FLUA (seasonal) | 15 (11) | RSV-A (1), AdV (1), hMPV (1) |
| FLUB | 1 (0.7) | No |
| RSV-A | 14 (10.8) | FLUA H1N1v (1), FLUA (1), CoV OC43/HKU1 (2), RHV-A/B (2), AdV (1), PIV-3 (1) |
| RSV-B | 3 (2.3) | FLUA H1N1v (1) |
| RHV-A/B | 13 (10) | RSV-A (2), AdV (4), CoV OC43/HKU1 (1), hMPV (2), PIV-3 (1) |
| AdV | 7 (5.4) | FLUA (1), RSV-A (1), RHV A/B (4), hMPV (3), PIV-3 (1) |
| CoV 229E/NL63 | 2 (1.5) | No |
| CoV OC43/HKU1 | 3 (2.3) | RSV-A (2), RHV-A/B (1), |
| hMPV | 12 (9.3) | FLUA (1), RHV-A/B (2), AdV (3) |
| PIV-1 | 1 (0.7) | No |
| PIV-2 | 1 (0.7) | No |
| PIV-3 | 7 (5.4) | RSV-A (1), RHV-A/B (1), AdV (1) |
Demographic and clinical data for patients with viral coinfections indicate that the median age was 2 years (age range, 6 months to 31 years) and 43% had comorbidities: asthma (n = 1), acute myeloblastic leukemia (n = 1), cerebral paralysis (n = 1), COPD (n = 1), and cardiopathy (n = 1).
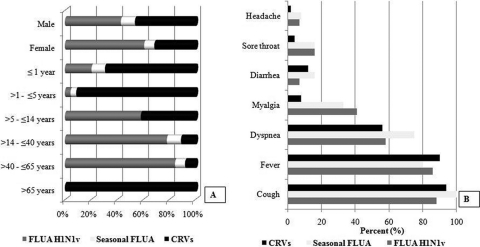
Table 2 shows demographic, clinical, and laboratory data for patients with FLUA H1N1v and CRV infections. Demographic data from infected patients showed that there was a predominance of females (58%) and adults among patients with FLUA H1N1v and that the patients infected with CRVs were more frequently younger than 5 years of age (Fig. 2A). Cough, fever, and dyspnea were the most frequent clinical findings and were distributed equally between the groups. Myalgia was less frequent in patients infected with CRVs (Fig. 2B).
Table 2.
Demographic, clinical, and laboratory data for patients infected with pandemic influenza A virus H1N1 and community respiratory viruses
| Data | FLUA H1N1v | CRV | P value |
|---|---|---|---|
| Gender (no. ♀/no. ♂a) | 39/28 | 25/37 | 0.04 |
| Age (yr) | |||
| Median | 30 | 1.5 | <0.001b |
| Interquartile range | 15–44 | 0.5–12.7 | |
| No. of patients with the following/total no.: | |||
| Cough | 59/61 | 59/63 | 0.99 |
| Fever | 55/61 | 58/63 | |
| Dyspnea | 37/61 | 39/63 | |
| Myalgia | 8/61 | 28/63 | 0.02 |
| Time from onset of illness to hospital admission (days) | |||
| Median | 4 | 3 | 0.007 |
| Interquartile range | 3–6 | 2–5 | |
| Time of hospitalization (days) | |||
| Mean | 5 | 4 | 0.22 |
| Interquartile range | 2–11 | 2–6 | |
| No. of patients with the following underlying medical condition/total no.: | |||
| Any underlying condition | 27/67 | 30/62 | 0.71 |
| COPD | 3/35 | 9/32 | 0.32 |
| Pregnancy | 7/39 | 1/25 | 0.13 |
| Obesity | 2/35 | 2/32 | |
| No. of patients with the following laboratory-determined conditions | |||
| Hypoxemia | 30/59 | 22/61 | <0.001 |
| Increased CK concn | 15/54 | 5/44 | 0.076 |
| Increased LDH concn | 19/52 | 7/47 | 0.021 |
| No. of patients with the following X-ray findings/total no.: | |||
| Interstitial pneumonia | 24/66 | 20/58 | 0.84 |
| Pulmonary consolidation | 10/66 | 7/58 | 0.80 |
| Mixed pattern | 14/66 | 6/58 | 0.09 |
| No. of patients with mechanical ventilation/total no. | 18/59 | 5/59 | 0.004 |
| Interval between the onset of illness and antiviral administration (days) | |||
| Median | 4 | 3 | 0.01 |
| Interquartile range | 2–7 | 2–5 |
♀, female; ♂, male.
Boldface indicates a statistically significant difference.
Fig. 2.
(A) Demographic data from infected and noninfected patients; (B) clinical manifestations in respiratory virus-infected patients.
The median time from onset of illness to hospital admission and the mean time of hospitalization were compared for the two groups, and the differences between the median times from onset of illness to hospital admission for the two viral infection groups were statistically significant. However, the mean time of hospitalization showed no significant difference between these two groups.
Among the 171 patients, 84 (49%) had one or more underlying medical conditions, among which chronic obstructive pulmonary disease (COPD) was the most common. There was a higher frequency of pregnancy in the group of FLUA H1N1v-infected women. On admission, obesity was present in 3.5% of patients and was distributed equally between the two groups.
The results of the laboratory analyses showed that some parameters displayed greater changes for patients infected with FLUA H1N1v than for those infected with CRVs. These included hypoxemia and creatine phosphokinase (CK) and lactate dehydrogenase (LDH) concentrations. This indicates the higher severity of pandemic FLUA infection.
Chest X ray was performed for 96% (164/171) of the patients, of whom 76% had some abnormality. Significant alterations such as interstitial pneumonia, pulmonary consolidation, and a mixed pattern were reported in patients infected with FLUA H1N1v and CRVs. There was no statistically significant difference between the groups.
Comparisons between the groups of FLUA H1N1v- and CRV-infected patients showed that the antiviral oseltamivir was given to 95% and 88% of the patients, respectively. Antibiotics were prescribed to 78% of the patients. For the FLUA H1N1v-infected patients the rate of prescription was 77%, and for the CRV-infected patients it was 69%. Mechanical ventilation was required in 18% of the patients. In the overall population, 17% (30/171) of the patients died (36% for FLUA H1N1v-infected patients versus 10% for CRV-infected patients; P = 0.0007).
To evaluate the risk of progression to death, FLUA H1N1v-infected patients were divided into two groups: survivors and nonsurvivors. The laboratory, clinical, and demographic data from both groups were compared. Cough, dyspnea, and myalgia were more frequent among patients who survived. X rays showing interstitial pneumonia and pulmonary condensation were similar in frequency for both groups. However, a mixed pattern on the X ray was more frequently detected in patients who died (P = 0.02). Furthermore, the use of antibiotics was more frequent in this group of patients (P = 0.002). Corticosteroids were rarely used in either group. Other clinical, laboratory, and demographic data with the results of univariate and multivariate analyses are shown in Table 3.
Table 3.
Clinical and laboratory data and percentage of abnormal findings in patients with pandemic FLUA H1N1v infectiona
| Clinical/laboratory observation | N | Value | % of patients with abnormal findings |
P value |
|
|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | ||||
| Temp ≥ 38 (°C) | |||||
| Survivors | 24/40 | 60 | NS | ||
| Nonsurvivors | 11/22 | 50 | |||
| Pulse rate > 100/min | |||||
| Survivors | 18/36 | 50 | NS | ||
| Nonsurvivors | 11/22 | 50 | |||
| Respiration > 30/min | |||||
| Survivors | 17/39 | 44 | NS | ||
| Nonsurvivors | 9/17 | 53 | |||
| Systolic BP ≤ 90 mm Hg | |||||
| Survivors | 21/28 | 75 | NS | ||
| Nonsurvivors | 16/21 | 76 | |||
| SpO2 ≤ 90% | |||||
| Survivors | 15/38 | 39 | 0.01 | NS | |
| Nonsurvivors | 15/21 | 71 | |||
| Creatine phosphokinase concn > 130 IU/ml | |||||
| Survivors | 4/35 | 11 | 0.01 | 0.003 | |
| Nonsurvivors | 11/21 | 52 | |||
| LDH concn > 225 IU/ml | |||||
| Survivors | 6/32 | 19 | 0.06 | NS | |
| Nonsurvivors | 13/20 | 65 | |||
| Comorbidities | |||||
| Survivors | 21/43 | 49 | NS | NS | |
| Nonsurvivors | 13/24 | 54 | |||
| Demographic data | |||||
| Median (IQR) age (yr) | |||||
| Survivors | 26 (8–36) | 0.0005 | 0.03 | ||
| Nonsurvivors | 42.5 (30–50) | ||||
| Gender (no. male:no. female) | |||||
| Survivors | 18:25 | NS | |||
| Nonsurvivors | 10:14 | ||||
| Median (IQR) time interval between onset of disease and hospitalization (days) | |||||
| Survivors | 4 (2–7) | NS | |||
| Nonsurvivors | 5 (4–6) | ||||
| Median (IQR) time interval between onset of disease and antiviral use (days) | |||||
| Survivors | 4 (2–6) | 0.03 | NS | ||
| Nonsurvivors | 5 (3.7–7.5) | ||||
N, number of patients with alterations/number with medical records with the information; BP, blood pressure; SpO2, oxygen saturation; IQR, interquartile range; NS, not significant. Boldface data indicate a statistically significant difference.
The Kaplan-Meier survival curves in Fig. 3 estimate the survival time by comparing the patients who received specific antiviral treatment within 3 days (first group) or after 3 days (second group) from the time of onset of illness. Overall, 21% of the patients who began medication in a later phase died, whereas 10% of those who started medication earlier died (P = 0.20). The median survival times were 16 days in the first group and 24 days in the second group (95% confidence interval [CI], 0.25 to 1.08), corresponding to a hazard ratio of 1.93 (95% CI, 0.66 to 5.58; P = 0.22).
Fig. 3.
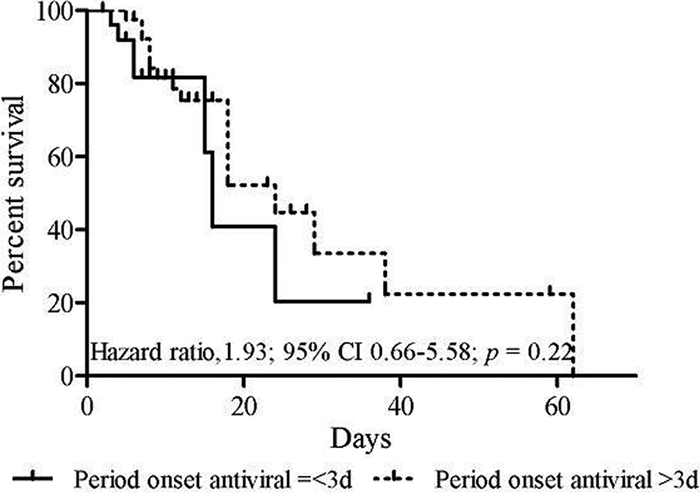
Kaplan-Meier estimates of survival time depending on the time of start of antiviral therapy.
DISCUSSION
Data comparing the epidemiological and clinical findings for patients infected by the pandemic 2009 influenza A virus and cocirculating seasonal respiratory viruses in the community setting are limited. Furthermore, there is little information concerning the impact of other cocirculating respiratory viral infections on the spread of pandemic FLUA H1N1v. These data would be useful to tailor rational decisions in the handling and control of epidemics and pandemics. In seeking to understand the severe clinical course of patients hospitalized with ARI in a tertiary care hospital (Hospital de Clínicas/UFPR) in southern Brazil, we performed more detailed laboratory analyses. We also investigated the clinical and epidemiological aspects of infection/coinfection with other respiratory viruses in two groups of patients: those infected with seasonal CRVs and those infected with pandemic FLUA H1N1v. It is important to mention that even though we describe the groups as nonpandemic influenza cases and community-acquired respiratory virus-infected cases separately, in both cases, all the patients in the study were presumably infected in the community; however, we used this description to highlight the differences between the two groups.
The study presented here began a few weeks after the confirmation of FLUA H1N1v circulation in Brazil, corresponding to the period of heightened surveillance of the pandemic virus circulation in the Southern Hemisphere. Concomitant with FLUA H1N1v spread, infections by other respiratory viruses were observed, especially after the peak of circulation for H1N1v FLUA. A similar occurrence has been reported by groups in Italy and Singapore (20, 24). The most frequently detected CRVs were seasonal FLUA, RSV, RHV, and hMPV, which usually circulate during the winter in this southern region of Brazil (7, 25). Apparently, the cocirculation of pandemic and seasonal viruses suggests the absence of the phenomenon of viral strain displacement or viral interference, which is contrary to the hypothesis of Linde et al. (16). However, more studies are needed to confirm these findings.
Unlike Nisii et al. (20), who reported a higher prevalence of FLUA H1N1v than CRV infections among hospitalized patients (70.9% versus 35%, respectively), we observed similar percentages of FLUA H1N1v and CRV infections (39% versus 36%, respectively). This was probably due to the naturally higher frequency of circulating CRVs during winter in the Southern Hemisphere. However, the epidemiological and clinical profiles of FLUA H1N1v-infected patients were altered, with a predominance of young adults and higher disease severity. Inpatients infected by CRVs were predominantly elderly and children, as previously reported (25). Nevertheless, a few studies have evaluated the role of viruses as causative agents or as cofactors in lower respiratory tract infections in adult patients in Brazil. Minosse et al. (18) had drawn attention to this problem in Italy, when they found an overall CRV prevalence of 42.2% in hospitalized adults.
Similar to other studies (16, 17), H1N1v FLUA infection and severe disease were rarely observed in elderly people in Brazil. This may be due to the partial cross-immunity of previous influenza A H1N1 virus infection in people who were alive prior to 1957 (12, 15). Previous studies (3, 19, 21) have demonstrated that preexisting conditions, such as obesity, underlying diseases, and pregnancy, were prognostic of a worse clinical outcome in both groups of infected patients (CRV and FLUA H1N1v); however, more studies are necessary to define the importance of these features in clinical evolution. Knowledge of the risk factors for fatality is fundamental to define the candidate group in an immunization program, which can vary according to the target population.
In agreement with the findings of Niisi et al. (20), viral and bacterial coinfections were not common in FLUA H1N1v-infected patients. This suggests that the severity of the infection was related to the viral variant and the intensity of the immune response. Viral coinfection has been associated with severe infections by CRVs, mainly in pediatric patients and those with underlying diseases (4, 11).
Secondary bacterial pneumonia is a common complication in influenza virus-infected patients (22). In light of this risk, most inpatients were treated with antibiotics, as recommended by other authors (3, 13). However, microbiological investigations (blood cultures and bacterial isolation from nasopharyngeal aspirate or bronchoalveolar lavage fluid specimens) of these patients did not demonstrate the presence of bacterial infection at admission.
The overall mortality rate due to pandemic influenza A virus in Brazil is within the average observed in other countries of the Americas (0.85/100,000 inhabitants) (2). However, in a different epidemic scenario, the mortality rate in southern Brazilian increased by more than 2-fold. Under a similar scenario, the United States, Chile, Australia, and Singapore did not show an increase in the mortality rate due to pneumonia and influenza virus infection (8, 12, 21, 24).
The higher incidence of FLUA H1N1v infections in southern of Brazil could be explained by more efficient laboratory surveillance as well as the characteristics of the pandemic itself. Previous studies suggest that there were geographic variations, which implies that this epidemic was heterogeneous (5). This heterogeneity can be explained by the climatic conditions of the winter, which contributed significantly to virus dissemination, as well as the degree of immunity of the local population to the circulating influenza virus and virus infectiousness (17).
The statistical analysis of the results showed no significant difference in the clinical manifestations between patients infected with FLUA H1N1v and those infected with CRVs, except for myalgia, which was more frequent in FLUA-infected patients. Previously, myositis with an increased creatine phosphokinase concentration has been reported in patients with influenza virus infections (15). Myoglobinuria and renal failure, feasible complications of the myositis, were not associated with H1N1v FLUA infection.
During the pandemic period, all patients with respiratory infections referred to the Hospital de Clínicas/UFPR were treated with oseltamivir. It has been reported that during periods of influenza outbreak in the community, the clinicians had a low threshold for suspecting, diagnosing, and treating the infection according to the recommended guidelines (23). In 2010, Perret (21) showed a high correlation between clinical findings and real-time PCR detection for diagnosis of influenza virus infections. The widespread use of antiviral therapy must be followed by epidemiological and laboratory surveillance to detect drug resistance. Several factors, such as late medical evaluation and a delay in antiviral administration, could underlie the higher level of severity of influenza virus infection observed in our study population.
The clinical and laboratory data concerning levels of oxygen saturation, creatine phosphokinase levels, appearance of a mixed pattern on X ray, median age, and time interval between onset of illness and antiviral administration were statistically different between survivor and nonsurvivor patients infected with H1N1v FLUA. However, in a multivariate analysis, only median age and levels of creatine phosphokinase remained statistically different, and both of these were associated with a severe outcome.
The mortality rate associated with FLUA H1N1v infection in the present study was higher than the rates for inpatients in the United States (14), Australia (8), and the United Kingdom (19) but comparable to that reported in China (29). Similar to Chinese patients, the patients who died during the present study were hospitalized at the fifth day of illness, which could contribute to the higher mortality rate that we observed. However, in contrast to the present study, the independent risk factors for hospital death in the Chinese study were diabetes, LDH levels, presence of septic shock, and altered mental status (29). A study designed to detect risk factors was carried out in the United Kingdom at the same time and showed that obesity, previous pulmonary diseases, the presence of pneumonia, and raised C-reactive protein levels were all associated with poor outcomes. However, 59% of all inpatient deaths occurred in previously healthy individuals (19).
The median time between the onset of symptoms and hospital presentation among survivor and nonsurvivor patients was more than 3 days for both groups. Therefore, early administration of neuraminidase inhibitors (within 48 h from symptom onset) was rare. This could explain the lack of significant differences in the Kaplan-Meier survival estimates, where the survival time was longer for patients who began antiviral drugs later. It is possible that less severe symptoms of disease contributed to patients seeking medical care later and that patients presenting more severe symptoms were hospitalized earlier but still too late to be able to respond to antiviral therapy. An analysis of greater casuistry is required to confirm these findings.
The influenza mitigation strategies used to control the epidemics in Paraná consisted of postponing attendance at school, having people avoid crowded places, and the use of antiviral therapy for everyone presenting with respiratory symptoms. A significant decrease in the number of hospitalizations and mortality rates was observed after these measures were followed. The impact of these strategies could be overestimated due to the late implementation (the beginning of the decline in the curve of cases) of such measures. However, an important relief in medical care, as well as an increased sense of security in society, was seen.
The pandemic of H1N1 influenza A virus introduced significant changes in the epidemiological and clinical presentation of viral respiratory infections. ARI remains a major and ongoing threat to public health. The results presented here highlight the importance of constant surveillance for the detection of these pathogens and increase the knowledge concerning their epidemiological dynamics. Together, these can help to lessen the impact of these infections on the general population. To our knowledge, this is the first report of clinical and laboratory findings from patients infected by pandemic influenza A virus and other community-acquired respiratory viruses during the first pandemic wave in the Southern Hemisphere.
ACKNOWLEDGMENTS
This work was supported by Carlos Chagas Institute, Fiocruz/Paraná. S.M.R. and C.N.D.D. are CNPq fellows.
We thank Denise Siqueira, Universidade Federal do Paraná, for her advice on the statistical analysis.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print on 19 January 2011.
REFERENCES
- 1. Allander T., et al. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. U. S. A. 105:12891–12896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brazilian Ministry of Health 2010. Boletin epidemiologico 47. Brazilian Ministry of Health, Brasilia, Brazil: http://portal.saude.gov.br/portal/arquivos/pdf/boletim_influenza_se_47.pdf Accessed 16 July 2010 [Google Scholar]
- 3. Centers for Disease Control and Prevention 2010. Deaths and hospitalizations related to 2009 pandemic influenza A (H1N1)—Greece, May 2009–February 2010. MMWR Morb. Mortal. Wkly. Rep. 59:682–686 [PubMed] [Google Scholar]
- 4. Cruz A. T., Cazacu A. C., McBride L. J., Greer J. M., Demmler G. J. 2006. Performance characteristics of a rapid immunochromatographic assay for detection of influenza virus in children during the 2003 to 2004 influenza season. Ann. Emerg. Med. 47:250–254 [DOI] [PubMed] [Google Scholar]
- 5. Dabanch J., Perret C. 2009. Influenza pandemic of novel (H1N1) 2009 virus in Chile. How we dealt with the first wave? Rev. Chil. Infect. 26:309–310 [PubMed] [Google Scholar]
- 6. Daubin C., et al. 2006. Epidemiology and clinical outcome of virus-positive respiratory samples in ventilated patients: a prospective cohort study. Crit. Care 10:R142 doi:10.1186/cc5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Debur M. C., Bordignon J., Duarte dos Santos C. N., Vidal L. R., Nogueira M. B., Almeida S. M., Raboni S. M. 2007. Acute respiratory infection by human metapneumovirus in children in southern Brazil. J. Clin. Virol. 39:59–62 [DOI] [PubMed] [Google Scholar]
- 8. Denholm J. T., et al. 2010. Hospitalised adult patients with pandemic (H1N1) 2009 influenza in Melbourne, Australia. Med. J. Aust. 192:84–86 [DOI] [PubMed] [Google Scholar]
- 9. De Roux A., et al. 2004. Viral community-acquired pneumonia in nonimmunocompromised adults. Chest 125:1343–1351 [DOI] [PubMed] [Google Scholar]
- 10. Fouchier R. A., et al. 2004. A previously undescribed coronavirus associated with respiratory disease in humans. Proc. Natl. Acad. Sci. U. S. A. 101:6212–6216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greenberg S. B. 2002. Respiratory viral infections in adults. Curr. Opin. Pulm. Med. 8:201–208 [DOI] [PubMed] [Google Scholar]
- 12. Hancock K., et al. 2009. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N. Engl. J. Med. 361:1945–1952 [DOI] [PubMed] [Google Scholar]
- 13. Ho Y. C., et al. 2009. Prognostic factors for fatal adult influenza pneumonia. J. Infect. 58:439–445 [DOI] [PubMed] [Google Scholar]
- 14. Jain S., et al. 2009. The 2009 pandemic influenza A(H1N1) Virus Hospitalizations Investigation Team: hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N. Engl. J. Med. 361:1935–1944 [DOI] [PubMed] [Google Scholar]
- 15. Lim W. S. and Committee Members of British Thoracic Society, British Infection Society and Collaborators 2007. Pandemic with an influenza-like illness during an influenza pandemic flu: clinical management of patients. Provisional guidelines from the British Infection Society, British Thoracic Society, and Health Protection Agency in collaboration with the Department of Health. Thorax 62:1–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Linde A., Rotzén-Östlund M., Zweygberg-Wirgart B., Rubinova S., Brytting M. 2009. Does viral interference affect spread of influenza? Euro Surveill. 14(40):pii=19354 [PubMed] [Google Scholar]
- 17. Miller M. A., et al. 2009. The signature features of influenza pandemics—implications for policy N. Engl. J. Med. 360:2595–2598 [DOI] [PubMed] [Google Scholar]
- 18. Minosse C., et al. 2008. Frequency of detection of respiratory viruses in the lower respiratory tract of hospitalized adults. J. Clin. Virol. 42:215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nguyen-Van-Tam J. S., et al. 2010. Risk factors for hospitalization and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May-September 2009). Thorax 65:645–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nisii C., et al. 2010. Frequency of detection of upper respiratory tract viruses in patients tested for pandemic H1N1/09 viral infection. J. Clin. Microbiol. 48:3383–3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perret C. P. 2010. Influenza pandémica a un año de la primera ola. ¿Qué podemos decir ahora? Rev. Chil. Infect. 27:144–147 [PubMed] [Google Scholar]
- 22. Rothberg M. B., Haessler S. D., Brown R. B. 2008. Complications of viral influenza. Am. J. Med. 121:258–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shinde V., et al. 2009. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005-2009. N. Engl. J. Med. 360:2616–2625 [DOI] [PubMed] [Google Scholar]
- 24. Tang J. W. T., et al. 2010. Differing symptom patterns in early pandemic vs seasonal influenza infections. Arch. Intern. Med. 170:861–867 [DOI] [PubMed] [Google Scholar]
- 25. Tsuchiya L. R. V., et al. 2005. Viral respiratory infection in Curitiba, southern Brazil. J. Infect. 51:401–407 [DOI] [PubMed] [Google Scholar]
- 26. Van den Hoogen B. G., et al. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7:719–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. WHO 2009. CDC protocol of realtime RTPCR for influenza A (H1N1). WHO, Geneva, Switzerland: http://www.who.int/csr/resources/publications/swineflu/realtime/ptpcr/en/index.html Accessed 24 July 2010 [Google Scholar]
- 28. Woo P. C. Y., et al. 2005. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 79:884–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xi X., et al. 2010. Hospitalized adult patients with 2009 influenza A(H1N1) in Beijing, China: risk factors for hospital mortality. BMC Infect. Dis. 10:256. [DOI] [PMC free article] [PubMed] [Google Scholar]