Abstract
Transmission of methicillin-resistant Staphylococcus aureus (MRSA) between humans and animals is increasingly recognized. We newly document that the transmission of MRSA between human and hamster is possible.
CASE REPORT
We describe a case of suggested transmission of methicillin-resistant Staphylococcus aureus (MRSA) between a human and a pet hamster. This finding was one of the results of a project where MRSA-positive patients seen as outpatients at a large southeastern-United States hospital were identified and contacted to determine if they had pets. If they had pets and consented to participate in the study, a visit was scheduled to obtain samples from pets to determine their MRSA statuses. The study developed as a collaboration between a medical school and a veterinary college and was approved by institutional review boards and animal care and use committees at both participating institutions.
The index patient was a 28-year-old Caucasian male with advanced cystic fibrosis who had undergone an initial bilateral lung transplant and a repeat left lung transplant. He also had chronic sinusitis that had required three previous surgical procedures, diabetes mellitus, and renal insufficiency, and he presented with postnasal drip, a cough, clear rhinorrhea, and headaches. He was diagnosed with chronic rhinosinusitis and underwent endoscopic ethmoidectomy, sphenoidotomy, and partial resection of bilateral nasal turbinates. Presurgical culture of the patient's sinus contents yielded MRSA, and the patient was therefore contacted.
The clinical MRSA isolate from the patient was collected from the Duke Clinical Microbiology Laboratory and stored (−80°C) until required for additional use. After written informed consent was provided by the patient, nasal and rectal swabs were collected from three hamsters at the patient's residence. Nasal swabs were also collected from the patient's housemate. Swabs from the animals were processed within 24 h at a microbiology laboratory in the North Carolina State University (NCSU) College of Veterinary Medicine Population Health and Pathobiology Department.
Identification of S. aureus was performed in accordance with routine laboratory techniques. Swabs were rolled on Trypticase soy agar plates (containing 5% sheep blood) and mannitol salt agar (BD, NJ) and incubated at 35°C to 37°C for 24 and 48 h. Colonies with typical S. aureus colony morphology were further analyzed using Gram stain, catalase, and tube coagulase tests. A diagnosis of S. aureus was confirmed by multiplex PCR targeting the thermonuclease (nuc) gene locus (11). Resistances to oxacillin and cefoxitin were determined in the S. aureus isolates by disk diffusion. S. aureus isolates were classified as MRSA if the inhibition zone was ≤21 mm for cefoxitin or ≤10 mm for oxacillin (3).
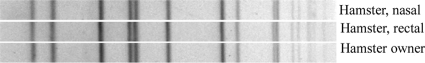
Nasal and rectal swabs from one hamster (female; 1.5 years of age) yielded MRSA. The other two hamsters and the housemate were S. aureus culture negative. mecA PCR was performed on the human and hamster MRSA isolates, and we evaluated their genetic relatedness using pulsed-field gel electrophoresis (PFGE) and spa typing as previously described (2, 8). The mecA gene was detected in both the hamster and patient MRSA isolates. The PFGE banding patterns of the human and hamster MRSA isolates were identical to each other (Fig. 1) but not equivalent to the most common hospital-acquired or community-associated MRSA types previously described by the CDC. All the isolates were spa type 2, clonal complex 5.
Fig. 1.
PFGE image comparing human and hamster SmaI DNA digestion patterns.
MRSA is a significant problem for both human and veterinary medicine. MRSA infection in several different animal species has been described, and MRSA transmission between humans and different species has also been suggested (1, 4–7, 10, 12–14). Most of our current knowledge on this topic is based on anecdotal reports, and several of the details of this interspecies exchange of MRSA are still unknown.
S. aureus has been previously isolated from hamsters (9). However, to the best of our knowledge there is no previous report of isolation of MRSA in a hamster. This study documents the first reported case of suggested MRSA transmission between a human and a hamster.
The genotypes of the hamster and human MRSA isolates were identical by PFGE banding patterns. The presence of MRSA with identical PFGE genotypes in both the patient and his hamster strongly implies that hamsters are capable of carrying MRSA and thus can potentially transmit it to pet owners. Conversely, patients who are colonized with MRSA may also be capable of transferring MRSA to hamsters.
The MRSA-positive hamster was acquired from the same source (a pet store) as the other two hamsters. In the household, the MRSA-positive hamster was housed in the same cage as her sister but separately from the other hamster. The three hamsters had daily contact with each other. The patient would feed and hold and/or play with the hamsters daily but was not responsible for cleaning their cages. He reported that he would always disinfect his hands with alcohol-based hand sanitizer after touching the hamster(s).
In the current case, we believe that the hamster most likely became a carrier after acquisition of MRSA by the patient, who was at high risk for long-term MRSA carriage, given his immunocompromised state and comorbidities. However, the hamster was not screened for MRSA at the time of acquisition and had been living with the patient for about 1 year and 4 months before the patient had his first (blood) MRSA-positive culture. Our assumption on the direction of transmission is therefore speculative. The possibility that both the hamster and the patient obtained their infections from a third party or perhaps from a fomite cannot be excluded.
We recognize that our study has other limitations. The hamster died while we were developing the study, which prevented us from collecting additional nasal swab samples, so we were unable to estimate the duration of colonization. On the other hand, the patient had multiple MRSA-positive samples (blood, sinus contents, nasal swabs, bronchoalveolar lavage) for a total period of approximately 1 year and 4 months, which included some months after the hamster's death.
Despite these limitations, this report makes an important observation: MRSA exchange between humans and hamsters is possible. Should testing of the pets of MRSA-positive patients be recommended? At this point, we recommend that MRSA-positive patients be informed that their companion animals can be potential sources of infection or reinfection. In the presence of a MRSA-positive human or animal, heightened hygiene practices should be instituted and unnecessary close contact should be avoided. Screening of household pets might be indicated in situations of recurrent MRSA infections despite adequate treatment or when immunocompromised patients live in the household. We speculate that the clinical significance of the findings are important for immunocompromised patients who keep pets in close proximity, but at this point we cannot determine the prevalence or clinical significance of this phenomenon.
Acknowledgments
This project was supported by R01-AI068804 (V.G.F.) and the North Carolina State University College of Veterinary Medicine. V.G.F. has received grant or research support from Astellas, Cubist, Merck, Theravance, Cerexa, Pfizer, Novartis, and Advanced Liquid Logic, is a paid consultant for Astellas, Cubist, Inhibitex, Merck, Johnson & Johnson, Leo Pharmaceuticals, NovaDigm, The Medicines Company, Baxter Pharmaceuticals, and Biosynexus, is on the speaker's bureau of Cubist, is employed by Duke University, has received honoraria from Arpida, Astellas, Cubist, Inhibitex, Merck, Pfizer, Targanta, Theravance, Wyeth, Ortho-McNeil, Novartis, and Vertex Pharmaceuticals, is a member of the advisory committee for Cubist, and has no ownership interests (e.g., stocks, stock options, or other ownership interests, excluding diversified mutual funds) or other relevant financial or material interests.
Footnotes
Published ahead of print on 16 February 2011.
REFERENCES
- 1. Atyah M. A. S., Zamri-Saad M., Siti-Zahrah A. 2010. First report of methicillin-resistant Staphylococcus aureus from cage-cultured tilapia (Oreochromis niloticus). Vet. Microbiol. 144:502–504 [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention 2001. Oxacillin-resistant Staphylococcus aureus on PulseNet (OPN): laboratory protocol for molecular typing of S. aureus by pulsed-field gel electrophoresis (PFGE). Centers for Disease Control and Prevention, Department of Health and Human Services, Washington, DC [Google Scholar]
- 3. Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial susceptibility testing; 20th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 4. Faires M., Gehring E., Mergl J., Weese J. S. 2009. Methicillin-resistant Staphylococcus aureus in marine mammals. Emerg. Infect. Dis. 15:2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faires M., Tater K., Weese J. S. 2009. An investigation of methicillin-resistant Staphylococcus aureus colonization in people and pets in the same household with an infected person or infected pet. J. Am. Vet. Med. Assoc. 235:540–543 [DOI] [PubMed] [Google Scholar]
- 6. Janssen D., et al. 2009. Methicillin-resistant Staphylococcus aureus skin infections from an elephant calf—San Diego, California, 2008. MMWR Morb. Mortal. Wkly. Rep. 58:194. [PubMed] [Google Scholar]
- 7. Juhász-Kaszanyitzky E., et al. 2007. MRSA transmission between cows and humans. Emerg. Infect. Dis. 13:630–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee J. H. 2006. Occurrence of methicillin-resistant Staphylococcus aureus strains from cattle and chicken, and analyses of their mecA, mecR1 and mecI genes. Vet. Microbiol. 114:155–159 [DOI] [PubMed] [Google Scholar]
- 9. Lin Y., et al. 25 August 2010, posting date Evidence of multiple virulence subtypes in nosocomial and community-associated MRSA genotypes in companion animals from the Upper Midwestern and Northeastern United States. Clin. Med. Res. doi:10.3121/cmr.2010.944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mulders M. N., et al. 2010. Prevalence of livestock-associated MRSA in broiler flocks and risk factors for slaughterhouse personnel in the Netherlands. Epidemiol. Infect. 138:743–755 [DOI] [PubMed] [Google Scholar]
- 11. Sasaki T., et al. 2010. Multiplex-PCR method for species identification of coagulase-positive staphylococci. J. Clin. Microbiol. 48:765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith T. C., et al. 2008. Methicillin-resistant Staphylococcus aureus (MRSA) strain ST398 is present in midwestern U.S. swine and swine workers. PLoS One 4:e4258 doi:10.1371/journal.pone.0004258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walther B., et al. 2008. Methicillin-resistant Staphylococcus aureus (MRSA) isolated from small and exotic animals at a university hospital during routine microbiological examinations. Vet. Microbiol. 127:171–178 [DOI] [PubMed] [Google Scholar]
- 14. Weese J. S., et al. 2006. An outbreak of methicillin-resistant Staphylococcus aureus skin infections resulting from horse to human transmission in a veterinary hospital. Vet. Microbiol. 114:160–164 [DOI] [PubMed] [Google Scholar]