Abstract
Candida spp. infect medical devices, such as venous and urinary catheters, by adhering to the surface and forming a community of drug-resistant cells surrounded by a matrix. The ability to measure drug activity during this biofilm mode of growth is of interest for the investigation of resistance mechanisms and novel antifungal therapies. The tetrazolium salt (XTT) reduction assay is the test most commonly used to estimate viable biofilm growth and to examine the impact of biofilm therapies. The primary goal of the current experiments was to identify assay variables that affect the XTT assay result in order to improve assay reproducibility, sensitivity, and throughput for the study of antifungal activity. The species used in the current studies included Candida albicans, C. parapsilosis, and C. glabrata. The assay variables that were studied included the impact of culture conditions, the duration of biofilm growth, the timing and frequency of drug administration, the XTT source and concentration, and the duration of XTT incubation. The conditions that impacted the assay readout and altered assay sensitivity included the duration of biofilm growth, the frequency of drug dosing, and the duration of XTT incubation. Several factors were found to reduce time and assay expense, including the elimination of washing steps, the shortening of incubation times, and the use of lower XTT concentrations. A description of assay pitfalls and troubleshooting is included. Recognition of these technical variables should allow investigators to better design reproducible biofilm therapeutic studies.
INTRODUCTION
The most clinically important phenotype of Candida biofilm cells is their remarkable resistance to antifungal drugs (1, 4, 17, 29, 39). Cells in this environment can survive up to 1,000-fold-higher concentrations of antifungals than nonbiofilm, planktonic cells. Because antifungal drugs typically are not effective against biofilm organisms, the recommended therapy for Candida biofilm infection of a medical device includes device removal, which is associated with increased procedural morbidity and health care expenditures (25). Novel drug targets and the development of new antifungal agents for the treatment of these recalcitrant infections are therefore of interest.
The use of the tetrazolium salt 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide inner salt (XTT) reduction assay to study Candida biofilms has been pioneered by labs in the mycology community (7, 10, 13, 33, 38). It is the method most commonly utilized for quantitative measurement of Candida biofilm mass, growth, and response to drug therapy (1, 9, 12, 18, 20, 26, 28, 37). Other techniques used to assay the biofilm cell burden include [3H]leucine incorporation, fluorescein diacetate, crystal violet staining, viable counts, dry weight measurements, and imaging using confocal or electron microscopy (5, 8, 10, 35, 36). The XTT assay has become the preferred tool due to the rapidity of the assay, the ability to use a high-throughput format (e.g., a 96-well plate), and more importantly, the ability to detect live yeast and hyphal organisms in the biofilm. Differentiation between viable and dead cells is particularly critical for testing drug efficacy. The assay is versatile and has been used for the measurement of biofilms on multiple substrates, including catheter material, denture strips, and contact lenses (5).
During our previous biofilm resistance studies, we noted several factors that appeared to influence the results of the XTT assay (21–23, 39). We hypothesized that systematically varying experimental conditions and comparing drug susceptibility outcomes would provide fundamental information useful for optimizing the assay. In these experiments we examined the impact of a wide variety of assay factors with the goals of (i) enhancing assay reproducibility, (ii) improving assay sensitivity and dynamic range, (iii) lowering assay cost, and (iv) improving efficiency (shortening the time). Ultimately, these experiments were designed to enhance the detection of drug susceptibility differences among strains and to simplify the assay for use in high-throughput screening of antifungal compounds.
MATERIALS AND METHODS
Organisms and culture conditions.
The Candida strains used for this study included Candida glabrata 5376, C. parapsilosis 5986, C. albicans strains K1 and DAY185, and the C. albicans FKS1/fks1Δ glucan synthase mutant (6, 24). The strains were stored in a 15% (vol/vol) glycerol stock at −80°C and were maintained on yeast extract-peptone-dextrose (YPD) medium (1% yeast extract, 2% peptone, and 2% dextrose) prior to experiments. Cultures were propagated overnight either in YPD medium supplemented with uridine or in RPMI 1640 medium buffered with morpholinepropanesulfonic acid (RPMI-MOPS) at 30°C or 37°C on an orbital shaker at 200 rpm.
Biofilm formation and drug treatment.
Biofilms were formed in 96-well polystyrene plates (23, 31). Cells were enumerated with a hemocytometer and were resuspended in RPMI-MOPS at a concentration of 106/ml. To each well of a 96-well microtiter plate, 100 μl of inoculum was added. After 6, 12, 18, or 24 h of incubation at 37°C, biofilms were gently washed twice with phosphate-buffered saline (PBS). Dilutions of antifungal agents or biocides and fresh RPMI-MOPS in a total volume of 200 μl were added to biofilms for 6, 24, or 48 h of incubation at 37°C. For a subset of assays, after 24 h of drug treatment, biofilms were washed with PBS, and drug dilutions were added for an additional 24 h of incubation. The concentrations studied were as follows: amphotericin B, 0.01 to 2 μg/ml; fluconazole, 4 to 1,000 μg/ml; H2O2, 4 to 1,000 mM.
XTT assay.
After the drug treatment and incubation period, an XTT reduction assay was performed as a measure of metabolic activity in order to estimate the burden of viable cells (23, 31). XTT and phenazine methosulfate stock solutions were prepared fresh for each set of assays and were kept away from light. XTT was added to PBS at a concentration of 0.25, 0.5, 0.75, or 1 mg/ml. XTT products from BioVectra, Biotium, MP Biomedicals, and Sigma were included. The solutions were centrifuged to remove any insoluble material prior to use. Phenazine methosulfate was dissolved in water (0.32 mg/ml). After drug dilutions were removed from each well, the biofilms were gently washed twice with PBS. To each well, 90 μl XTT and 10 μl phenazine methosulfate were added. The plates were incubated in the dark at 37°C for 0.5 h, 1 h, 2 h, or 4 h. Absorbances at 492 nm were recorded using an automated plate reader.
Statistical analysis.
Drug treatment wells were compared to no-drug controls, and the impact of the drug was calculated as a percentage of reduction. Data are presented as biofilm growth or the percentage of biofilm remaining after drug treatment, calculated as 1 − percentage of reduction. Assays were performed in triplicate on at least two occasions. Significant differences were measured by Student's t test or analysis of variance (ANOVA) with pairwise comparisons using the Holm-Sidak method. For a subset of experiments, we determined the drug concentration associated with a 50% reduction in optical density (OD) from that for the no-drug control wells (50% effective concentration [EC50]). To further describe drug impact over a concentration range, assays were also analyzed using a trapezoidal calculation, with the mathematical product of the concentration and the percentage of reduction summed over a concentration range [AUC = ∑(percentage of reduction × n), where AUC is the area under the concentration-time curve and n is the drug concentration].
RESULTS
Subculture conditions.
To determine if the subculture medium and temperature conditions impact the susceptibility of Candida biofilms to drugs, we examined the effects of an antifungal (amphotericin B) and a biocide (hydrogen peroxide) on C. albicans, C. parapsilosis, and C. glabrata biofilms. The liquid conditions for preparation of the inocula prior to the addition of cells to the wells of microtiter plates included incubation at 30°C or 37°C and growth in YPD medium plus uridine or RPMI-MOPS. The medium and temperature used during the biofilm formation and drug treatment steps were identical in all experiments (RPMI-MOPS and 37°C). Using an EC50 endpoint, we did not detect major differences between the susceptibilities of the biofilms formed under the various subculture conditions (Table 1). Alterations in subculture conditions resulted in, at most, a 2-fold difference in the EC50 for C. glabrata or C. parapsilosis. The largest difference in susceptibility due to variation in subculture conditions was observed for C. albicans and hydrogen peroxide. C. albicans strain K1 grown under subculture conditions at 37°C was 4-fold more resistant to hydrogen peroxide than the same strain grown at 30°C.
Table 1.
Impact of subculture medium and temperature on the susceptibility of Candida biofilms
| Strain | Subculture condition |
EC50a of: |
||
|---|---|---|---|---|
| Temp (°C) | Mediumb | Amphotericin B (μg/ml) | H2O2 (mM) | |
| C. albicans DAY185 | 30 | YPD | 0.06 | 250 |
| 30 | RPMI | 0.06 | 250 | |
| 37 | YPD | NA | NA | |
| C. albicans K1 | 30 | YPD | 0.06 | 125 |
| 30 | RPMI | 0.13 | 125 | |
| 37 | YPD | 0.13 | 500 | |
| C. glabrata 5376 | 30 | YPD | 0.13 | 500 |
| 30 | RPMI | 0.06 | 1,000 | |
| 37 | YPD | 0.13 | 500 | |
| C. parapsilosis 5986 | 30 | YPD | 0.13 | 250 |
| 30 | RPMI | 0.13 | 500 | |
| 37 | YPD | 0.13 | 500 | |
Drug concentration associated with a 50% reduction in optical density from that for no-drug control wells. NA, not applicable.
YPD, yeast extract-peptone-dextrose medium (1% yeast extract, 2% peptone, 2% dextrose supplemented with uridine); RPMI, RPMI 1640 medium buffered with morpholinepropanesulfonic acid.
For several of the C. albicans subculture conditions, XTT experiments could not be performed because the cultures were hyphal and could not be enumerated by hemocytometer counts. Hyphae were the predominate cellular type when strain K1 was grown in RPMI-MOPS at 37°C. C. albicans DAY185 cultures contained hyphae if grown at 37°C regardless of the medium selected. Under these conditions, mats of tangled hyphae present in the subculture persisted despite vortexing. Accurate hemocytometer counts could not be obtained, and the starting inoculum could not be standardized.
Washing of cells.
With the goal of simplifying the XTT assay procedure, we examined the impact of eliminating the cell-washing step prior to hemocytometer enumeration and biofilm formation. We compared the outcomes of the assay with and without the washing step for determination of the susceptibilities of C. albicans K1 and DAY185 to amphotericin B and fluconazole. Exclusion of the washing step did not impact the susceptibility results for either of the strains or drugs tested (Table 2). An example of the susceptibility of DAY185 to amphotericin B with omission of this washing step is shown in Fig. 1.
Table 2.
Impact of a subculture washing step on the Candida biofilm susceptibility result
| C. albicans strain | Subculture condition | EC50a (μg/ml) of: |
|
|---|---|---|---|
| Amphotericin B | Fluconazole | ||
| DAY185 | Washing | 0.25 | >1,000 |
| No washing | 0.25 | >1,000 | |
| K1 | Washing | 0.13 | >1,000 |
| No washing | 0.13 | >1,000 | |
Drug concentration associated with a 50% reduction in optical density from that for no-drug control wells.
Fig. 1.
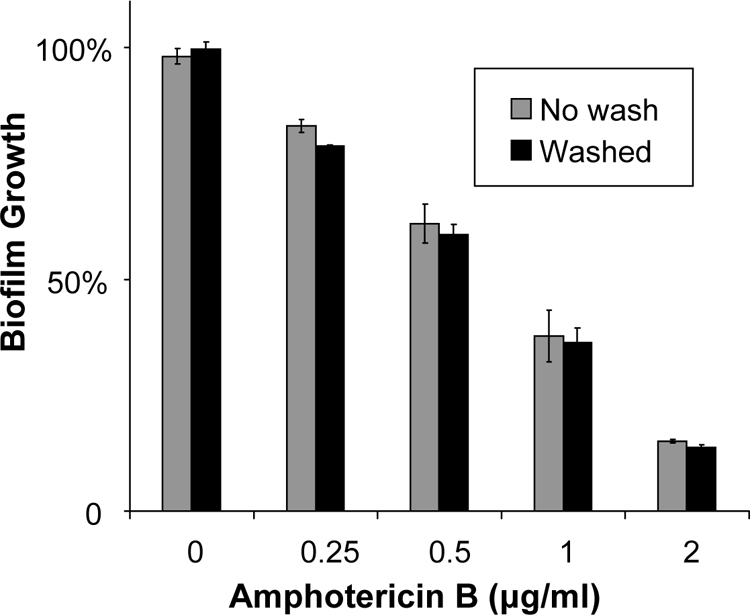
Impact of omission of cell washes prior to biofilm formation. C. albicans DAY185 was grown overnight in YPD medium plus uridine at 30°C. Cells either were washed with sterile PBS or were directly enumerated with a hemocytometer. Biofilms were grown for 6 h and were treated with amphotericin B for 24 h. Absorbance at 492 nm was measured following incubation with XTT (0.75 mg/ml) for 1 h. Student's t test was used to compare the effects of no wash to washing at each concentration; the results were not significant.
Duration of biofilm growth.
We next tested the impact of altering the duration of biofilm growth on XTT assay results. We hypothesized that shortening the time for biofilm formation would result in less-mature biofilms, improving the assay sensitivity and decreasing total assay time. A C. albicans reference strain (DAY185) and a glucan synthase mutant (FKS1/Δfks1) were selected for investigation, and biofilms were allowed to form over a 6-, 12-, 18-, or 24-h period prior to addition of the antifungal drug. In response to fluconazole treatment following 6 h of biofilm growth, the growth of mutant biofilm was reduced by 40%, while the reference strain was not impacted (Fig. 2). The difference between the strains was smaller after 12 h of biofilm growth and was difficult to detect if the biofilms were allowed to grow for 18 h or longer.
Fig. 2.
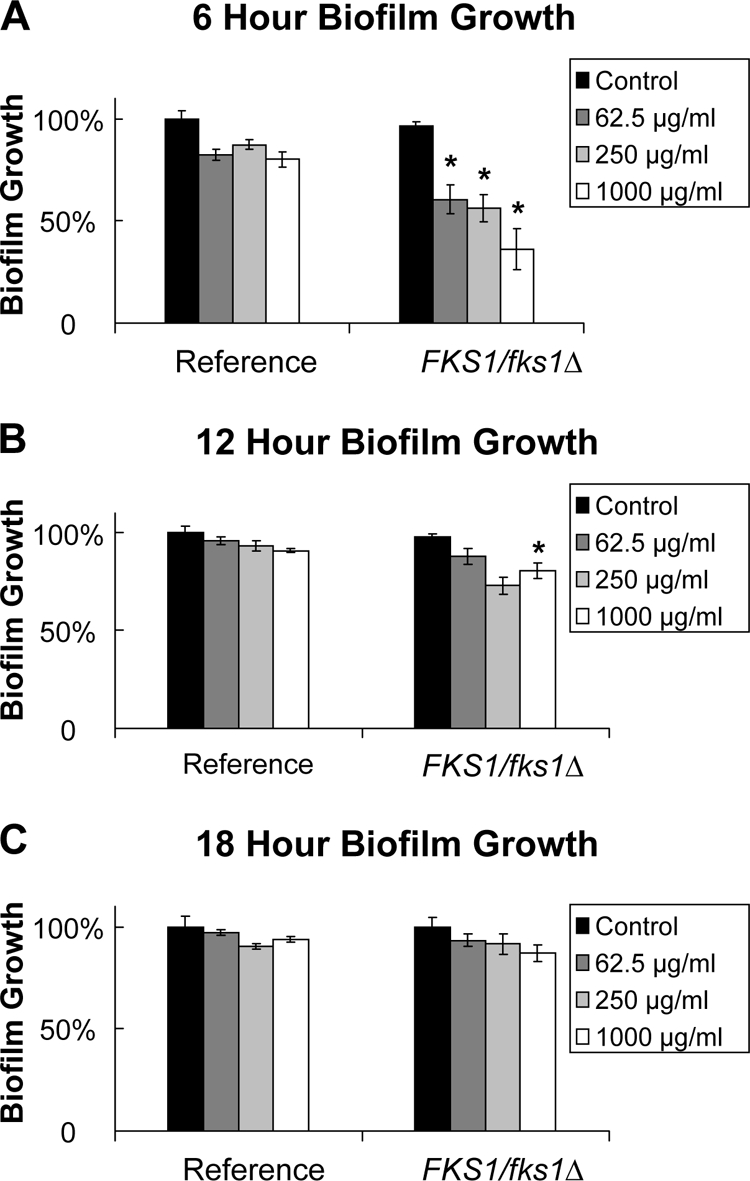
Impact of the duration of biofilm growth on the susceptibility of C. albicans biofilms. The reference strain DAY185 and the FKS1/fks1Δ glucan synthase mutant were grown overnight in YPD medium plus uridine at 30°C, and cells were enumerated with a hemocytometer. Following biofilm formation for 6, 12, or 18 h (A, B, or C, respectively), biofilms were treated with serial dilutions of fluconazole for 24 h. (Fluconazole concentrations are given in the keys.) Absorbance at 492 nm was measured following incubation with XTT (0.75 mg/ml) for 1 h. Student's t test was used to compare drug-treated biofilms with untreated controls. *, P < 0.05.
Drug administration regimen.
The next set of experiments was designed to determine the impact of the frequency of drug administration on biofilm susceptibility. We hypothesized that either drug instability or a saturable resistance mechanism may compromise the impact of a single drug administration. We further theorized that providing additional drug exposures may enhance drug delivery and improve the ability of the XTT assay to reflect the antifungal effect. The susceptibilities of the C. albicans reference strain and a glucan synthase mutant (FKS1/Δfks1) to two fluconazole dosing regimens were compared. Treatment with a single dose of fluconazole for 48 h reduced the growth of the mutant biofilm as much as 50% but only minimally impacted the reference strain biofilm (Fig. 3A). However, a much larger difference in susceptibility was apparent when fresh drug and medium were added midway through the 48-h drug incubation (Fig. 3B). With this dosing regimen, the growth of the mutant biofilm was reduced by 90%, while the reference stain remained resistant.
Fig. 3.
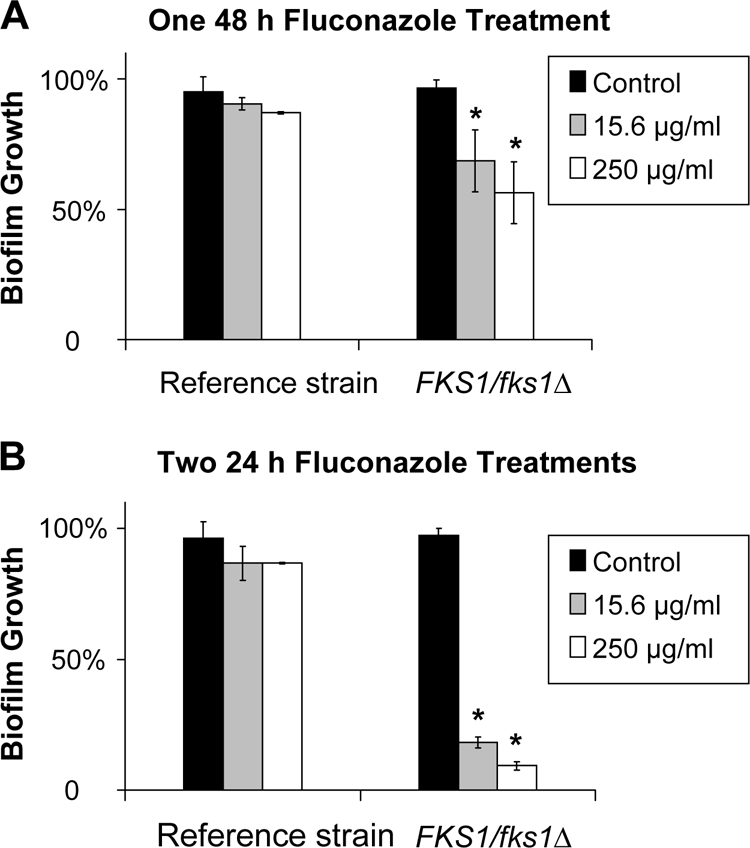
Impact of the antifungal dosing regimen on the susceptibility of C. albicans biofilms. The reference strain DAY185 and the FKS1/fks1Δ glucan synthase mutant were grown overnight in YPD medium plus uridine at 30°C, and cells were enumerated with a hemocytometer. After a 6-h biofilm formation period, biofilms were treated with serial dilutions of fluconazole for 48 h. Fluconazole was supplied either as a single 48-h treatment (A) or as two 24-h treatments (B). Absorbance at 492 nm was measured following incubation with XTT (0.75 mg/ml) for 1 h. Student's t test was used to compare drug-treated biofilms with untreated controls. *, P < 0.05.
Impact of XTT concentration, incubation time, and manufacturer.
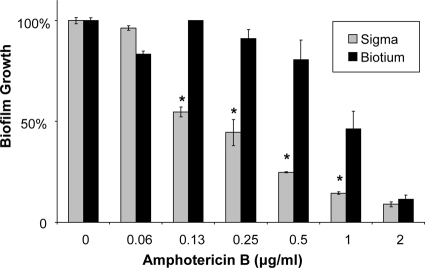
We next hypothesized that there would be an optimum XTT exposure for the quantification of biofilm mass and that the use of XTT concentrations or exposure times outside the optimal range may limit assay reproducibility and sensitivity. In addition, we wanted to identify the lowest XTT concentration and the shortest XTT incubation time that could be used reliably in a high-throughput assay. We chose to study C. albicans DAY185 and tested the impact of several XTT concentrations (0.25, 0.5, 0.75, or 1 mg/ml) and XTT exposure times (0.5, 1, 2, or 4 h) on the susceptibility of biofilms to hydrogen peroxide and amphotericin B. When a 1-h incubation time was used, the susceptibility results were similar for all XTT concentrations tested (Fig. 4). In the studies with variable XTT incubation times, a shorter duration (0.5 h) increased the sensitivity of the assay, which detected percentages of reduction as much as 4-fold higher than those with longer incubation periods (1 to 4 h) (Fig. 5). However, the EC50 endpoints were identical regardless of the XTT incubation time chosen. Another variable we considered for XTT administration was the manufacturer. Differences in antifungal susceptibility were observed based on the manufacturer and the lot. An example is shown for the reference strain and amphotericin B susceptibility in Fig. 6.
Fig. 4.

Impact of the XTT concentration on the susceptibility of C. albicans biofilms. The reference strain DAY185 was grown overnight in YPD medium plus uridine at 30°C, and cells were enumerated with a hemocytometer. After a 24-h biofilm formation period, biofilms were treated with serial dilutions of amphotericin B for 24 h. Absorbance at 492 nm was measured following incubation with XTT (0.25 to 1 mg/ml, as shown in the key) for 1 h. ANOVA with pairwise comparisons using the Holm-Sidak method was used to compare XTT concentrations at each drug concentration. The results were not significant.
Fig. 5.
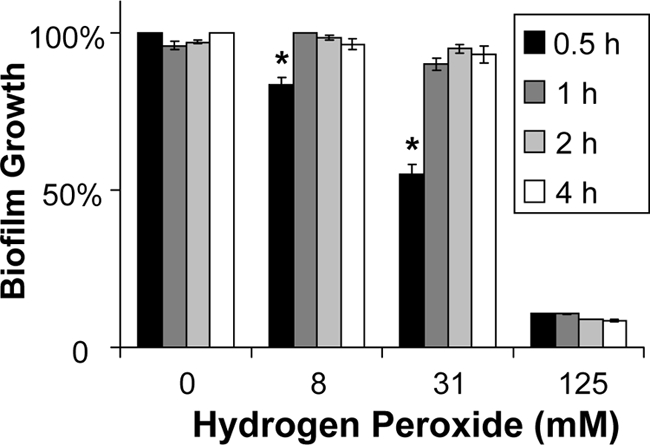
Impact of the duration of XTT incubation on the susceptibility of C. albicans biofilms. The reference strain DAY185 was grown overnight in YPD medium plus uridine at 30°C, and cells were enumerated with a hemocytometer. After a 24-h biofilm formation period, biofilms were treated with serial dilutions of hydrogen peroxide for 24 h. Absorbance at 492 nm was measured following incubation with XTT (0.75 mg/ml) for 0.5 to 4 h. ANOVA with pairwise comparisons using the Holm-Sidak method was used to compare XTT incubation times at each drug concentration. *, P < 0.05.
Fig. 6.
Impact of the XTT manufacturer on assay results. The reference strain C. albicans DAY185 was grown overnight in YPD medium plus uridine at 30°C, and cells were enumerated with a hemocytometer. After a 24-h biofilm formation period, biofilms were treated with serial dilutions of amphotericin B for 24 h. Absorbance at 492 nm was measured following 1 h of incubation with XTT (0.75 mg/ml) from Sigma or Biotium. Student's t test was used to compare Sigma XTT with Biotium XTT at each amphotericin B concentration. *, P < 0.05.
Colorimetric measurement.
Following incubation of the biofilm cells with XTT, current protocols call for transfer of the cellular supernatant to a new plate for colorimetric measurement by an automated plate reader. This step requires additional time for transfer of the supernatant and washing of the biofilm, as well as the cost of an additional microtiter plate. We tested the impact of eliminating this step on the susceptibility of C. albicans biofilms to amphotericin B. When this step was omitted, the EC50 endpoint did not change (Fig. 7). However, significant differences were found for percentages of reduction in the steepest part of the dose-response curve.
Fig. 7.
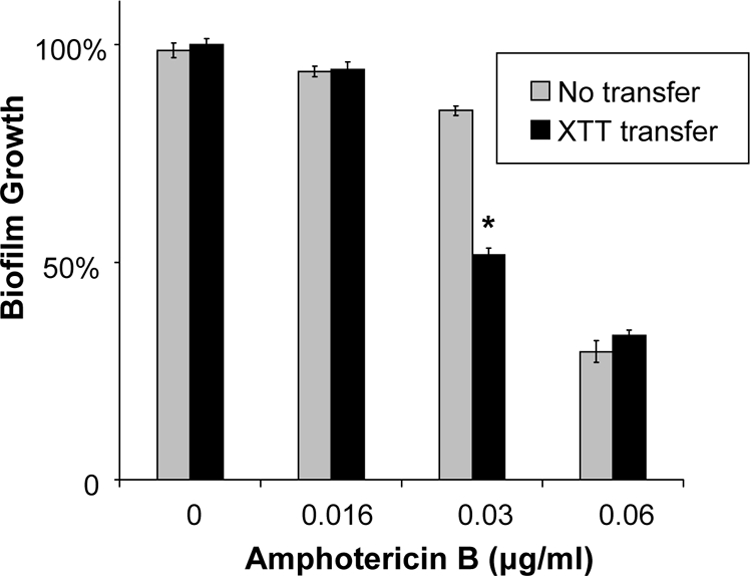
Impact of omission of the XTT supernatant transfer step. The reference strain C. albicans DAY185 was grown overnight in YPD medium plus uridine at 30°C, and cells were enumerated with a hemocytometer. After a 6-h biofilm formation period, biofilms were treated with serial dilutions of amphotericin B for 24 h. XTT (0.75 mg/ml) was added, and plates were incubated for 1 h. Colorimetric absorbance was measured either directly in the 96-well plate or following the transfer of the XTT cellular supernatant to a fresh plate. Student's t test was used to compare transfer versus no transfer at each concentration. *, P < 0.05.
Endpoint determination.
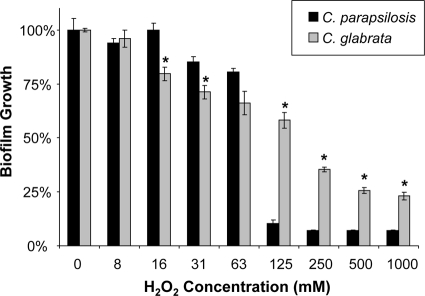
We considered three methods for analyzing the impact of a drug and comparing results for different strains. For these calculations, we chose to examine the impact of hydrogen peroxide on C. parapsilosis and C. glabrata (Fig. 8). One endpoint commonly used is the concentration required for a 50% reduction (EC50), which is considered similar to the MIC50 (MIC at which 50% of isolates are inhibited) endpoint for planktonic organisms. In the example provided, the EC50 endpoints are 125 and 250 mM for C. parapsilosis and C. glabrata, respectively.
Fig. 8.
Biofilm treatment endpoint determination. C. parapsilosis 5986 and C. glabrata 5376 were grown overnight in YPD medium plus uridine at 30°C, and cells were enumerated with a hemocytometer. After a 24-h biofilm formation period, biofilms were treated with serial dilutions of hydrogen peroxide for 24 h. Absorbance at 492 nm was measured following incubation with XTT (0.75 mg/ml) for 1 h. Biofilm susceptibilities were compared between strains by using the EC50, the percentage of reduction, and the AUC as endpoints (see Results). Student's t test was used to compare percentages of reduction between strains at each H2O2 concentration. *, P < 0.05.
The second method requires choosing a concentration in the dynamic range and comparing the percentages of reduction for the two strains. For example, following treatment with 125 mM H2O2, the growth of the C. parapsilosis biofilm was reduced by 90%, while that of the C. glabrata biofilm was reduced by only 40%. However, this calculation may not fully describe the dose-effect relationship. If a lower concentration (16 to 63 mM) were chosen for analysis, C. glabrata would be considered the more susceptible strain.
The third method, a modified trapezoidal calculation, was used to capture the impact of a drug across the entire dose-response curve. By summing the products of the concentration and the percentage of reduction at that concentration, the trapezoid rule accounts for the entire dose-response curve: AUC = ∑(percentage of reduction × n), where n stands for the drug concentration. Summing these products (percentage of reduction and drug concentration) for C. parapsilosis and C. glabrata across the 0 to 1,000 mM range yields 1,765 and 1,389, respectively. In the trapezoidal AUC analysis, C. glabrata appears to be the more susceptible strain in this assay.
DISCUSSION
The 96-well microtiter plate biofilm model and XTT assay have been invaluable tools for the quantitation of Candida biofilms and the testing of their susceptibility to antifungal drugs (1, 9, 20, 24, 28, 39). However, our previous studies identified variability in assay results. Our experience has been informally confirmed via communication with numerous laboratories. We examined our experience with these assays over the past several years and attempted to identify experimental variables that might account for these inconsistencies. The primary goal of the current set of experiments was to investigate potential assay factors that might account for the observed variability in study accuracy and sensitivity in order to improve the assay for antifungal drug testing. The second objective of the present study was to develop a high-throughput XTT assay to be used for screening the susceptibility of biofilms to drugs. By systematically varying the assay conditions, we did identify factors that impact the results of the 96-well plate assay. These variables include (i) the duration of biofilm growth prior to antifungal administration, (ii) the antifungal dosing regimen, (iii) the XTT incubation time, (iv) transfer of the supernatant and washes prior to colorimetric measurement, and (v) the XTT manufacturer and lot. Experimental variables that did not appear to alter the XTT results significantly include (i) subculture medium and temperature conditions, (ii) washing of the subculture prior to the inoculation of wells, (iii) the XTT concentration, and (iv) transfer of the XTT supernatant to a new plate for colorimetric measurement.
Previous unpublished studies in our laboratory identified outcome variability with different subculture conditions prior to biofilm formation. We hypothesized that this may be due to the impact of the subculture medium and temperature on the presence or degree of filamentation, which has been shown to influence biofilm formation (16, 32). We found that the susceptibility patterns of C. albicans, C. glabrata, and C. parapsilosis biofilms were not profoundly impacted by subculture conditions (Table 1). Small differences were observed, possibly related to changes in cellular morphology or biofilm architecture. In our experience, altering the subculture conditions can be helpful for certain strains (Tables 3 and 4). For example, C. albicans K1 biofilms formed under RPMI-MOPS subculture conditions are more durable and easier to wash without disruption. We have found this phenotype for strains of C. parapsilosis and C. glabrata as well. However, for certain C. albicans strains, such as DAY185, the formation of hyphae under subculture conditions at 37°C or in RPMI-MOPS complicates hemocytometer enumeration. In sum, propagation of Candida spp. at 30°C in YPD medium plus uridine results primarily in yeast cells, which are easily enumerated with a hemocytometer. However, subculture in RPMI-MOPS may be useful for strains that are not forming consistent biofilms with YPD-plus-uridine subcultures.
Table 3.
Summary of Candida biofilm microtiter plate model and XTT assay variables for experimental design
| Objective | Proposed change in variables |
|---|---|
| Increase assay sensitivity | Shorten duration of biofilm formation |
| Increase drug concn | |
| Increase antifungal treatment period | |
| Shorten interval between drug doses | |
| Shorten XTT incubation time | |
| Increase biofilm formation | Try alternative subculture conditions (RPMI-MOPS or 37°C) |
| Lengthen duration of biofilm formation | |
| Decrease time and cost | Use cells directly from subculture without additional washes |
| To avoid having to filter sterilize XTT, prepare it fresh daily | |
| Use XTT at 0.25 mg/ml | |
| Shorten XTT incubation time to 30 min | |
| Record absorbance directly in original 96-well plate to avoid transfer to new plate |
Table 4.
Techniques for XTT assay troubleshooting
| Problem | Possible reasons | Options |
|---|---|---|
| Biofilm disruption during washing | Too-vigorous washing | Use gentle washes |
| Subculture conditions not optimal for biofilm formation | Consider alternative subculture conditions | |
| Medium not permissive for growth | Use uridine or other nutrient supplementation as required for strains | |
| Adhesion- or biofilm-defective strain | ||
| Susceptibility not detected | Strain resistant to antifungal concn | Shorten duration of biofilm growth |
| Increase antifungal concn | ||
| Decrease drug dosing interval | ||
| XTT incubation too long | Shorten XTT incubation time | |
| Resistance not detected | Immature biofilms | Increase duration of biofilm growth |
| Strain susceptible to concn and dose | Decrease antifungal concn | |
| Fluctuating results | Too-vigorous washing | Use gentle washes |
| Subculture conditions not optimal for biofilm formation | Consider alternative subculture conditions | |
| Medium not permissive for growth | Use uridine or other nutrient supplementation as required for strains | |
| XTT not in solution | Filter or centrifuge XTT to remove insoluble material | |
| Mix XTT prior to use | ||
| Agents exposed to light | Add assay agent in the dark | |
| Poor biofilm formation in outer wells | Liquid in outer wells evaporates during incubation | Avoid using outer wells for experiments |
| Add liquid to outer wells to prevent evaporation from inner wells | ||
| Use Parafilm and/or foil around plate to prevent evaporation |
A number of studies have shown that the susceptibility of Candida biofilms to drugs may be dependent on maturation or the biofilm growth phase. For example, the drug efflux pumps Cdr1p, Cdr2p, and Mdr1p appear to play a role in early biofilm drug resistance (20). Strains with disruption of these pumps are more susceptible to fluconazole during early biofilm formation but remain resistant if treated when biofilms are fully mature (20, 27). We hypothesized that testing C. albicans earlier in biofilm formation (6 h) may increase XTT sensitivity for the detection of other resistance mechanisms or for some antifungal compounds. For a C. albicans glucan synthase mutant, we observed increased XTT assay sensitivity for the detection of fluconazole antibiofilm activity when the biofilm growth period was reduced from 24 h to 6 h (Fig. 2) (24). The increased susceptibility of early-phase biofilms has been described previously and may be related to decreased matrix deposition, a smaller biofilm mass, or other biofilm phenotypic properties (19, 20). A shorter biofilm maturation time may be especially useful for the testing of azole drugs, such as fluconazole. C. albicans biofilms are frequently resistant to azoles at concentrations near drug solubility, and testing of higher drug concentrations may not be possible (2, 3, 15, 27). We considered a decrease in the starting inoculum as an alternative method of reducing the biofilm mass. However, quorum sensing is critical for biofilm formation, and the use of a smaller inoculum may result in uneven or inconsistent biofilm formation (11, 30). Candida biofilms are more susceptible to amphotericin B and echinocandins than to azoles. We consistently find a dose-response curve with concentrations higher than the planktonic MIC. Therefore, we have found altering the duration of biofilm growth prior to drug treatment less helpful for optimizing resistance testing for these drugs.
Another factor that appeared to improve the sensitivity of the triazole antibiofilm activity assay was altering the drug dosing scheme. The application of additional antifungal drug or redosing improved the ability to discern differences in antifungal susceptibility between C. albicans strains (Fig. 3). It is possible that the redosing replaces drug that has become inactive (unstable) over the treatment period (34). Another possibility is that the mechanism of resistance is saturable and that additional antifungal dosing is capable of “overcoming” the biofilm resistance. Additional studies with the glucan synthase mutant suggest the presence of a saturable resistance mechanism for the strains examined in the current studies (24). However, we cannot rule out the impact of drug stability on this study result. As described above, Candida biofilms may be resistant to azole concentrations approaching solubility, and techniques to improve the sensitivity of the assay for the detection of susceptibility can be especially useful for this drug class (2, 3, 15, 27).
Another assay variable that impacted the biofilm susceptibility results was the XTT concentration. We were somewhat surprised to find that variation in the XTT concentration over a range from 0.25 to 1 mg/ml produced similar endpoint results (Fig. 4). Although absorbance values were lower for treatment with XTT at 0.25 mg/ml (optical density at 492 nm [OD492], approximately 1.5) than at 1 mg/ml (OD492, approximately 2.5), it appears that both XTT concentrations produce colorimetric products in the dynamic range. Therefore, lower XTT concentrations may be considered in the assay design; this change would decrease the XTT cost 4-fold for high-throughput assays, saving more than $10 per 96-well plate. Adjustments in the XTT concentration may also be helpful for Candida strains with absorbance values too low or too high to accommodate a full dose range effect.
We also tested the impact of the duration of the XTT incubation period. We found that the greatest assay sensitivity was observed with incubations of approximately 30 min for C. albicans, a duration considerably shorter than that often described in previous publications (Fig. 5) (10, 31). The shorter incubation period improved the dynamic range for the detection of susceptibility for the strains and drugs we studied over a wide concentration range. This assay change both improved sensitivity and reduced the time necessary for assay completion (Tables 3 and 4). In addition, we found that the use of XTT from different manufacturers and lots impacts the XTT endpoint, and we suggest that comparisons among antifungals and strains should be made using XTT from the same manufacturer and lot number. Pilot testing of new lots may be helpful for optimal study design and interpretation (Fig. 6).
An important consideration for interpretation of the activity of antifungal drugs against biofilm cells is the selection of the endpoint. The endpoint most commonly used is the antifungal concentration associated with a 50% reduction in optical density from that for an untreated control (EC50). The EC50 endpoint gives a single value that often reflects a steep part of the dose-response curve. It can be used for comparisons among strains or drugs in a manner similar to the use of planktonic MICs. Another method of comparison is determination of the percentage of reduction at a single drug concentration. This approach easily allows statistical analysis when experiments are performed in triplicate. However, the percentage of reduction at a single given concentration may not best represent the entire dose-response curve. Comparison of multiple points along the dose-response curve will provide a more complete reflection of drug impact. The trapezoid-AUC rule considers the percentage of reduction at each of the drug concentrations tested and estimates the area for the dose-response curve. This value can be used for comparisons of the entire drug-effect relationship.
In summary, the XTT assay and Candida biofilm microtiter plate model have been useful tools for the study of drug activity against biofilms. However, assay inconsistency has been reported. The current experiments identified a number of variables that may be altered so as to optimize the assay for studying drug efficacy and comparing genetically modified strains for the investigation of drug resistance mechanisms. Although we considered many experimental variables, the assay variables investigated were by no means exhaustive. We focused on factors that we had hypothesized or observed to influence the XTT assay in a positive manner. We limited our study to include two antifungals with differing antibiofilm activities (fluconazole and amphotericin B) and one biocide (hydrogen peroxide). We believe the techniques for increasing assay sensitivity, troubleshooting, and saving time can be applied to the study of other antifungal drugs and biocides. Our experiments included C. glabrata and C. parapsilosis but focused on C. albicans. The variables found to increase assay sensitivity for C. albicans may not apply directly to the other strains. For example, a shorter biofilm growth period for C. glabrata may result in poor biofilm formation, considering the lower growth rate of this species. The processing of XTT may differ among Candida strains, and thus, direct correlations should be made with caution (14). XTT requires uptake and processing by the Candida cells. It is possible that strains with genetic alterations in the cell wall or biofilm matrix may limit uptake, but we have not observed this phenomenon.
These experiments identify factors for optimizing and troubleshooting the XTT assay for the assessment of drug activity against biofilms. The techniques have been useful for teaching new students and helping other labs adopt this method of susceptibility testing.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (RO1 AI073289-01) and the Veterans Affairs Women's Health Fellowship.
Footnotes
Published ahead of print on 12 January 2011.
REFERENCES
- 1. Al-Fattani M. A., Douglas L. J. 2006. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J. Med. Microbiol. 55:999–1008 [DOI] [PubMed] [Google Scholar]
- 2. Andes D., et al. 2004. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect. Immun. 72:6023–6031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baillie G. S., Douglas L. J. 1998. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob. Agents Chemother. 42:1900–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chandra J., et al. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chandra J., Mukherjee P. K., Ghannoum M. A. 2008. In vitro growth and analysis of Candida biofilms. Nat. Protoc. 3:1909–1924 [DOI] [PubMed] [Google Scholar]
- 6. Davis D., Wilson R. B., Mitchell A. P. 2000. RIM101-dependent and -independent pathways govern pH responses in Candida albicans. Mol. Cell. Biol. 20:971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hawser S. 1996. Adhesion of different Candida spp. to plastic: XTT formazan determinations. J. Med. Vet. Mycol. 34:407–410 [PubMed] [Google Scholar]
- 8. Hawser S. P., Douglas L. J. 1995. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 39:2128–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hawser S. P., Norris H., Jessup C. J., Ghannoum M. A. 1998. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J. Clin. Microbiol. 36:1450–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Honraet K., Goetghebeur E., Nelis H. J. 2005. Comparison of three assays for the quantification of Candida biomass in suspension and CDC reactor grown biofilms. J. Microbiol. Methods 63:287–295 [DOI] [PubMed] [Google Scholar]
- 11. Hornby J. M., et al. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacobson M. J., Steckelberg K. E., Piper K. E., Steckelberg J. M., Patel R. 2009. In vitro activity of micafungin against planktonic and sessile Candida albicans isolates. Antimicrob. Agents Chemother. 53:2638–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jin Y., Samaranayake L. P., Samaranayake Y., Yip H. K. 2004. Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch. Oral Biol. 49:789–798 [DOI] [PubMed] [Google Scholar]
- 14. Kuhn D. M., Balkis M., Chandra J., Mukherjee P. K., Ghannoum M. A. 2003. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J. Clin. Microbiol. 41:506–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuhn D. M., Ghannoum M. A. 2004. Candida biofilms: antifungal resistance and emerging therapeutic options. Curr. Opin. Investig. Drugs 5:186–197 [PubMed] [Google Scholar]
- 16. Kumamoto C. A., Vinces M. D. 2005. Alternative Candida albicans lifestyles: growth on surfaces. Annu. Rev. Microbiol. 59:113–133 [DOI] [PubMed] [Google Scholar]
- 17. LaFleur M. D., Kumamoto C. A., Lewis K. 2006. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 50:3839–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Melo A. S., Colombo A. L., Arthington-Skaggs B. A. 2007. Paradoxical growth effect of caspofungin observed on biofilms and planktonic cells of five different Candida species. Antimicrob. Agents Chemother. 51:3081–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mowat E., et al. 2008. Phase-dependent antifungal activity against Aspergillus fumigatus developing multicellular filamentous biofilms. J. Antimicrob. Chemother. 62:1281–1284 [DOI] [PubMed] [Google Scholar]
- 20. Mukherjee P. K., Chandra J., Kuhn D. M., Ghannoum M. A. 2003. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 71:4333–4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nett J., et al. 2007. Putative role of β-1,3 glucans in Candida albicans biofilm resistance. Antimicrob. Agents Chemother. 51:510–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nett J. E., Crawford K., Marchillo K., Andes D. R. 2010. Role of Fks1p and matrix glucan in Candida albicans biofilm resistance to an echinocandin, pyrimidine, and polyene. Antimicrob. Agents Chemother. 54:3505–3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nett J. E., Guite K. M., Ringeisen A., Holoyda K. A., Andes D. R. 2008. Reduced biocide susceptibility in Candida albicans biofilms. Antimicrob. Agents Chemother. 52:3411–3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nett J. E., Sanchez H., Cain M. T., Andes D. R. 2010. Genetic basis of Candida biofilm resistance due to drug-sequestering matrix glucan. J. Infect. Dis. 202:171–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pappas P. G., et al. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perumal P., Mekala S., Chaffin W. L. 2007. Role for cell density in antifungal drug resistance in Candida albicans biofilms. Antimicrob. Agents Chemother. 51:2454–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramage G., Bachmann S., Patterson T. F., Wickes B. L., Lopez-Ribot J. L. 2002. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 49:973–980 [DOI] [PubMed] [Google Scholar]
- 28. Ramage G., Lopez-Ribot J. L. 2005. Techniques for antifungal susceptibility testing of Candida albicans biofilms. Methods Mol. Med. 118:71–79 [DOI] [PubMed] [Google Scholar]
- 29. Ramage G., Saville S. P., Thomas D. P., Lopez-Ribot J. L. 2005. Candida biofilms: an update. Eukaryot. Cell 4:633–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramage G., Saville S. P., Wickes B. L., Lopez-Ribot J. L. 2002. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 68:5459–5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramage G., Vande Walle K., Wickes B. L., Lopez-Ribot J. L. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramage G., VandeWalle K., Lopez-Ribot J. L., Wickes B. L. 2002. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol. Lett. 214:95–100 [DOI] [PubMed] [Google Scholar]
- 33. Ramage G., Vandewalle K., Wickes B. L., Lopez-Ribot J. L. 2001. Characteristics of biofilm formation by Candida albicans. Rev. Iberoam. Micol. 18:163–170 [PubMed] [Google Scholar]
- 34. Rementeria A., et al. 2007. Comparison of tablet and disk diffusion methods for fluconazole and voriconazole in vitro activity testing against clinical yeast isolates. J. Chemother. 19:172–177 [DOI] [PubMed] [Google Scholar]
- 35. Richard M. L., Nobile C. J., Bruno V. M., Mitchell A. P. 2005. Candida albicans biofilm-defective mutants. Eukaryot. Cell 4:1493–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Samaranayake Y. H., Ye J., Yau J. Y., Cheung B. P., Samaranayake L. P. 2005. In vitro method to study antifungal perfusion in Candida biofilms. J. Clin. Microbiol. 43:818–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seneviratne C. J., Jin L. J., Samaranayake Y. H., Samaranayake L. P. 2008. Cell density and cell aging as factors modulating antifungal resistance of Candida albicans biofilms. Antimicrob. Agents Chemother. 52:3259–3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tellier R., Krajden M., Grigoriew G. A., Campbell I. 1992. Innovative endpoint determination system for antifungal susceptibility testing of yeasts. Antimicrob. Agents Chemother. 36:1619–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Uppuluri P., Nett J., Heitman J., Andes D. 2008. Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob. Agents Chemother. 52:1127–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]