Abstract
Bacteriological diagnosis of brucellosis is performed by culturing animal samples directly on both Farrell medium (FM) and modified Thayer-Martin medium (mTM). However, despite inhibiting most contaminating microorganisms, FM also inhibits the growth of Brucella ovis and some B. melitensis and B. abortus strains. In contrast, mTM is adequate for growth of all Brucella species but only partially inhibitory for contaminants. Moreover, the performance of both culture media for isolating B. suis has never been established properly. We first determined the performance of both media for B. suis isolation, proving that FM significantly inhibits B. suis growth. We also determined the susceptibility of B. suis to the antibiotics contained in both selective media, proving that nalidixic acid and bacitracin are highly inhibitory, thus explaining the reduced performance of FM for B. suis isolation. Based on these results, a new selective medium (CITA) containing vancomycin, colistin, nystatin, nitrofurantoin, and amphotericin B was tested for isolation of the main Brucella species, including B. suis. CITA's performance was evaluated using reference contaminant strains but also field samples taken from brucella-infected animals or animals suspected of infection. CITA inhibited most contaminant microorganisms but allowed the growth of all Brucella species, to levels similar to those for both the control medium without antibiotics and mTM. Moreover, CITA medium was more sensitive than both mTM and FM for isolating all Brucella species from field samples. Altogether, these results demonstrate the adequate performance of CITA medium for the primary isolation of the main Brucella species, including B. suis.
INTRODUCTION
Brucellosis is a worldwide zoonosis affecting mainly low-income countries (23). Besides two new species (Brucella ceti and B. pinnipedialis) isolated recently from marine mammals, the genus Brucella includes six classical species named according to their host preference (18). Among them, B. melitensis, B. abortus, B. suis, and B. ovis (which preferentially infect sheep and goats, cattle, pigs, and sheep, respectively) are the most important from a socioeconomic standpoint, since in addition to decreasing productivity in animals, the first three species are the main ones responsible for brucellosis in human beings (19). In infected animals, brucellae can be isolated from vaginal discharges, placental and fetal tissues, milk, and semen of live animals and, after necropsy, from the lymph nodes, spleen, liver, mammary glands, epididymides, and male sexual glands. Due to its specificity, the isolation and identification of Brucella in these animal fluids and tissues are the only incontestable demonstration of brucellosis in a given animal or flock. Since primary Brucella isolation requires 4 to 7 days of incubation, the presence in the above field samples of overgrowing fungi as well as commensal and environmental bacteria explains the frequent contamination of culture plates and the reduced sensitivity of bacteriological diagnosis. Thus, the use of adequate selective culture media is of paramount importance for a proper bacteriological diagnosis of brucellosis.
The simultaneous use of Farrell's medium (FM) (7) and modified Thayer-Martin medium (mTM) (15) is currently considered the strategy of choice for primary Brucella isolation from field veterinary samples (21). FM is probably the most widely used selective medium for the bacteriological diagnosis of brucellosis. This excellent medium, initially developed for the isolation of B. abortus bv. 2 from contaminated sources (6, 7), contains antibiotics that are highly inhibitory for most overgrowing contaminants present in field veterinary samples (14). Moreover, because it is translucent, it facilitates the presumptive identification of Brucella by assessing the colony morphology (1). However, due mainly to the nalidixic acid and bacitracin contained in its formulation, FM is inhibitory for B. ovis and also for some B. melitensis and B. abortus strains (15). Considering that mTM significantly improves the sensitivity of FM for the isolation of B. ovis and B. melitensis (3, 14), the simultaneous use of both culture media has been recommended for the routine bacteriological diagnosis of disease (21). However, mTM is significantly less inhibitory than FM for contaminant microorganisms. Moreover, mTM is not translucent due to the presence of hemoglobin as a basal component and thus is unsuitable for direct observation of colony morphology, probably the most practical procedure for the presumptive identification of Brucella (1). Finally, the performance of both FM and mTM for isolating B. suis has been the object of debate but has never been investigated thoroughly. One of the objectives of this work was to evaluate this subject and to investigate the susceptibility of B. suis to the different antibiotics contained in both selective media. Using the results obtained, we formulated a new selective culture medium (CITA medium) which, in addition to being translucent, allows the growth of all Brucella species and simultaneously inhibits most contaminant microorganisms present in veterinary samples.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and medium compositions.
The Brucella reference and field strains used are summarized in Table 1. All Brucella strains were typed by standard bacteriological (1) and molecular (8) procedures and were kept lyophilized at 4°C in the culture collection of the CITA de Aragón (Zaragoza, Spain) until use. After rehydration, the strains were cultured on blood agar base plates (BAB no. 2; Biolife, Italy) supplemented with 5% sterile newborn calf serum (CS; PanBiotech, Germany) (BAB-CS) as described previously (9). Brucella suspensions were prepared in sterile buffered saline solution (BSS; pH 6.85) and adjusted to the desired concentration by spectrophotometry (suspensions with an optical density at 600 nm [OD600] of 0.170 usually contain 1 × 109 CFU/ml), using BSS or Mueller-Hinton broth (MHB; Difco) supplemented with 0.5% yeast extract (YE; Difco) and 5% CS (MHB-YE-CS). The exact number of colonies (CFU/ml) contained in each bacterial suspension was determined retrospectively, as described elsewhere (9).
Table 1.
Brucella reference and field strains used in this work
| Species | Biovara | Strain | No. of strains or isolates |
|---|---|---|---|
| Reference strains | |||
| B. abortus | 1 | 2308 | |
| 1 | S19 | ||
| 3 | Tulya | ||
| B. melitensis | 1 | 16 M | |
| 1 | Rev1 | ||
| 2 | 63/9 | ||
| 3 | Ether | ||
| B. suis | 1 | 1330 | |
| 2 | Thomsen | ||
| 3 | 680 | ||
| 4 | 40 | ||
| 5 | 513 | ||
| B. ovis | NA | Bow | |
| NA | Reo 198 | ||
| B. canis | NA | 6/66 | |
| Strains isolated from field samples | |||
| B. abortus | 1 | S19 | 2 |
| 1 | RB51 | 26 | |
| 3 | 7 | ||
| B. melitensis | 1 | 61 | |
| 3 | 148 | ||
| 1 | Rev1 | 28 | |
| B. suis | 1 | 5 | |
| 2 | 25 | ||
| 3 | 7 | ||
| 4 | 3 | ||
| B. ovis | NA | 32 |
NA, not applicable.
The microorganisms used as typical contaminants of veterinary samples were Escherichia coli (ATCC 285922), Staphylococcus aureus (ATCC 6538), Aspergillus niger (ATCC 16404), Bacillus subtilis (ATCC 6633), Enterococcus faecalis (ATCC 29212), Pseudomonas aeruginosa (ATCC 27853), and Proteus spp. (MicroBioLogics). All of these strains were provided by the Spanish Type Culture Collection (ECCT; University of Valencia, Spain) and cultured as recommended by the supplier. The growth or inhibition of these contaminant microorganisms on the corresponding selective culture media was assessed after 1 week of incubation at 37°C in both air and a 10% CO2 atmosphere.
Both FM and mTM were prepared as described previously (15), and the compositions of these media are shown in Table 2.
Table 2.
Compositions of the selective culture media compared in this study
| Component | Concn or descriptiona |
||
|---|---|---|---|
| FM | mTM | CITA | |
| Basal medium | BMB-CS | GC-H | BAB-CS |
| Nalidixic acid (mg/liter) | 5 | ||
| Bacitracin (IU/liter) | 25,000 | ||
| Polymyxin B (IU/liter) | 5,000 | ||
| Cycloheximide (mg/liter) | 100 | ||
| Natamycin (mg/liter) | 50 | ||
| Vancomycin (mg/liter) | 20 | 3 | 20 |
| Nystatin (IU/liter) | 100,000 | 100,000 | 100,000 |
| Colistin (mg/liter) | 7.5 | 7.5 | |
| Nitrofurantoin (mg/liter) | 10 | 10 | |
| Amphotericin B (mg/liter) | 4 | ||
FM, Farrell's medium (7); BMB-CS, brucella medium base (Oxoid, United Kingdom) supplemented with 5% CS (PanBiotech, Germany); mTM, modified Thayer-Martin medium (15); GC-H, GC agar base supplemented with 2 g Bacto agar and 1% hemoglobin (all from Difco); CITA, new selective culture medium; BAB-CS, blood agar base number 2 (Biolife, Italy) supplemented with 5% CS. FM was prepared using commercial antibiotic supplements containing either natamycin or cycloheximide (Oxoid). All antimicrobial agents not included in the commercial FM supplements were from Sigma.
Susceptibility of B. suis to both FM and mTM.
Bacterial suspensions of the 5 B. suis reference strains and 40 field strains tested (Table 1) were prepared in BSS, and dilutions containing around 1 × 103 CFU/ml were cultured (0.1 ml/plate) in triplicate on plates of FM, mTM, and BAB-CS (control). The mean and standard deviation (SD) for the number of CFU/plate were determined for each strain in each culture medium after incubation of plates at 37°C for 3 days in a 10% CO2 atmosphere. In addition, the 40 B. suis field strains were also studied for individual susceptibilities to each of the 9 antimicrobial agents contained in both FM and mTM, as well as to amphotericin B (Sigma Aldrich) (Table 2). This experiment was carried out in both liquid and solid media, as follows.
(i) Liquid medium assay.
Bacterial suspensions of the above-mentioned B. suis strains in MHB-YE-CS were adjusted to 5 × 105 CFU/ml as described above, and 0.1 ml of each suspension was mixed in a 96-well sterile plate (Nunc microplate; ThermoFisher Scientific S.L.U., Spain) with 0.1 ml of MHB-YE-CS supplemented with adequate antibiotic concentrations. For this purpose, concentrated suspensions of each antibiotic were prepared in MHB-YE-CS (40 mg/liter nalidixic acid, 200,000 IU/liter bacitracin, 40,000 IU/liter polymyxin B, 800 mg/liter cycloheximide, 400 mg/liter natamycin, 800,000 IU/liter nystatin, 160 mg/liter vancomycin, 60 mg/liter colistin, 80 mg/ml nitrofurantoin, and 32 mg/liter amphotericin B) (all from Sigma Aldrich) and then 2-fold serially diluted in MHB-YE-CS. Wells containing only bacteria, antibiotics, or MHB-YE-CS were used as controls in each plate. Plates were incubated at 37°C in 10% CO2, and bacterial growth was determined spectrophotometrically (OD595 readings) 4 and 7 days after incubation and confirmed by streaking out 25 μl/well in triplicate onto BAB-CS plates, which were incubated at 37°C in 10% CO2 for 5 to 7 days. Isolation of at least one CFU was considered a positive result.
(ii) Solid medium assay.
Bacterial suspensions of the above-mentioned B. suis strains were adjusted in BSS to 1 × 103 CFU/ml, and 0.1 ml of each suspension was smeared in triplicate on MH agar plates (MHA; Difco) supplemented with YE-CS as described above (MHA-YE-CS) and supplemented or not with the corresponding concentration of each antibiotic (Table 2). Nalidixic acid and bacitracin (both from Sigma Aldrich) were also tested at concentrations 2-fold above those contained in FM. The mean number of CFU/plate for each culture medium was determined after incubation at 37°C for 5 to 7 days in a 10% CO2 atmosphere. The mean (n = 3) individual number of CFU/plate was assessed for each Brucella strain in each culture medium. Once equivalent numbers of CFU were obtained in the control BAB-CS medium for the 45 B. suis strains tested, the mean and SD for the number of CFU/plate were determined for each antimicrobial agent. Statistical comparisons of means were performed by a two-way (strain-medium interaction) analysis of variance (ANOVA) followed by post hoc Fisher's protected least significant difference (PLSD) test.
Development of new selective culture medium (CITA).
Considering the importance and practical interest of using translucent media, the performances of the following basal culture media were assessed for isolating the main Brucella species: brucella medium base (BMB; Oxoid, United Kingdom), GC medium base (GC; Difco), Trypticase soy broth supplemented with 0.5% yeast extract (both products from Biolife, Italy) and 1.5% agar (Difco) (TSA-YE), and BAB. All of these basal components were supplemented with 5% sterile CS, and the resulting culture media (BMB-CS, GC-CS, TSA-YE-CS, and BAB-CS) were compared for Brucella growth. Adjusted suspensions (see above) of representative B. abortus, B. melitensis, B. suis, and B. ovis strains (Table 1) were plated in the corresponding basal media and incubated for 5 to 7 days at 37°C in 10% CO2, and the resulting mean CFU/plate was determined as described elsewhere (9). With the exception of GC-CS, which was somewhat inhibitory, the remaining media performed similarly for isolation of all Brucella species (results not shown). With its adequate performance and availability, BAB-CS was considered the basal component of choice for ensuing experiments.
In the second experimental phase, suspensions of representative Brucella strains (Table 1) and contaminants were inoculated on BAB-CS plates supplemented with all of the antibiotics contained in mTM (Table 2), but increasing the vancomycin (Sigma Aldrich) concentration to 20 mg/liter (as in FM) and adding several concentrations (2.5, 4, and 5 mg/liter) of amphotericin B (Sigma Aldrich) as a complementary antifungal agent. The same mTM formulation without amphotericin B (Sigma Aldrich) and BAB-CS alone were used as controls in this experiment. The resulting CFU of the corresponding contaminants and brucellae were determined in triplicate experiments after incubation at 37°C in 10% CO2 for 3 and 5 days, respectively.
According to the results obtained in these previous experiments, 4 mg/liter of amphotericin B (Sigma Aldrich) and 20 mg/liter of vancomycin (Sigma Aldrich) were considered adequate enough to inhibit the contaminant microorganisms while allowing the growth of all Brucella species and strains tested. Thus, the CITA medium was formulated with BAB-CS as a basal component and with the following antibiotic supplements: vancomycin (20 mg/liter), colistin methanesulfonate (7.5 mg/liter), nitrofurantoin (10 mg/liter), nystatin (100,000 IU/liter), and amphotericin B (4 mg/liter) (all from Sigma Aldrich).
Diagnostic performance of CITA medium.
The performance of the new selective culture medium was compared with those of both FM and mTM, using representative reference and field strains (i.e., 38 B. abortus, 35 B. melitensis, 45 B. suis, and 34 B. ovis strains). Adequate suspensions of these strains were cultured in triplicate, and the numbers of CFU per plate in each selective medium and in BAB-CS as a control were determined and statistically compared as described previously (9). Also, the growth or inhibition of contaminant microorganisms was determined for each culture medium after 72 h of incubation at 37°C in a 10% CO2 atmosphere.
In the final step, the performance of CITA medium for direct primary isolation was compared to those of FM and mTM, with the last being supplemented with 4 mg/liter of amphotericin B (Sigma Aldrich) (mTMA). For this purpose, a total of 4,483 fresh field samples were obtained either in vivo (milk, vaginal swabs, and semen) or after necropsy (uterus/epididymides, spleen, and lymph nodes) from (i) rams experimentally infected with B. ovis PA (n = 528 samples), (ii) sheep suspected of B. melitensis infection (n = 3,247 samples), (iii) swine suspected of B. suis infection (n = 602 samples), and (iv) aborted cattle in an area where B. abortus is endemic and cattle are vaccinated with B. abortus RB51 (n = 106 samples) (see Table 4). All of these samples were taken and processed as described previously (2), and the same sample amounts (massively in the case of vaginal swabs and 0.5 ml of tissue homogenate/plate for the remaining samples) were cultured in duplicate plates of CITA and either mTMA or FM (see Table 4). One culture was considered positive when at least one CFU of Brucella was isolated. The suspected Brucella colonies isolated were identified and typed using both standard microbiological (1) and molecular (8) methods. The number of contaminated plates of each selective medium was also recorded.
Table 4.
Numbers of animal samples cultured in CITA and either FM or mTMA and the corresponding Brucella sp. strains isolateda
| Brucella organism(s) detected (no. of isolates) | No. of samples cultured | No. of Brucella strains isolated (no. of contaminated plates) |
||||
|---|---|---|---|---|---|---|
| CITA alone | FM alone | mTMA alone | Both FM and mTM | Total | ||
| B. ovis | 528b | 93 (0) | ND | 50 (22) | 151 (10) | 294 (32) |
| B. melitensis bv. 1 strain Rev1 (28), field strains (331), and bv. 3 (139) | 3,247c | 157 (37) | 39 (45) | ND | 302 (19) | 498 (101) |
| B. suis bv. 2 | 602d | 46 (22) | 16 (14) | ND | 49 (11) | 111 (47) |
| B. abortus bv. 1 strains S19 (1) and RB51 (24) and bv. 3 (7) | 106e | 11 (21) | 0 (13) | ND | 21 (10) | 32 (44) |
See Table 2 for the exact composition of each selective culture medium. mTMA, mTM supplemented with 4 mg/liter of amphotericin B. ND, not determined.
Semen and necropsy samples from rams experimentally infected with B. ovis PA.
Vaginal swabs and milk and necropsy samples from sheep suspected of B. melitensis infection.
Vaginal swabs and milk and necropsy samples from swine suspected of B. suis infection.
Vaginal swabs and milk samples from cattle from areas where B. abortus is endemic and who were vaccinated with RB51.
RESULTS
B. suis studies.
The mean ± SD (n = 45) CFU/plate obtained with representative B. suis strains in FM (11.3 ± 5.7) was significantly lower (P < 0.0001) than those obtained with both mTM (69.4 ± 21) and BAB-CS (69.3 ± 20). The liquid medium assay evidenced that all B. suis strains were highly susceptible to nalidixic acid and bacitracin at concentrations close to and above those contained in FM (data not shown). These results correlated with those obtained in the solid medium assays (Table 3), in which the number of B. suis CFU was significantly inhibited (P < 0.001) by both antibiotics at the concentrations used in FM and at 2-fold higher concentrations (Table 3). In contrast, the remaining antibiotics tested did not show significant inhibitory effects on B. suis.
Table 3.
Susceptibility of B. suis to the different antibiotics contained in FM and mTM
| Antibiotic | Concn | Growth of B. suis (no. of CFU/plate [mean ± SD])a |
|---|---|---|
| Nalidixic acid | 5 mg/liter | 71.7 ± 8.1* |
| 10 mg/liter | 2.9 ± 0.6** | |
| Bacitracin | 25,000 IU/liter | 70.9 ± 6.9* |
| 50,000 IU/liter | 13.8 ± 3.7** | |
| Polymyxin B | 5,000 IU/liter | 81.4 ± 7.9 |
| Cycloheximide | 100 mg/liter | 80.8 ± 7.5 |
| Natamycin | 50 mg/liter | 78.8 ± 7.3 |
| Vancomycin | 20 mg/liter | 74.3 ± 9.8 |
| Nystatin | 100,000 IU/liter | 80.1 ± 7.7 |
| Colistin | 7.5 mg/liter | 79.6 ± 7.9 |
| Nitrofurantoin | 10 mg/liter | 80.7 ± 8.1 |
| Amphotericin B | 4 mg/liter | 80.7 ± 6.6 |
| None (control) | 80.5 ± 7.8 |
Growth of 40 representative B. suis strains (Table 1) in MHA-YE-CS supplemented with the indicated antibiotics at the concentrations used in FM or mTM. Nalidixic acid and bacitracin were also tested at 2-fold higher concentrations than those in FM or mTM. *, P < 0.001; **, P < 0.0001 with respect to MHA-YE-CS agar control plates without the corresponding antibiotic.
Development and performance of CITA selective medium.
B. suis bv. 2 strains were significantly (P < 0.0001) inhibited when they were cultured on GC-CS basal medium (not shown). However, when hemoglobin was added to this basal medium, all B. suis strains grew as in BAB-CS (not shown). Although the difference was not significant, the BMB-CS basal medium negatively affected the growth of B. ovis (not shown). Thus, both media were discarded as basal components for formulating the new CITA medium. In contrast, both BAB-CS and TSA-YE-CS media showed similar performances (in terms of CFU numbers and colonial morphology and size) in supporting the growth of all Brucella species (not shown). For practical reasons, availability, and ease of preparation, BAB-CS was selected as the basal medium for CITA formulation.
The antibiotics contained in mTM (Table 2) allowed the growth of A. niger and E. faecalis while inhibiting the remaining contaminant microorganisms tested (not shown). However, A. niger was inhibited when 4 or 5 mg/liter (but not 2.5 mg/liter) of amphotericin B was added to either mTM or BAB-CS (not shown). Moreover, E. faecalis growth was fully inhibited when 20 mg/liter (but not lower concentrations) of vancomycin was added to either mTM or BAB-CS. Therefore, in addition to the antibiotics contained in mTM (Table 2), amphotericin B (4 mg/liter) and vancomycin (20 mg/liter) were selected as the antibiotic combination of choice for the definitive formulation of the new CITA selective medium.
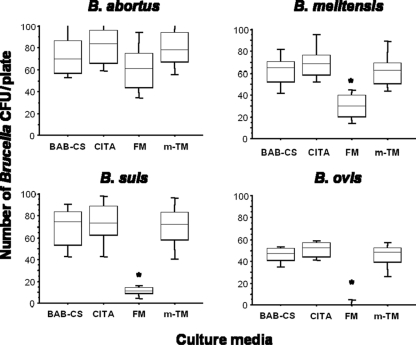
The comparative efficacies of mTM, FM, and CITA medium to support the growth of the different Brucella species and biovars are shown as a box-plot graph in Fig. 1. Boxes represent the two central quartiles, the data between whiskers are 90% of the data obtained for each Brucella species (Table 1) in each culture medium, and horizontal lines inside boxes indicate the median values (CFU/plate) obtained. As expected, FM fully inhibited the growth of B. ovis and significantly reduced the growth of B. suis and B. melitensis (P < 0.0001), as well as that of some strains of B. abortus (not evident in Fig. 1), with respect to that on BAB-CS control medium. Moreover, both CITA medium and mTM were as effective as BAB-CS medium without added antibiotics at supporting the growth of all Brucella species and biovars tested (Fig. 1). In testing the ability of these culture media to inhibit the growth of contaminants, both FM and CITA medium fully inhibited the standard contaminant microorganisms tested, while mTM allowed the growth of A. niger and E. faecalis (results not shown).
Fig. 1.
Growth (number of CFU/plate) of representative Brucella species (see Table 1 for descriptions) on BAB-CS and three selective culture media: mTM (15), FM (15), and CITA (the new selective medium developed in this work). Statistical comparisons of means (numbers of CFU/plate) obtained for a given Brucella sp. for the different culture media were performed by Fisher's PLSD test. *, P < 0.0001 between FM and any other culture medium.
The comparative diagnostic performances of the three selective culture media for isolating Brucella from field samples are summarized in Table 4. The number of culture plates discarded due to the presence of overgrowing contaminants was lower for CITA medium than for mTM for samples from B. ovis-exposed rams but was similar to that for FM for the remaining samples. Regardless of the Brucella species considered, the new CITA medium showed a higher overall diagnostic sensitivity than the other selective media compared (Table 4).
DISCUSSION
The use of selective culture media is needed to increase the probability of success of bacterial culture, and it is compulsory for the adequate bacteriological diagnosis of brucellosis. The different studies conducted with the several selective culture media described until now (1, 3, 5, 7, 10, 11, 12, 13, 15, 16, 22, 24, 25, 26, 27) have concluded that the simultaneous use of both FM and mTM results in maximal sensitivity to isolate the most relevant Brucella species and biovars from field veterinary samples (21). FM is probably the most widely used selective medium for isolating Brucella, as it inhibits most contaminants present in field veterinary samples. However, some of the antibiotics contained in this medium are also highly inhibitory for B. ovis and some B. melitensis and B. abortus strains, including the RB51 vaccine strain (11, 15). In the cases of B. abortus and B. melitensis, this inhibitory effect is due mainly to the presence in FM of nalidixic acid and bacitracin at concentrations close to or higher than (Table 3) those allowing the growth of all strains of these Brucella species (15). The results obtained here confirm the above findings showing that FM fully inhibits the growth of B. ovis and significantly reduces the growth of B. melitensis, and moreover, they demonstrate that it is also highly inhibitory for B. suis (Fig. 1). Despite a somewhat reduced growth of B. abortus in FM (Fig. 1), the differences obtained were not statistically significant in this study.
As indicated above, FM is the preferred culture medium for the primary isolation of B. abortus from contaminated sources. Due to the presence of calf serum (7), this medium is very effective for isolating serum-dependent strains of B. abortus bv. 2 (6, 7). The presence of cycloheximide in the antibiotic supplement makes this medium highly effective for inhibiting the growth of fungi, which are among the main contaminants found in field veterinary samples. This agent inhibits the translation of mRNA by ribosomes, preventing protein synthesis of fungi. However, this drug has potentially toxic effects on mammalian cells, including teratogenesis, DNA damage, birth defects, sperm toxicity, and several others (20). The existence of these risks has resulted in the increasingly reduced availability and use of cycloheximide in recent years. Natamycin has been proposed as an alternative to cycloheximide for inclusion in commercially available antibiotic supplements for Brucella selective media (26). In fact, an antibiotic supplement containing natamycin is currently available (Oxoid), although it has been reported that this drug is somewhat less inhibitory than cycloheximide for fungal growth (26). Amphotericin B has been proposed as a safer and cheaper antifungal agent and has been recommended as a component of culture media for the primary isolation of Mycobacterium spp. (4) and Campylobacter spp. (17). This drug interacts with ergosterol, a steroid present in fungal membranes, and results in a loss of both membrane selective permeability and cytoplasmic components. However, amphotericin B does not affect bacteria, with the exception of Mycoplasma species. As demonstrated here, amphotericin B at 4 mg/liter was inhibitory for the reference fungal contaminants tested but did not affect the growth of B. suis (Table 3) or the remaining Brucella species tested when it was incorporated into the new CITA formulation (Fig. 1).
Altogether, the results of the different experiments allowed us to select the most adequate components to formulate a new selective medium (CITA) suitable for the isolation of all Brucella species. In addition to a suitable antibiotic combination (Table 2), this new medium contains BAB-CS as the basal component, resulting in a translucent medium, which is important for facilitating the presumptive identification of brucellae by assessing colony morphology under stereoscopic magnification (1). The addition of serum is also relevant, since this component allows the growth of the most fastidious Brucella strains, including B. ovis. These components of the CITA medium were proven highly effective in the final experiment assessing its diagnostic performance on a large collection of animal field samples (Table 4).
Both FM and mTM have been reported as highly inhibitory for the B. abortus RB51 vaccine strain (10, 11). Our results agree with these reports, since FM inhibited over half of the B. abortus strains isolated, most of which were RB51 (Table 4). In conclusion, the new CITA selective culture medium should be considered a useful tool for successful isolation of all Brucella species from field veterinary samples. However, the simultaneous use of CITA plus FM or mTMA results in the best diagnostic performance for isolating B. melitensis and B. suis or B. ovis.
ACKNOWLEDGMENTS
This work was supported in part by MICINN-CICYT, Spain (AGL2008-04514-C03-01 and AGL2010-20247), and by the joint Costa Rica-Spain Bilateral Cooperation (CRUSA-CSIC) (2008CR0006). Contract support for P.M.M. from the Instituto Nacional de Investigaciones Agrarias (INIA) of Spain (DR08-0064) and the Grupo Consolidado de Brucelosis (A-14) of the Aragón Government is also gratefully acknowledged.
Footnotes
Published ahead of print on 26 January 2011.
REFERENCES
- 1. Alton G. G., Jones L. M., Angus R. D., Verger J. M. 1988. Techniques for the brucellosis laboratory. INRA, Paris, France [Google Scholar]
- 2. Barrio M. B., et al. 2009. Rough mutants defective in core and O-polysaccharide synthesis and export induce antibodies reacting in an indirect ELISA with smooth lipopolysaccharide and are less effective than Rev 1 vaccine against Brucella melitensis infection of sheep. Vaccine 27:1741–1749 [DOI] [PubMed] [Google Scholar]
- 3. Brown G. M., Ranger C. R., Kelley D. J. 1971. Selective media for the isolation of Brucella ovis. Cornell Vet. 61:265–280 [PubMed] [Google Scholar]
- 4. Drancourt M., Raoult D. 2007. Cost-effectiveness of blood agar for isolation of Mycobacteria. PLoS Negl. Trop. Dis. 1:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ewalt D. R., Packer R. A., Harris S. K. 1983. An improved selective medium for isolating Brucella sp. from bovine milk, p. 577–589 In Proceedings of the Third International Symposium of the World Association of Veterinary Laboratory Diagnosticians College of Veterinary Medicine, Iowa State University, Ames, IA [Google Scholar]
- 6. Farrell I. D., Robertson L. 1972. A comparison of various selective media, including a new selective medium for the isolation of brucellae from milk. J. Appl. Bacteriol. 35:625–630 [DOI] [PubMed] [Google Scholar]
- 7. Farrell I. D. 1974. The development of a new selective medium for the isolation of Brucella abortus from contaminated sources. Res. Vet. Sci. 16:280–286 [PubMed] [Google Scholar]
- 8. Garcia-Yoldi D., et al. 2006. Multiplex PCR assay for the identification and differentiation of all Brucella species and the vaccine strains Brucella abortus S19 and RB51 and Brucella melitensis Rev1. Clin. Chem. 52:779–781 [DOI] [PubMed] [Google Scholar]
- 9. Grilló M. J., et al. 2006. Increases of efficacy as vaccine against Brucella abortus infection in mice by simultaneous inoculation with avirulent smooth bvrS/bvrR and rough wbkA mutants. Vaccine 24:2910–2916 [DOI] [PubMed] [Google Scholar]
- 10. Her M., et al. 2010. The development of a selective medium for the Brucella abortus strains and its comparison with the currently recommended and used medium. Diagn. Microbiol. Infect. Dis. 67:15–21 [DOI] [PubMed] [Google Scholar]
- 11. Hornsby R. L., Jensen A. E., Olsen S. C., Thoen C. O. 2000. Selective media for isolation of Brucella abortus strain RB51. Vet. Microbiol. 73:51–60 [DOI] [PubMed] [Google Scholar]
- 12. Jones L. M. 1958. A preliminary report on a selective medium for the culture of Brucella, including fastidious types. Bull. World Health Organ. 19:200–203 [PMC free article] [PubMed] [Google Scholar]
- 13. Kuzdas C. D., Morse E. V. 1953. A selective medium for the isolation of brucellae from contaminated materials. J. Bacteriol. 66:502–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marín C. M., Jiménez-de-Bagüés M. P., Barberán M., Blasco J. M. 1996. Comparison of two selective media for the isolation of Brucella melitensis from naturally infected sheep and goats. Vet. Rec. 138:409–411 [DOI] [PubMed] [Google Scholar]
- 15. Marín C. M., Alabart J. L., Blasco J. M. 1996. Effect of antibiotics contained in two Brucella selective media on growth of Brucella abortus, Brucella melitensis, and Brucella ovis. J. Clin. Microbiol. 34:426–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin J. E., Billings T. E., Hackney J. F., Thayer J. D. 1967. Primary isolation of N. gonorrhoeae with a new commercial medium. Public Health Rep. 82:361–364 [PMC free article] [PubMed] [Google Scholar]
- 17. Martin K. W., Mattick K. L., Harrison M., Humphrey T. J. 2002. Evaluation of selective media for Campylobacter isolation when cycloheximide is replaced with amphotericin B. Lett. Appl. Microbiol. 34:124–129 [DOI] [PubMed] [Google Scholar]
- 18. Moreno E., Moriyón I. 2002. Brucella melitensis: A nasty bug with hidden credentials for virulence. Proc. Natl. Acad. Sci. U. S. A. 99:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moreno E., Moriyón I. 2006. The genus Brucella, p. 315–456 In Falkow S., et al. (ed.), The prokaryotes, vol. 5 Springer-Verlag, New York, NY [Google Scholar]
- 20. Murray P. R., et al. (ed.). 2003. Manual of clinical microbiology, 8th ed., vol. 1, p. 1212 ASM Press, Washington, DC [Google Scholar]
- 21. OIE 2009. Manual of diagnostic tests and vaccines for terrestrial animals, mammals, birds and bees, 6th ed., p. 589–597 World Organisation for Animal Health, Paris, France [Google Scholar]
- 22. Painter G. M. 1966. Comparison of several media for the isolation of Brucella. Can. J. Comp. Med. Vet. Sci. 30:218–223 [PMC free article] [PubMed] [Google Scholar]
- 23. Pappas G., Akritidis N., Bosilkovski M., Tsianos E. 2005. Medical progress: brucellosis. N. Engl. J. Med. 352:2325–2336 [DOI] [PubMed] [Google Scholar]
- 24. Robertson F. J., Farrell I. D., Hinchliffe P. M. 1977. The isolation of brucellae from contaminated sources. A review. Br. Vet. J. 133:193–200 [DOI] [PubMed] [Google Scholar]
- 25. Ryan W. J. 1967. A selective medium for the isolation of Brucella abortus from milk. Bull. Minist. Health 26:33–39 [PubMed] [Google Scholar]
- 26. Stack J. A., Harrison M., Perrett L. L. 2002. Evaluation of a selective medium for Brucella isolation using natamycin. J. Appl. Microbiol. 92:724–728 [DOI] [PubMed] [Google Scholar]
- 27. Thayer J. D., Martin J. E. 1966. Improved medium selective for cultivation of N. gonorrhoeae and N. meningitidis. Public Health Rep. 81:559–562 [PMC free article] [PubMed] [Google Scholar]