Abstract
We report the first fatal case of Campylobacter rectus infection due to a subdural empyema and ruptured mycotic intracranial aneurysm and two cases of limb-threatening C. rectus necrotizing soft tissue and bone infection and empyema thoracis that responded to amoxicillin-clavulanate and surgical debridement and drainage. All three strains were identified by 16S rRNA sequencing.
CASE REPORTS
Case 1.
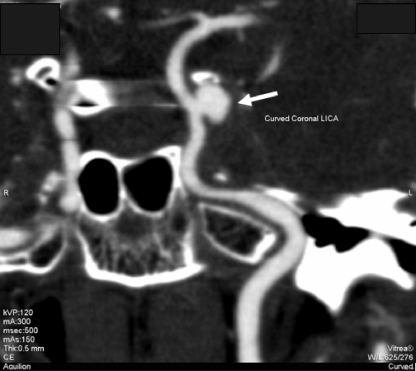
A previously healthy 41-year-old Indonesian woman was admitted to the hospital in July 2006 because of left frontal headache, left eye ptosis, and diplopia lasting for 2 weeks. On admission, she was afebrile. Examination revealed palsy of the left side of the third, fourth, and sixth cranial nerves with pupil involvement. Blood tests showed leukocytosis with neutrophil predominance (white cell count, 13.0 × 109/liter; 90% neutrophils), normal hemoglobin level (12.0 g/dl) and platelet count (378 × 109/liter), hyperglycemia (random glucose, 20.9 mmol/liter), and elevated erythrocyte sedimentation rate (ESR) (55 mm/h) and C-reactive protein (CRP) level (43.6 mg/liter). Renal and liver function tests were normal. A contrast cranial computed tomography (CT) scan with angiography showed a 9- by 6- by 6-mm laterally pointing saccular aneurysm over the cavernous portion of the left internal carotid artery (Fig. 1). Urgent percutaneous coil embolization of the aneurysm was performed, with a postembolization angiogram showing obliteration of the aneurysmal sac. The patient's headache initially improved; however, 10 days later, her headache recurred and she developed fever. A cranial CT scan showed the presence of blood in the interhemispheric fissure and supratentorial regions, with thin rims of hyperdensity present in the left temporal and occipital regions, suggesting a subdural hematoma. A digital subtraction angiogram showed two new aneurysms arising from the cavernous part of the left internal carotid artery. Transthoracic echocardiography revealed good left ventricular function with no vegetations. Parent artery occlusion of the cervical part of the left internal carotid artery by coil embolization was performed. A blood culture was performed, and empirical treatment with intravenous vancomycin plus ceftriaxone was initiated. Her condition did not improve, and repeated cranial CT scans showed an increase in size of the left subdural collection causing midline shift. A decompressive craniotomy was performed, and foul-smelling pus mixed with old blood was noted. A craniectomy with evacuation of the residual subdural empyema was performed. Empirical treatment with intravenous metronidazole was added. Unfortunately, the patient's condition continued to worsen, and she died from transtentorial herniation 3 weeks after admission. The anaerobic blood culture bottle eventually yielded a mixed growth of Porphyromonas gingivalis and an unidentified anaerobic Gram-negative rod (strain 1); culture of the subdural pus also grew the same unidentified organism as well as Propionibacterium acnes.
Fig. 1.
CT angiogram of brain showing a laterally pointing saccular aneurysm over the cavernous portion of the left internal carotid artery (arrow).
Case 2.
A previously healthy 64-year-old Chinese man was admitted to the hospital in November 2009 because of right anterior thigh pain lasting for 2 weeks. On admission, he was afebrile, and physical examination revealed some local tenderness over the proximal right thigh without any signs of inflammation. His oral hygiene was poor, with gingivitis and multiple dental caries. An X ray of the right femur showed osteolytic lesions involving the right proximal femoral shaft. He had an elevated white cell count (15.9 × 109/liter) with neutrophil predominance (79%) and elevated levels of alkaline phosphatase (161 IU/liter) and alanine aminotransferase (63 IU/liter), while renal function tests and serum glucose were normal. Serum inflammatory markers were elevated (ESR, 66 mm/h; CRP, 237 mg/liter). Tumor markers, including alpha-fetoprotein, carcinoembryonic antigen, and prostate-specific antigen, were all within normal limits. No serum paraprotein or urinary Bence-Jones protein was detected. The patient experienced sudden worsening in right thigh pain and swelling after admission. A CT scan showed a fracture of the right femoral shaft, with abnormal air densities within the intermuscular septa, subcutaneous layer, and medullary cavity of the fractured femur. A rim-enhancing intramuscular fluid collection was also noted. Blood culture was performed, and empirical treatment with intravenous amoxicillin-clavulanate plus ciprofloxacin was started. An emergency above-knee amputation of the right leg was performed; intraoperative findings included a pathological fracture of the right femur, a large amount of purulent material over the subcutaneous and muscle layers, and necrosis of the deep fascia and muscular tissues. A blood culture grew Porphyromonas gingivalis, while cultures of the necrotic tissue and pus of the right thigh grew the same organism as well as diphtheroid bacilli and coagulase-negative staphylococci. Subsequently, an unidentified anaerobic Gram-negative rod (strain 2) was also recovered from the tissue culture. The patient was continued on amoxicillin-clavulanate and received multiple surgical operations. He eventually recovered and was discharged after 59 days of hospitalization. Antibiotic treatment was continued orally for an additional month. He was referred to the dentist for further management of his poor dental condition. A follow-up CT scan showed no residual collection or gas pockets.
Case 3.
A previously healthy 56-year-old Chinese man was admitted to the hospital in December 2009 because of right pleuritic chest pain and malaise lasting for 1 week. He had a productive cough, progressive shortness of breath, and weight loss of 9 kg in the previous 4 weeks. On admission, he was afebrile. His oral hygiene was poor, with gingivitis and multiple dental caries. There were percussion dullness and decreased breath sounds over his right chest. Chest radiography showed near-complete opacification of his right lung field. Blood tests showed leukocytosis with neutrophil predominance (white cell count, 30.3 × 109/liter, 90% neutrophils), normochromic normocytic anemia (hemoglobin, 7.8 g/dl), thrombocytosis (platelet count, 644 × 109/liter), hyperglycemia (serum glucose, 20.9 mmol/liter), hypoalbuminemia (albumin, 19 g/liter), and raised alkaline phosphatase (144 IU/liter) and serum creatinine (120 μmol/liter) levels. Blood and sputum cultures were performed, and empirical treatment with intravenous amoxicillin-clavulanate was started. A thoracic CT scan with contrast revealed a huge empyema occupying most of the right lung with collapse/consolidation. A chest drain was inserted, with 2.1 liters of pus drained. Blood culture grew Streptococcus constellatus, while sputum cultures yielded only mixed oral flora. Cultures of the pus grew Fusobacterium nucleatum and an unidentified anaerobic Gram-negative rod (strain 3). Intravenous amoxicillin-clavulanate and drainage of the empyema were both continued, and the patient was eventually discharged after 29 days of hospitalization. He was referred to a dentist for further management of his poor dental condition. Antibiotic treatment was continued orally for an additional month.
Microbiological data.
Clinical specimens were collected and handled according to standard protocols (6). All suspicious colonies were identified by standard conventional biochemical methods (6). The three strains of unidentified anaerobic Gram-negative rods grew on blood agar as tiny nonhemolytic gray colonies after 7 days of incubation at 37°C in an anaerobic environment but did not grow in ambient air or 5% CO2. Gram smears of the colonies showed small, pleomorphic Gram-negative rods, with some showing a curved appearance. The organisms were catalase negative, oxidase positive, and motile. They did not yield any recognizable biochemical profile when subjected to routine identification by systems such as the API 20A (bioMérieux, Marcy-I'Etoile, France) and the Vitek 2 ANC card (bioMérieux, Marcy-I'Etoile, France).
16S rRNA gene sequencing.
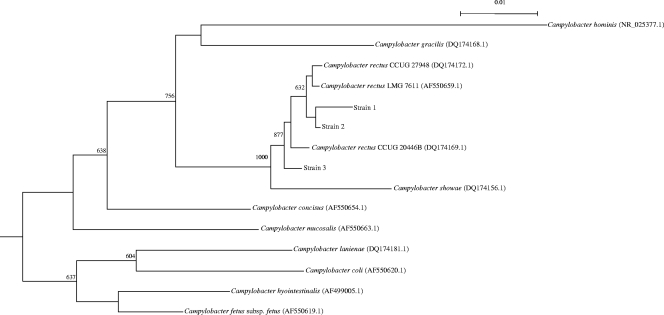
Bacterial DNA extraction, PCR amplification, and DNA sequencing of the 16S rRNA gene of the three anaerobic Gram-negative rods were performed according to our previous publications (17, 18), using LPW57 (5′-AGTTTGATCCTGGCTCAG-3′) and LPW182 (5′-AGTCGCTGATTCCACTGTGG-3′) (Sigma-Proligo, Singapore) as the PCR and sequencing primers. DNase I-treated distilled water was used as the negative control. The sequences of the PCR products were compared with sequences of closely related species in GenBank by multiple sequence alignment using ClustalX 1.83 (15). Phylogenetic relationships were determined using the neighbor-joining method. Sequencing of the 16S rRNA genes of the three isolates showed that they were of different lengths (1,354 bp, 1,551 bp, and 1,562 bp, respectively). Intervening sequences of 189 bp, identical to those of reported strains of Campylobacter rectus, were present in two of the three isolates (PMID 16627635). With the intervening sequences excluded, there were 5 to 17 (0.38 to 1.29%) base differences between the 16S rRNA gene sequences of the three isolates and those of other strains of Campylobacter rectus (GenBank accession no. AF550659.1, DQ174169.1, and DQ174172.1), 25 to 34 (1.90 to 2.58%) base differences between the 16S rRNA gene sequences of the three isolates and that of Campylobacter showae (GenBank accession no. DQ174156.1), 58 to 67 (4.40 to 5.08%) base differences between the 16S rRNA gene sequences of the three isolates and that of Campylobacter concisus (GenBank accession no. AF550654.1), 60 to 67 (4.55 to 5.08%) base differences between the 16S rRNA gene sequences of the three isolates and that of Campylobacter gracilis (GenBank accession no. DQ174168.1), and 71 to 78 (5.38 to 5.91%) base differences between the 16S rRNA gene sequences of the three isolates and that of Campylobacter mucosalis (GenBank accession no. AF550663.1), indicating that the three isolates were strains of C. rectus (Fig. 2).
Fig. 2.
Phylogenetic tree showing the relationships of the anaerobic Gram-negative rods isolated from the three patients in this study and closely related species. The tree was inferred from 16S rRNA data by the neighbor-joining method and rooted using the 16S rRNA gene sequence of Bacteroides ureolyticus (GenBank accession no. FN401327.1). Bootstrap values were calculated from 1,000 trees. The scale bar indicates the estimated number of substitutions per 100 bases. Names and accession numbers are given as cited in the GenBank database.
Literature search.
We conducted a search of the literature in PubMed using the keywords “Campylobacter rectus” and “Wolinella recta” in combination with “human infection” through May 2010. All the original papers were retrieved, and the clinical details and patient outcomes were noted.
Campylobacter rectus was first described in 1981 as Wolinella recta, a small, unbranched, straight, nonsporulating, anaerobic, Gram-negative rod with rapid darting motility by means of a single, polar flagellum. Some strains can grow in the presence of 5% O2 but not in air enriched with 10% CO2 (14). The organism was transferred to the genus Campylobacter in 1991 based on phylogenetic studies (16). C. rectus is a member of the human oral flora and has been found in areas such as the periodontal sulcus, tongue, cheek mucosa, and saliva (4, 9). To date, five other Campylobacter species (C. concisus, C. curvus, C. gracilis, C. showae, and C. sputorum) have been isolated from the human oral cavity (10). C. rectus is associated with human periodontal disease, being present in higher numbers in diseased than in healthy subgingival sites (10, 12). One report described a case of oral abscess with coisolation of alpha-hemolytic streptococci in a patient with underlying gastroesophageal carcinoma (11). In another study, C. rectus was isolated from infected skin and a soft tissue bite wound in humans, showing its propensity to cause localized disease in extraoral sites (7). Only three cases of invasive C. rectus infections have been reported in the literature, and all three patients survived (5, 8, 13). In this article, we report three cases of severe C. rectus infections, with one being fatal.
Our current report presents the first two cases of limb- and life-threatening C. rectus infections in the literature, with one patient who suffered from necrotizing soft tissue and bone infection requiring above-knee amputation and another patient who died of transtentorial herniation due to a subdural empyema from a ruptured mycotic aneurysm. Among the six cases of invasive C. rectus infections, five had underlying immunocompromising conditions, including chronic alcoholism, postchemotherapy neutropenia, and undiagnosed diabetes mellitus (Table 1). Poor oral hygiene also appeared to be an important predisposing factor, being present in three (50%) of the six cases (Table 1). It is possible that chronic dental sepsis acted as a source of the organism in some cases, leading to subsequent spread of the infection to other parts of the body. It is also noteworthy that in culture, C. rectus was frequently coisolated with other oral flora, many of which may also contribute to abscess formation. Bostanci et al. have shown that the cytokine production from human monocytes induced by C. rectus was suppressed by Porphyromonas gingivalis, suggesting that mixed infections may impair host immune responses (2, 3). The interactions between C. rectus and other bacteria in the pathogenesis of invasive infection deserve further investigations.
Table 1.
Summary of reported cases of invasive Campylobacter rectus infections
| Case | Reference | Sexa/age (yr) | Underlying condition or risk | Diagnosis | Other bacteria isolated | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | Spiegel and Telford (13) | M/62 | Poor oral hygiene, alcoholism (with history of losing consciousness) | Left chest wall abscess with pleural and extrapleural components and rib destruction | Actinomyces viscosus | Drainage, antibiotics (penicillin) | Discharged after 38 days of hospitalization |
| 2 | Han et al. (8) | F/32 | Lymphoma, neutropenic, nipple piercing | Left breast abscess | Non-group A beta-hemolytic streptococci | Drainage, antibiotics (vancomycin, clindamycin, aztreonam) | Discharged after clinical remission |
| 3 | de Vries et al. (5) | M/24 | History of chronic otitis media, meningoradiculitis of unknown cause | Vertebral abscess | Actinomyces species, Eubacterium brachy | Hemilaminectomy, antibiotic (clindamycin) | Discharged after 2 weeks of hospitalization |
| 4 | Present case 1 | F/41 | Hyperglycemia | Ruptured mycotic intracranial aneurysm with subdural empyema | Porphyromonas gingivalis, Propionibacterium acnes | Percutaneous arterial embolization, drainage, antibiotics (vancomycin, ceftriaxone, metronidazole) | Died from transtentorial herniation |
| 5 | Present case 2 | M/64 | Poor oral hygiene | Necrotizing soft tissue infection of right thigh | Diphtheroid bacilli, coagulase-negative staphylococci, Porphyromonas gingivalis | Above-knee amputation, surgical debridement, antibiotic (amoxicillin- clavulanate) | Discharged after 59 days of hospitalization |
| 6 | Present case 3 | M/56 | Poor oral hygiene, hyperglycemia | Right empyema thoracis | Streptococcus constellatus, Fusobacterium nucleatum | Drainage, antibiotic (amoxicillin- clavulanate) | Discharged after 29 days of hospitalization |
M, male; F, female.
Treatment of complicated infections due to C. rectus usually required surgical drainage together with prolonged courses of antibiotic therapy. There was no standardized antibiotic regimen, but β-lactam antibiotics and clindamycin were commonly used with success. Antibiotic susceptibility data for C. rectus is scarce, and there are no currently accepted guidelines for testing and interpretation of results. Based on the limited studies, C. rectus has been shown to be susceptible to amoxicillin-clavulanate, cefoxitin, clindamycin, imipenem, levofloxacin, and metronidazole. β-Lactamase activity was not detected in one study (1). We have performed limited susceptibility testing on our three isolates, and the MICs for all the isolates to amoxicillin-clavulanate were low, as determined by the Etest method (AB Biodisk, Sweden) (MIC, 0.064 μg/ml). As two of the three patients who received amoxicillin-clavulanate together with surgical drainage had satisfactory clinical outcomes, we propose that combinations of β-lactam and β-lactamase inhibitor antibiotics may be a reasonable therapeutic choice for treating invasive infections causing by C. rectus. Referral for dental assessment to eradicate any dental source of the organism is also advisable.
The present report highlights the role of 16S rRNA sequencing in gaining a better understanding of the epidemiology and disease spectrum of bacteria such as C. rectus, which are rarely encountered in clinical microbiology laboratories. None of the three isolates could be identified by phenotypic methods or commercial identification systems and were unambiguously identified as C. rectus only by 16S rRNA gene sequencing. Of the three isolates in previously reported cases of invasive infections, two were also identified using this technique (5, 8), and an intervening sequence was also present in the 16S rRNA gene in one of them (8), similar to the findings for two of our three isolates. We speculate that invasive infections associated with C. rectus may be underestimated as a result of its fastidious growth and the difficulties in identifying this bacterium to the species level in clinical microbiology laboratories. As 16S rRNA gene sequencing techniques become more readily available, more cases of C. rectus infections may be recognized.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences of the three isolates have been deposited in the GenBank sequence database under accession numbers HQ890329 to HQ890331.
Acknowledgments
This work was supported partly by the HKSAR Research Fund for the Control of Infectious Diseases of the Health, Welfare and Food Bureau and by the University Development Fund, The University of Hong Kong. We thank Sally Leung, Kit-Wah Leung, and Hazel Yeung for technical help.
Footnotes
Published ahead of print on 26 January 2011.
REFERENCES
- 1. Blandino G., et al. 2007. Antimicrobial susceptibility and beta-lactamase production of anaerobic and aerobic bacteria isolated from pus specimens from orofacial infections. J. Chemother. 19:495–499 [DOI] [PubMed] [Google Scholar]
- 2. Bostanci N., et al. 2007. Porphyromonas gingivalis antagonises Campylobacter rectus induced cytokine production by human monocytes. Cytokine 39:147–156 [DOI] [PubMed] [Google Scholar]
- 3. Bostanci N., et al. 2007. Interleukin-1alpha stimulation in monocytes by periodontal bacteria: antagonistic effects of Porphyromonas gingivalis. Oral Microbiol. Immunol. 22:52–60 [DOI] [PubMed] [Google Scholar]
- 4. Cortelli J. R., et al. 2008. Etiological analysis of initial colonization of periodontal pathogens in oral cavity. J. Clin. Microbiol. 46:1322–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Vries J. J. C., Arents N. L. A., Manson W. L. 2008. Campylobacter species isolated from extra-oro-intestinal abscess: a report of four cases and literature review. Eur. J. Med. Microbiol. Infect. Dis. 27:1119–1123 [DOI] [PubMed] [Google Scholar]
- 6. Fitzgerald C., Nachamkin I. 2007. Campylobacter and Arcobacter, p. 933–946 In Murray P. R., Baron E. J., Jorgensen J. H., Landry M. L., Pfaller M. A. (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC [Google Scholar]
- 7. Goldstein E. J., et al. 2001. Comparative in vitro activity of ertapenem and 11 other antimicrobial agents against aerobic and anaerobic pathogens isolated from skin and soft tissue animal and human bite wound infections. J. Antimicrob. Chemother. 48:641–651 [DOI] [PubMed] [Google Scholar]
- 8. Han X. Y., Tarrand J. J., Rice D. C. 2005. Oral Campylobacter species involved in extraoral abscess: a report of three cases. J. Clin. Microbiol. 43:2513–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Könönen E., et al. 2007. Population-based study of salivary carriage of periodontal pathogens in adults. J. Clin. Microbiol. 45:2446–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Macuch P. J., Tanner A. C. R. 2000. Campylobacter species in health, gingivitis, and periodontitis. J. Dent. Res. 79:785–792 [DOI] [PubMed] [Google Scholar]
- 11. Mahlen S. D., Clarridge J. E., III 2009. Oral abscess caused by Campylobacter rectus: case report and literature review. J. Clin. Microbiol. 47:848–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moore W. E., et al. 1983. Bacteriology of moderate (chronic) periodontitis in mature adult humans. Infect. Immun. 42:510–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spiegel C. A., Telford G. 1984. Isolation of Wolinella recta and Actinomyces viscosus from an actinomycotic chest wall mass. J. Clin. Microbiol. 20:1187–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tanner A. C. R., et al. 1981. Wolinella gen. nov., Wolinella succinogenes (Vibrio succinogenes Wolin et al.) comb. nov., and description of Bacteroides gracilis sp. nov., Wolinella recta sp. nov., Campylobacter concisus sp. nov., and Eikenella corrodens from humans with periodontal disease. Int. J. Syst. Bacteriol. 31:432–445 [Google Scholar]
- 15. Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vandamme P., et al. 1991. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: emendation of generic descriptions and proposal of Arcobacter gen. nov. Int. J. Syst. Bacteriol. 41:88–103 [DOI] [PubMed] [Google Scholar]
- 17. Woo P. C. Y., et al. 2009. First reports of Tsukamurella keratitis: association between T. tyrosinosolvens and T. pulmonis and ophthalmologic infections. J. Clin. Microbiol. 47:1953–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Woo P. C. Y., et al. 2002. Thermo-tolerant Campylobacter fetus bacteraemia identified by 16S ribosomal RNA gene sequencing: an emerging pathogen in immunocompromised patients. J. Med. Microbio. 51:740–746 [DOI] [PubMed] [Google Scholar]