Abstract
PCR assays designed for the diagnosis of invasive aspergillosis (IA) in high-risk patients have to detect minute amounts of target DNA to reach sufficient analytical sensitivity to be of clinical use. This prospective study assessed the use of a novel strategy for selective pathogen DNA enrichment for enhancing the performance of diagnostic PCR in a direct comparison with a highly sensitive in-house quantitative PCR (qPCR) assay and the galactomannan enzyme-linked immunosorbent assay (ELISA). Surprisingly, and in contrast to experience with other patient groups, the novel protocol for selective pathogen DNA enrichment did not enhance but instead significantly impaired sensitivity. This could be explained by the small amounts of host DNA in the specimens, which were derived mostly from severely neutropenic patients. In the qPCR assay, positive samples required an average of 43.5 amplification cycles (range, 39.2 to 50) for detection in the in-house PCR. Repetitive testing of selected samples showed test positivity to be variable, most likely due to the small amounts of target DNA. Despite this, the in-house protocol proved helpful in the diagnosis of IA, detecting 2 out of 3 patients with probable IA and 10 out of 19 patients with possible IA. Our results underline the necessity for diagnostic PCR protocols that help diagnose IA to be highly sensitive and show that selective pathogen DNA enrichment using affinity purification may not be useful in severely neutropenic patients.
INTRODUCTION
Despite invasive aspergillosis (IA) being the most common cause of infection-related mortality in patients being treated for hematological malignancies (8), diagnosis of this infectious disease is still a clinical challenge. Because radiology and classical microbiology, including detection of galactomannan (GM) antigen, have varied sensitivities and specificities, the detection of fungal DNA in patient samples has long been considered an attractive diagnostic option. Consequently, several PCR assays have been described as possible diagnostic tools for the detection of IA. However, most of them were evaluated only with spiked samples or a very limited number of patient-derived specimens (7). Furthermore, there is currently no consensus about the definition of a standardized molecular assay for the diagnosis of IA, and protocols in use vary widely, which is the main reason why PCR assays are still not included in the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria for the diagnosis of IA (4, 7). Recently, some initiatives dedicated to the standardization of real-time PCR and, in particular, to the definition of a diagnostic IA PCR standard have been taken (3, 10). A major obstacle in the design of molecular assays for the diagnosis of IA is the fact that the pathogen load in clinical specimens, especially in blood, is relatively low (1, 6), even in patients with proven or probable disease. Thus, there is a need to develop extremely sensitive assays. This has been achieved mainly by targeting multicopy genes, such as the ribosomal gene cluster, and with the use of new technology, such as molecular-beacon-based assays or assays based on nucleic acid sequence-based amplification (10–12). A novel protocol for selective pathogen DNA enrichment based on affinity purification has successfully been used for the diagnosis of sepsis (9). This protocol is based on affinity purification of unmethylated microbial DNA due to selective binding to a protein. However, there are currently no data on the use of selective pathogen DNA enrichment for the diagnosis of IA in neutropenic patients. In this study, we compared selective pathogen DNA enrichment combined with a standard PCR assay with an in-house DNA extraction and real-time PCR protocol, using prospectively collected blood specimens (n = 536) from high-risk hematological-malignancy patients.
MATERIALS AND METHODS
Study design.
Patients after allogeneic stem cell transplantation (allo-SCT) and patients receiving myeloablative chemotherapy with an expected duration of neutropenia (leukocyte count of <1,000/μl) of at least 10 days were included in the study after informed consent was obtained for a maximum of 100 days after allo-SCT or until the termination of neutropenia. Between January and August 2009, blood samples from patients with a high risk of invasive fungal disease, together with clinical data, were collected in order to categorize the patients according to the EORTC/MSG criteria (4). Blood and serum samples were taken twice weekly, with a maximum of 30 blood samples taken per patient. Blood specimens of 3 to 5 ml were used for each of the PCR assays, and serum samples were used for galactomannan quantification. The study was approved by the ethics committee at the University Hospital in Würzburg, Germany.
Assay 1 (bead-based DNA extraction and quantitative PCR [qPCR] protocol). (i) DNA extraction from blood specimens.
All extraction steps were performed under a class 2 laminar-flow cabinet. In each extraction procedure, at least one negative control was included. Extraction performance was monitored by occasional testing of both unidentified and identified simulated positive specimens. Three milliliters of fresh EDTA blood was added to 10 ml of hypotonic red cell lysis buffer (RCLB) (10 mM Tris [pH 7.6], 5 mM MgCl2, 10 mM NaCl) and incubated on a shaker for 5 min. After centrifugation at 3,000 × g for 10 min, the supernatant was removed and the pellet resuspended in 10 ml of RCLB. The red cell lysis and centrifugation were repeated. The supernatant was removed, and the pellet was resuspended in 1 ml of freshly prepared white cell lysis buffer (WCLB) (10 mM Tris [pH 7.6], 10 mM EDTA, 50 mM NaCl, 0.2% SDS, 200 μg of proteinase K per ml) and incubated at 65°C for 30 min. Samples were centrifuged at 5,000 × g for 10 min, and the supernatant was removed. Ceramic beads (MagNA Lyser Green beads; Roche) were added to the pellet and vigorously vortexed for 90 s to lyse the fungal cells. DNA was purified by using the High Pure PCR template preparation kit (Roche) according to the manufacturer's instructions with the following modifications: 200 μl of binding buffer and 50 μl of reconstituted proteinase K were added to the vortexed beads, incubation was prolonged to 15 min at 70°C, and the elution volume was adjusted to 100 μl.
(ii) ITS semiquantitative real-time PCR assay.
Aspergillus-specific oligonucleotide primers and probes were designed to target the multicopy ribosomal operon in the region from internal transcribed spacer 1 (ITS1) to the 5.8S region and were synthesized by TIB Molbiol (Germany). The primer and probe sequences were Asp fum F (5′-GCAGTCTGAGTTGATTATCGTAATC-3′), Fungi5.8_R (5′-CAGGGGGCGCAATGTGC-3′), Fungi5.8_FL (5′-AATGCGATAAGTAATGTGAATTGCAGA-FL-3′, where FL is fluorescein), and Fungi5.8_LC640 (5′-LC Red640-TCAGTGAATCATCGAGTCTTTGAACGC-PH-3′, where PH is phosphate). Real-time PCR (qPCR) amplification was performed in 20 μl using a LightCycler machine (model 1.5; Roche). Each 20-μl reaction mixture contained 2 μl of LightCycler FastStart DNA Master HybProbe (Roche), 62.5 nM Asp fum F, 125 nM Fungi5.8_R, 150 nM (each) probe Fungi5.8_FL and Fungi5.8_LC640, 1.25 μl of dimethyl sulfoxide (DMSO) (Sigma), 4 μl of MgCl2 (25 mM), and 10 μl of extracted DNA. The amplification was carried out as follows: initial Taq polymerase activation was at 95°C for 9 min, followed by 55 cycles of 95°C for 10 s, 54°C for 30 s, and 72°C for 25 s and then melting curve analysis. In each run, negative and positive controls were included. Aspergillus-specific amplification yielded DNA amplicons of 153 bp in length. Different Aspergillus species could be distinguished by their specific melting temperatures: 64°C for DNA of A. fumigatus, A. versicolor, and A. nidulans, 58°C for A. terreus and A. niger, and 54°C for A. flavus. Negative and positive controls were included in each run. PCR mean efficiency was calculated by using the equation 10(−1/slope) − 1.
Assay 1 has only subtle differences from the protocol proposed by the European Aspergillus PCR Initiative (EAPCRI) for Aspergillus-diagnostic PCRs (10). Therefore, the performance of this assay can be expected to fulfill the quality standards of the EAPCRI protocol.
Modified assay 1.
For additional tests, the LightCycler assay was converted into a hydrolysis probe assay to be run on a different cycler. The reason for this is that the diagnostic cyclers, for quality control reasons, may not be used for research applications. The probe was designed using Primer Express software (Applied Biosystems) with probe ITS-PF (5′-FAM-CAGCGAAATGCGATAAGTAATGTGAATTGCA-TAMRA-3′, where FAM is 6-carboxyfluorescein and TAMRA is 6-carboxytetramethylrhodamine). Real-time PCR amplification was performed in a 21-μl mixture using a StepOnePlus machine (Applied Biosystems). Each 21-μl reaction mixture contained 10 μl TaqMan GenEx master mix (Applied Biosystems), 300 nM Asp fum F, 600 nM Fungi5.8_R, 143 nM probe ITS-PF, and 10 μl of extracted DNA. Amplification was carried out as follows: uracil-DNA glycosylase (UNG) activation was at 50°C for 2 min, and the initial Taq polymerase activation was at 95°C for 10 min, followed by 55 cycles of 95°C for 15 s, 54°C for 30 s, and 72°C for 30 s. In each run, negative and positive controls were included. Aspergillus-specific amplification yielded DNA amplicons of 153 bp in length. Each sample was analyzed in triplicate.
Assay 2 (bead-based DNA extraction with pathogen-specific selective DNA enrichment and multiplex PCR). (i) DNA extraction from blood specimens by the protocol provided by the manufacturer of assay 2.
Blood cells (5 ml blood) were mechanically lysed using glass beads in a FastPrep-24 (MP Biomedicals). The blood lysates were loaded onto membranes. DNA bound to the membrane at 99.9% efficiency, allowing removal of PCR inhibitors by subsequent washing. DNA was eluted from the membrane with a high-ionic-strength solution. To obtain the highest purity, the eluted DNA was desalted and concentrated by precipitation. Fungal DNA was enriched via a specific binding matrix based on DNA affinity chromatography using a matrix-immobilized DNA binding protein (Looxster; SIRS Lab) which recognizes characteristic motifs within fungal DNA. When a complex DNA mixture is applied onto the matrix, more than 90% of the human background DNA is removed by two wash steps (9), altering the final ratio of fungal DNA to human DNA, potentially increasing the sensitivity of the downstream protocols.
(ii) Fungal multiplex-PCR assay.
Enriched DNA was amplified by multiplex PCR and detected via gel electrophoresis. The multiplex-PCR assay comprised primer pairs specific for A. fumigatus, Candida krusei, and a panfungal primer (Pan Fungi) set (a commercial assay in development). Amplification was carried out as follows: initial Taq polymerase activation was at 95°C for 15 min, followed by 30 cycles of 94°C for 30 s, 54°C for 90 s, and 72°C for 45 s and a final extension at 72°C for 10 min. These conditions were optimized for multiplexing and specifically designed to allow easy integration of other specific fungal primer sets in future assay add-ons. As an internal extraction and amplification control, Bacteroides fragilis was used, yielding an amplicon of 635 bp in length, whereas amplicon lengths for fungi were 326 bp for A. fumigatus, 241 bp for Candida krusei, and 143 bp for Pan Fungi.
GM ELISA.
Galactomannan (GM) was quantified in serum samples using the Platelia Aspergillus GM enzyme-linked immunosorbent assay (ELISA) (Bio-Rad) according to manufacturer's instructions, with a cutoff of 0.5.
RESULTS
Patient details and incidence of invasive aspergillosis.
Five hundred thirty-six specimens from 46 patients at high risk for invasive fungal infection were collected (Table 1). The cohort comprised 29 men (mean age of 51 years; range, 21 to 77 years) and 17 women (mean age of 58 years; range, 20 to 78 years). The main underlying disease was acute leukemia; 25 patients had acute myeloid leukemia (AML) and 10 patients had acute lymphoblastic leukemia (ALL). Patient episodes resulted in a mean of 11.6 specimens (range, 1 to 30). During the study period, 3 cases of probable IA, 19 cases of possible IA, and 24 unclassified cases were identified according to EORTC/MSG criteria. Apart from one case of microbiologically documented candidemia, no other fungal infections were definitively documented throughout the study period.
Table 1.
Patient characteristics
| Parameter | Value(s) |
|---|---|
| No. of patients | 46 |
| No. of males | 29 |
| No. of females | 17 |
| Median age (yr) of males (range) | 51 (21–77) |
| Median age (yr) of females (range) | 58 (20–78) |
| No. of blood specimens | 536 |
| No. of specimens per patient (range, mean) | 1–30, 11.6 |
| No. of patients with fewer than 3 specimens | 6 |
| No. of AML patients | 25 |
| No. of ALL patients | 10 |
| No. of patients with other hematological malignanciesa | 11 |
| No. of allogeneic stem cell transplant recipients | 29 |
Malignancies included chronic lymphoblastic leukemia (CLL), osteomyelofibrosis (OMF), non-Hodgkin lymphoma (NHL), myelodysplastic syndrome (MDS), and lymphoblastic lymphoma (LBL).
Performance of the galactomannan assay.
The galactomannan ELISA yielded 6 positive results (1.1%) (Table 2); for three samples, no serum was available (0.6%). One patient (V064) showed 2 positive results. This patient was neutropenic (according to EORTC/MSG criteria) and showed radiologically dense pulmonary lesions with positive halos and could therefore be classified as a case of probable IA when the galactomannan results were taken into account. The patient had received posaconazole prophylaxis. Consequently, treatment was initiated with voriconazole and anidulafungin during the study period. Four patients (M030, M033, T091, T095) showed a single positive result in the galactomannan assay. Of those, patients M030 and M033 had undergone allo-SCT (M030 also received T-cell-suppressive medication), and their pulmonary radiology results were positive, with dense lesions; thus, in combination with the positive galactomannan results, they fulfilled the EORTC/MSG criteria for probable IA. In addition, patient M033 was diagnosed with a Candida bloodstream infection during the study period. Patient T095 was an allo-SCT recipient who had additionally received T-cell-suppressive therapy. Radiologically, the patient presented with infiltrates; these were, however, classified as nonspecific. The patient did not formally fulfill the criteria for possible IA and was therefore grouped as a case of possible IA/invasive fungal disease (IFD) outside the EORTC/MSG criteria. Based on a clinical decision, posaconazole was initiated for this patient. Patient T091, who had undergone allo-SCT and received T-cell-suppressive therapy, had a single positive galactomannan test (index, 1.98). This patient did not present clinical signs of IA, and no anti-Aspergillus therapy was initiated. In summary, the galactomannan ELISA detected 3 of 3 cases of probable IA (all three were classified as probable due to the positive galactomannan results) and 1 of 19 possible cases. False-positive results in the galactomannan ELISA have been linked to the use of beta-lactam–beta-lactamase inhibitor combinations. Of the 5 patients with positive galactomannan ELISA results, three (M030, T095, V064) received such antibiotics and one was treated with a beta-lactam alone (M033) during the study period.
Table 2.
Summary of selected patients in this studya
| Group | Patient | Final classificationb | No. of specimens positive by assay 1c | No. of specimens positive by the GM ELISAd | Risk factor(s)e | Clinical sign(s)f | AM at first classification or prophylaxisg |
|---|---|---|---|---|---|---|---|
| Possible/probable-IA patients detected by assay 1 | M030 | Probable | 5h | 1 | a, t | d | P |
| V064 | Probable | 4h | 2 | n | d, h | P | |
| M028 | Possible | 1 | None | a, t | d | P | |
| M035 | Possible | 3h (A. fumigatus, A. flavus, A. terreus) | None | a, n | d, h, c | V | |
| M036 | Possible | 1 | None | a, n, t | d, h | V | |
| M037 | Possible | 2 | None | n | d, h | V | |
| M039 | Possible | 1 | None | a, co, n, t | d, ac | ||
| T095 | Possible | 1i | 1 | a, t | Nonej | P | |
| V060 | Possible | 2 | None | n | d | ||
| V062 | Possible | 2 | None | n | d | P | |
| V063 | Possible | 1 | None | n | d, h | V | |
| V069 | Possible | 1 (A. flavus) | None | n | d | ||
| Possible/probable-IA patients not detected by assay 1 | M033 | Probable | 0 | 1 | a | d | E |
| M029 | Possible | 0 | None | a, n, t | d, h | P | |
| M038 | Possible | 0 | None | a, n, t | d, h | ||
| M040 | Possible | 0 | None | n | d | V | |
| T090 | Possible | 0 | None | n | d, h | ||
| T098 | Possible | 0 | None | a, t | d | P | |
| T099 | Possible | 0 | None | n, a, t | d | ||
| V049 | Possible | 0 | None | n, a | d, h, c | V | |
| V065 | Possible | 0 | None | a, t | d | P | |
| V070 | Possible | 0 | None | n | d | V | |
| Unclassified patients detected by assay 1 | T094 | Not classified | 1 | None | a, t | None, AMk | |
| V058 | Not classified | 1 | None | a, n, t | None | ||
| V067 | Not classified | 1 | None | n | None | ||
| M032 | Not classified | 1 | None | a, t | None | ||
| M021 | Not classified | 1 | None | Nonel | None | ||
| V042 | Not classified | 1 | None | n | None, AMk | ||
| Unclassified patients detected by galactomannan ELISA | T091 | Not classified | 0 | 1 | a, t | None |
All patients that had either clinical signs of invasive aspergillosis (possible, probable, or proven IA according to EORTC/MSG criteria) or positive diagnostic results.
The final classification of the patient according to EORTC/MSG criteria as having proven, probable, or possible invasive aspergillosis.
Species detected are given in parentheses if they are not A. fumigatus.
A positive GM ELISA result is a microbiological criterion for IA per EORTC/MSG criteria.
Risk factors for invasive aspergillosis according to EORTC/MSG criteria: a, allogeneic stem cell transplantation; co, corticosteroid treatment; n, neutropenia; t, T-cell-suppressive therapy.
Clinical signs for invasive aspergillosis according to EORTC/MSG criteria (all referring to chest CT scans): ac, positive air crescent sign; c, cavitation; d, dense well circumscribed lesions; h, positive halo sign.
AM, antimycotic therapy was initiated at the time the patient was first classified as possibly or probably having IFI. V, voriconazole; P, posaconazole; E, echinocandin. All patients received fluconazole and/or topical polyene prophylaxis. Unclassified patients received antifungal prophylaxis at the time of the positive assay 1/galactomannan ELISA result.
These patients had consecutive positive assay 1 results.
Patient T095 also had one positive assay 2 result.
For patient T095, only nonspecific infiltrates were detected in a chest CT scan. Due to the multiplicity of positive biomarkers, we grouped this patient with the patients who possibly had IA despite T095's not formally fulfilling the EORTC/MSG criteria.
AM, antimycotic treatment with anti-Aspergillus efficacy was initiated in patients T094 (echinocandin) and V042 (voriconazole).
This patient was included due to an expected duration of neutropenia of >10 days; however, neutropenia then turned out to be <10 days.
Performance of the PCR assays.
PCR assay 1 had a sample positivity of 5.6% (30 positive samples). Six patients showed 2 or more positive results (range, 2 to 5) (M030, M035, M037, V060, V062, V064) (Table 2). Three of these had consecutively positive results (M030, M035, V064), and all three were classified as possible (M035 had 3 positive assay 1 results) or probable (M030 had 5 positive assay 1 results, and V064 had 4 positive assay 1 results) cases of IA (Table 2). The two probable cases also had positive galactomannan results and are described above. Patient M035, a neutropenic allo-SCT recipient receiving T-cell-suppressive treatment during the study episode presented with dense pulmonary lesions with a positive halo sign and later with cavitation. Thus, although the patient formally fulfilled only the criteria for possible IA, fungal infection was clinically highly likely, and the patient was consequently treated with voriconazole. Of note, this patient showed three positive PCR tests, one each detecting DNA from A. fumigatus/A. versicolor/A. nidulans, A. terreus/A. niger, and A. flavus, pointing toward the possibility of a mixed infection. In patients M030 and V064, only DNA from A. fumigatus/A. versicolor/A. nidulans was detected (Table 2).
The other patients (M037, V060, V062) (Table 2) had 2 positive PCR assay 1 results, all with a second positive PCR result more than 10 days after the first positive result. Patient M037 (AML) suffered from prolonged neutropenia (>14 days) and repeatedly showed pulmonary lesions by computed tomography (CT) that were described as dense (once with a positive halo sign) and judged by the radiologists to be suggestive of fungal infection. Two positive assay 1 results were obtained for this patient in the same neutropenic episode, with a period of 18 days (and two negative assay 1 tests) between the two positive tests. The patient was formally classified as having possible IA due to the lack of a mycological criterion. Clinically, the patient was judged to likely suffer from fungal infection and was treated with voriconazole and later with a combination of voriconazole and echinocandin. Patient V060 showed prolonged neutropenia (>27 days) and later short neutropenic phases due to treatment for AML. While not presenting clinical signs for IA, two positive assay 1 results were obtained for this patient, with a period of >4 weeks and several negative results between the positive tests. Clinically, the patient was judged as unproblematic and did not receive anti-Aspergillus treatment. More than 4 weeks after the second positive assay 1 result, patient V060 showed new, dense pulmonary lesions by CT during a short period of neutropenia, and voriconazole treatment was started. At this time point, neither PCR nor galactomannan ELISA suggested fungal infection. This patient was classified as a possible case due to the presence of risk factors and suggestive radiology. Patient V062 (AML) was neutropenic throughout inclusion in the study (25 days). Two positive assay 1 results from this patient were obtained, with a lag period of 20 days (4 negative tests) between them. Based on a clinical decision, therapy with voriconazole (later switched to posaconazole) was initiated in this patient at the time of the first positive assay 1 result. Radiologically dense pulmonary lesions were observed by CT at the time of the second positive assay 1 result, and the patient was classified as possibly having IA.
A single positive PCR assay 1 result was recorded for 12 patients (Table 2). Of those, 6 were classified as possible cases of invasive aspergillosis. Five (M028, M036, M039, V063, V069) were classified due to a combination of risk factors and clinical signs. Noticeably, in patient V069, DNA from A. flavus was detected. One patient (T095) did not present any conclusive clinical sign (and was thus not formally classifiable based on EORTC/MSG criteria) but had also shown a positive galactomannan assay result and was therefore grouped with the patients possibly having IA/IFD outside the EORTC/MSG criteria (see above). The only positive assay 2 result (0.2% positivity) was also detected for this patient (T095). A relatively high number of 48 specimens (9%) did not give any conclusive results in assay 2.
Correlation of diagnostic assays with clinical data.
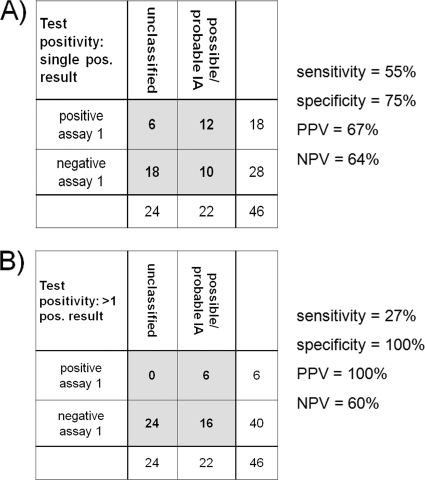
In general, the correlation of the results of the three diagnostic assays used in this study was low. Three patients with a high probability of invasive aspergillosis (V064, M030, T095) were detected by both assay 1 and galactomannan testing. Due to the strict EORTC/MSG criteria, one of those (T095) could be classified only as a possible case, but the only sample positive in assay 2 also came from this case. Of 3 probable cases of IA in this study, 2 were detected by both the galactomannan ELISA and assay 1, and one was detected by just the galactomannan ELISA (Table 2). Of 19 possible cases of IA in this study, one was detected in parallel by the galactomannan ELISA and assay 1, 9 were detected only by assay 1, and 9 were not detected by any of the diagnostic assays (Table 2). Consequently, the galactomannan ELISA detected 3 of 3 probable-IA patients (and was also the reason for those patients to be classified as probably having IA), 1 of 19 possible-IA patients, and 1 of 24 unclassified patients (Table 2). PCR assay 1 detected 2 of 3 probable-IA patients, 10 of 19 possible-IA patients, and 6 of 24 unclassified patients (Table 2; Fig. 1). A good correlation was observed between clinical data, the galactomannan assay, and PCR assay 1 when only 2 or more positive assay 1 results were taken into account. This strategy identified only patients classified as probably having IA (2 of 3) or possibly having IA (4 of 19). Three of those patients (2 probable, 1 possible) were also positive by the galactomannan ELISA (Fig. 1).
Fig. 1.
Performance of assay 1 for diagnosis of possible/probable IA according to EORTC/MSG criteria in the study population. Whereas the assay is of only moderate diagnostic value when single positive tests are taken into account (A), two or more positive tests indicate patients at high risk for IA (B). PPV, positive predictive value; NPV, negative predictive value.
Specificities of in-house PCR assays.
One reason for the high number of positive specimens with PCR assay 1 might be a lack of specificity. To exclude this possibility, the analytical specificity of the PCR assays was tested before initiation of this study by adding 5 ng of genomic DNA from the following fungi to the PCR assays: Aspergillus fumigatus, A. flavus, A. terreus, A. versicolor, A. niger, Candida albicans, Candida glabrata, Candida tropicalis, Candida krusei, Candida parapsilosis, Fusarium solani, Fusarium oxysporum, Cryptococcus albidus, C. humicola, Alternaria alternata, Mucor fragilis, Penicillium chrysogenum, and Scedosporium sp. The presence of DNA was also tested by performing a panfungal PCR in parallel (5), with slight modifications (see Materials and Methods). No cross-reactivity with other species was observed, whereas the panfungal control PCR performed in parallel showed amplicons of the expected lengths for all fungal specimens. In addition, no cross-reactivity with human DNA could be detected by assay 1.
LODs of the molecular assays.
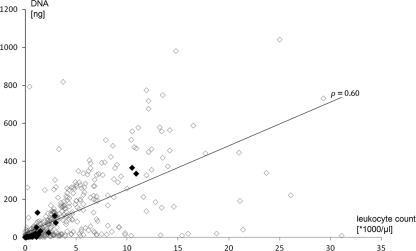
Based on the data so far, the most likely explanation for the difference in performance of the two PCR assays was that they had different limits of detection (LODs). To determine the LOD of assay 1, we tested blood spiked with different counts of Aspergillus fumigatus spores. Fungal DNA was amplified from all samples containing ≥20 CFU/ml (detection limit) and from 62.5% of spiked samples containing 5 CFU/ml. Similar results were found for modified assay 1. In contrast, assay 2 with spiked blood samples showed a detection limit of 100 CFU/ml. Noticeably, this detection limit increased to >1,000 CFU/ml when spiked erythrocyte/plasma mixtures were used to mimic conditions in neutropenic patients, indicating a dramatic increase in the LOD of assay 2 when blood samples from neutropenic patients were tested. A retrospective analysis of data from assay 1 indicated that positive samples were detected at an average quantification cycle (Cq) of 43.5 (range, 39.2 to 50). To test, whether these small amounts of fungal DNA might affect the diagnostic accuracy of highly sensitive assay 1, a collection of 15 samples from a non-study patient (V077) fulfilling the criteria for probable IA were analyzed by both assay 1 (once per sample) and modified assay 1 (each sample in triplicate). Assay 1 detected Aspergillus DNA in three nonconsecutive samples (samples 5, 10, and 13) of this patient. Retesting of these samples by modified assay 1 in triplicate yielded 3 positive results for sample 13, but only one of three parallel tests were positive for samples 5 and 10 (Table 3). In addition, samples 2 (1 of 3 tests was positive), 11 (3 of 3 tests were positive), and 12 (3 of 3 tests were positive) were positive by retesting (Table 3). These data indicate that fungal DNA load may in many samples be close to the detection limit of even highly sensitive PCR protocols. If the impact of neutropenic samples on the LOD of assay 2 is taken into account, the deficient performance of assay 2 in this study is clearly explained. The limited amounts of total DNA in the patient specimens of this study might thus have contributed to the failure of a selective pathogen DNA enrichment approach. Amounts of DNA isolated from the specimens were clearly correlated to the leukocyte count (r = 0.60; P < 0.01) (Fig. 2). In this study focusing on a patient cohort with a high prevalence of disease and/or treatment-associated neutropenia, 42% and 79% of all specimens had leukocyte counts of ≤1,000/μl and ≤5,000/μl, respectively. Whereas an average of 314 μg DNA could be isolated from the specimens with >5,000/μl leukocytes, specimens below this threshold had an average of 49 μg DNA (P < 0.01). As enrichment of unmethylated pathogen DNA from a large excess of methylated host DNA is the major benefit of the employed purification protocol (9), the lack of contaminating host DNA might well result in lower efficacy.
Table 3.
Results of multiple PCR testinga
| Sample from patient V077 | Initial PCR result | PCR result in: |
GM ELISA result | No. of leukocytes (×1,000/μl) | ||
|---|---|---|---|---|---|---|
| Rep. 1 | Rep. 2 | Rep. 3 | ||||
| 1 | Neg. | Neg. | Neg. | Neg. | Neg. | 0 |
| 2 | Neg. | Pos. | Neg. | Neg. | Neg. | 0 |
| 3 | Neg. | Neg. | Neg. | Neg. | Neg. | 0 |
| 4 | Neg. | Neg. | Neg. | Neg. | Pos. | 0 |
| 5 | Pos. | Pos. | Neg. | Neg. | Pos. | 0 |
| 6 | Neg. | Neg. | Neg. | Neg. | Pos. | 0 |
| 7 | Neg. | Neg. | Neg. | Neg. | Pos. | 0 |
| 8 | Neg. | Neg. | Neg. | Neg. | Pos. | 0 |
| 9 | Neg. | Neg. | Neg. | Neg. | Pos. | 0 |
| 10 | Pos. | Pos. | Neg. | Neg. | Neg. | 0 |
| 11 | Neg. | Pos. | Pos. | Pos. | Pos. | 0.1 |
| 12 | Neg. | Pos. | Pos. | Pos. | Pos. | 0 |
| 13 | Pos. | Pos. | Pos. | Pos. | Pos. | 1.9 |
| 14 | Neg. | Neg. | Neg. | Neg. | Pos. | 6.8 |
| 15 | Neg. | Neg. | Neg. | Neg. | Pos. | 6.4 |
Samples 1 to 15 were taken with a time span of 3 to 4 days between consecutive samples. Initial results represent the first results of the Aspergillus PCR (assay 1). For all samples, PCR was repeated in triplicate (replicates [Rep.] 1 to 3) using the initially isolated DNA as the template.
Fig. 2.
Total DNA extracted from blood specimens correlated to the patients' leukocyte counts (r = 0.60; P < 0.01). Black boxes represent specimens positive by assay 1, and white boxes represent specimens negative by assay 1.
DISCUSSION
The diagnosis of IA in patients at risk is still a major clinical challenge. Several studies have indicated that PCR-based detection of pathogen DNA in clinical samples may both improve the quality and increase the speed of diagnosis (7). The aim of this study was to evaluate a novel protocol for pathogen-specific DNA enrichment for its ability to increase the diagnostic sensitivity of PCR diagnosis in a setting in which patients, including severely neutropenic patients, are at high risk for IA. In contrast to previously used protocols for enrichment of fungal DNA (e.g., differential lysis/centrifugation), this novel protocol is based on affinity purification. A protein which is attached to the column matrix binds selectively to nonmethylated DNA and therefore retains fungal DNA to a much higher degree than it does human DNA. This novel protocol was compared to an established method using differential cell lysis. In both protocols, fungal elements were disrupted by a bead-beating step. This method has previously been shown to be well suited to guaranteeing sufficient recovery of fungal DNA from clinical specimens despite the rigid fungal cell wall (10). Performance of selective DNA enrichment by affinity purification has been promising so far. In an initial proof of concept (39 samples), the rate of positive samples could be increased from 26% to 74% (9), and a commercial multiplex-PCR assay for the diagnosis of bacterial and fungal sepsis has been approved for clinical diagnostics by EU regulatory authorities (CE mark). In this study, we assessed the use of affinity purification-based pathogen-specific DNA enrichment for the diagnosis of invasive aspergillosis. The competitor assay (assay 1) was a real-time PCR protocol almost identical to the method proposed by the EAPCRI working group (10). This assay detected 2 out of 3 probable-IA patients and 10 out of 19 possible-IA patients. Furthermore, 6 patients with no clinical evidence for IA had a single positive assay 1 result. Analysis of the diagnostic sensitivity of this assay is hampered by the lack of a diagnostic gold standard, and sensitivity could also be influenced by antifungal prophylaxis, which most of the patients in this study received at some point. Furthermore, when the EORTC/MSG criteria are used, the competing galactomannan ELISA is by itself a case-defining criterion. In fact, all cases of probable IA in this study could be classified only into this category because of a positive galactomannan ELISA result. However, clinical data from this study suggest that the galactomannan ELISA most likely misses some cases that very likely represent invasive fungal infections (IFI). In contrast, most likely, several of the cases with single positive assay 1 results do not represent IFI. A good correlation with clinical case evaluations was seen for cases with two (or more) positive assay 1 results. This strategy identified only patients classified as probably having IA (2) or possibly having IA (4) (Fig. 1), corresponding to an incidence of 13% in the selected patient group at high risk for IA, which seems more reasonable than either the 6% incidence suggested by galactomannan ELISA results alone or the 40% incidence suggested with a single positive assay 1 result being used as a diagnostic criterion. The patient cohort in this study included a high proportion of neutropenic patients and patients with decreased leukocyte counts, which hampered the performance of pathogen-specific DNA enrichment-based assay 2. Whereas the sensitivity of assay 2 was a detection limit of ≤100 CFU/ml for conidium-spiked whole-blood samples, this sensitivity decreased to ≤1,000 CFU/ml for neutropenic samples. This clearly indicates that the pathogen-specific DNA enrichment protocol requires some amounts of “contaminating” host DNA for full efficacy.
For this reason, no direct comparison between the assays (e.g., by using identical protocols for DNA extraction) was included in this study other than as originally planned. However, as the novel assay based on a multiplex-PCR protocol could outcompete a highly sensitive qPCR only if pathogen-specific DNA enrichment had really improved target DNA concentrations, this study part was skipped to save resources. It has, however, to be noted that the effect of a lack of host DNA on the results of pathogen-specific DNA enrichment might well be overcome by the presence of larger amounts of pathogen DNA in clinical samples. In a recently published study, the protocol used in our study was employed to isolate DNA from ascites specimens for subsequent detection of bacterial DNA (2). In that study, 8 out of 14 ascites specimens positive for bacterial DNA had leukocyte counts of <250/μl, which did not affect the positive PCR result. However, amounts of bacterial DNA in infected ascites specimens are likely to be significantly higher than the notoriously small amounts of blood fungal DNA in IA patients. Consequently, the use of pathogen-specific DNA enrichment for the detection of low pathogen numbers in neutropenic blood samples may result in a dramatic loss of sensitivity. This problem will especially affect the yield of multiplex-PCR assays, which often cannot be tuned to maximum sensitivity for each target. As this problem can easily be detected by quantification of the total DNA content (which is routinely performed during the isolation procedure), it might be possible to circumvent it, e.g., by adding external carrier DNA to samples below a certain threshold of total DNA. However, approaches like this need to be thoroughly evaluated before they can be introduced into a clinical routine. Therefore, at the moment, we recommend not using pathogen-specific DNA enrichment protocols for purification of DNA from neutropenic samples. As a direct consequence of this study, the manuals of commercially available kits using the evaluated selective pathogen DNA enrichment protocol for diagnostic purposes other than for IA now include a criterion of at least 1,500 cells/ml or >20 μg total DNA as a prerequisite for diagnostic samples. Indeed, the small amounts of fungal DNA in clinical samples also have an impact on real-time-PCR-based methods. As shown here by repeated testing of identical samples, even a 55-cycle qPCR does not reliably detect fungal DNA in patient samples due to the low pathogen DNA concentration. These data indicate that, although economically unaffordable, triplicate testing of patient samples with highly sensitive PCR assays might be necessary to gain full sensitivity from molecular diagnostic tools. In summary, this study clearly underlines the absolute necessity of optimizing protocols for DNA amplification-based diagnosis of IA with regard to sensitivity. This can be achieved only by combining highly efficient protocols for DNA purification with PCR protocols designed to detect even minute amounts of pathogen DNA.
ACKNOWLEDGMENTS
This work would not have been possible without the assistance of Annett Österlein, Susanne Endres, and Angelika Hansen from the Diagnostic Molecular Biology Laboratory at the Institute of Hygiene and Microbiology in Würzburg, Germany.
The study was funded by SIRS-Lab GmbH. Additional work in the lab of O.K. was funded by the Federal Ministry for Education and Science (grant BMBF 03Z2JN21), and work in the labs of J.L. and T.R.R. was funded by the EU project Development of Novel Management Strategies for Invasive Aspergillosis (MANASP, grant LSHE-CT-2006-037899).
Footnotes
Published ahead of print on 29 December 2010.
REFERENCES
- 1. Baskova L., Landlinger C., Preuner S., Lion T. 2007. The Pan-AC assay: a single-reaction real-time PCR test for quantitative detection of a broad range of Aspergillus and Candida species. J. Med. Microbiol. 56:1167–1173 [DOI] [PubMed] [Google Scholar]
- 2. Bruns T., et al. 2009. Identification of bacterial DNA in neutrocytic and non-neutrocytic cirrhotic ascites by means of a multiplex polymerase chain reaction. Liver Int. 29:1206–1214 [DOI] [PubMed] [Google Scholar]
- 3. Bustin S. A., et al. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55:611–622 [DOI] [PubMed] [Google Scholar]
- 4. De Pauw B., et al. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Einsele H., et al. 1997. Detection and identification of fungal pathogens in blood by using molecular probes. J. Clin. Microbiol. 35:1353–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loeffler J., et al. 2000. Quantification of fungal DNA by using fluorescence resonance energy transfer and the light cycler system. J. Clin. Microbiol. 38:586–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mengoli C., Cruciani M., Barnes R. A., Loeffler J., Donnelly J. P. 2009. Use of PCR for diagnosis of invasive aspergillosis: systematic review and meta-analysis. Lancet Infect. Dis. 9:89–96 [DOI] [PubMed] [Google Scholar]
- 8. Pagano L., et al. 2006. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 91:1068–1075 [PubMed] [Google Scholar]
- 9. Sachse S., et al. 2009. Truncated human cytidylate-phosphate-deoxyguanylate-binding protein for improved nucleic acid amplification technique-based detection of bacterial species in human samples. J. Clin. Microbiol. 47:1050–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. White P. L., et al. 2010. Aspergillus PCR: one step closer to standardization. J. Clin. Microbiol. 48:1231–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao Y., et al. 2009. Rapid real-time nucleic acid sequence-based amplification–molecular beacon platform to detect fungal and bacterial bloodstream infections. J. Clin. Microbiol. 47:2067–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao Y., et al. 2010. Detection of Aspergillus fumigatus in a rat model of invasive pulmonary aspergillosis by real-time nucleic acid sequence-based amplification. J. Clin. Microbiol. 48:1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]