Abstract
Kingella kingae is an emerging osteoarticular pathogen in young children. Its isolation by traditional culture methods remains difficult, underscoring the need to implement other diagnostic methods for its detection and identification, such as nucleic acid amplification tests. Although the genome of this bacterium has not yet been sequenced, a toxin named RTX has been identified. The goal of this study was to develop sensitive, specific, and rapid molecular methods based on the rtxA toxin gene sequence to diagnose this infection. Two real-time PCR assays (SYBR green and TaqMan chemistries) targeting this gene are reported. Sensitivity and specificity were first evaluated successfully with 67 strains: 31 Kingella kingae isolates and 36 strains from other bacterial species. Then, 52 clinical specimens positive or negative by culture and/or PCR (16S rRNA and cpn60 genes) were tested with these assays. A nested PCR assay with subsequent sequencing was also developed to confirm the presence of Kingella kingae isolates in these clinical specimens. The results obtained demonstrate that these assays are accurate for the diagnosis of Kingella kingae infection.
INTRODUCTION
Experience accumulated over the past 2 decades has clearly demonstrated that the direct inoculation of joint exudates in blood culture bottles significantly enhances the recovery of bacterial pathogens from children with osteomyelitis and septic arthritis (23, 26). These studies revealed that Kingella kingae, a normal inhabitant of the oropharynx, is involved in osteoarticular infections (OAI) in healthy children aged 6 to 36 months (1, 10, 22, 24, 25). These acute pediatric infections require a fast and sensitive diagnosis allowing an appropriate treatment directed against the causative pathogen (12).
Nucleic acid amplification tests are now increasingly relevant for the detection and identification of K. kingae, particularly in view of the lack of sensitivity of culture for detecting this emerging pediatric pathogen (8, 11, 12, 18). Thus, the microbiological diagnosis of K. kingae infections relies mainly upon analysis of articular fluid by molecular methods. Since the first PCR was reported in 1998, nucleic acids targeting either the 16S rRNA gene (11, 12, 20) or the cpn60 gene (4, 8, 14) have further improved the detection of K. kingae and have firmly placed this fastidious organism as the main and yet underestimated cause of OAI in young children (<3 years) (19, 21), followed by Staphylococcus aureus (4, 8, 19).
A recent study revealed that K. kingae expresses a toxin named RTX which has been shown to be responsible for in vitro cytotoxicity on respiratory epithelial, synovial, and macrophage-like cells, with sensitivity levels up to 4-fold higher for synovial and macrophage-like cells than for respiratory cells (15). It should be noted that synovial and macrophage-like cells are the cells that K. kingae is likely to interact with the course of a septic arthritis (5). These observations suggest that the RTX toxin could play a role in the pathogenicity of this bacterium in breaching the epithelial barrier and destroying the synovium (15), making this toxin a highly specific target to develop tools for the diagnosis of K. kingae infection in general.
Recently, real-time PCR assays (TaqMan chemistry) targeting the rtxA and rtxB genes (7) confirmed the presence of RTX toxin genes in invasive K. kingae strains and demonstrated their importance in the diagnosis of 23 cases of K. kingae OAI (6). However, although these methods seem very promising, the authors concluded that a larger cohort is required to adequately study new PCR assays based on RTX genes. Accordingly, and to improve the detection of K. kingae, the present study describes two real-time PCR assays based on SYBR green and TaqMan chemistries, targeting the toxin-encoding gene rtxA of K. kingae. These assays were compared with those previously reported (6, 7), and the accuracy of this rtxA toxin gene as a marker of K. kingae OAI was evaluated with a larger number of isolates, including other Kingella species, and clinical specimens.
MATERIALS AND METHODS
Bacterial strains.
Several strains of Kingella species, including K. kingae (n = 31) (Table 1), Kingella oralis type strain UB-38 (CIP 103803), Kingella denitrificans type strain A358/72 (CIP 103473), and Kingella potus type strain 3/SID/1128 (CIP 108935), were used in this study. Strains were cultured on Trypticase soy blood agar (bioMérieux, Marcy l'Étoile, France) for 24 to 72 h at 37°C in an atmosphere enriched in CO2.
Table 1.
Description of the Kingella kingae isolates used in this study (n = 31) according to clinical diagnosis and origin
| Straina | Specimen (clinical diagnosisc) | Origin | GenBank accession no. for rtxA gene (∼1,100 bp) |
|---|---|---|---|
| 4177/66Tb | Nasal swab (NA) | Oslo, Norway | GQ325000 |
| 5530b | Blood (NA) | Oslo, Norway | GQ325001 |
| 2941b | Blood (NA) | Oslo, Norway | GQ325002 |
| A2471b | Bone lesion (osteomyelitis) | Oslo, Norway | GQ325003 |
| 449-79b | Blood (NA) | Besançon, France | GQ325004 |
| Cléb | Blood (NA) | Nancy, France | GQ325005 |
| 553.85b | Blood (NA) | Grenoble, France | GQ325006 |
| 474-86b | NA (osteomyelitis) | Paris, France | GQ325007 |
| 475-86b | NA (osteomyelitis) | Paris, France | GQ325008 |
| 08/09-BRIH | Blood (endocarditis) | Bordeaux, France | GQ325009 |
| 0801-260938-BOD | AF (arthritis of the right ankle) | Poitiers, France | GQ325010 |
| 0608-141565-SER | AF (arthritis of the right wrist) | Poitiers, France | GQ325011 |
| 12-05-04-MAR | Blood (NA) | Rochefort/mer, France | GQ325012 |
| 166739 | AF (arthritis of the right ankle) | Paris, France | GQ325013 |
| 19477 | AF (arthritis, localization NA) | Paris, France | GQ325014 |
| 116811 | AF (arthritis of the left shoulder) | Tours, France | GQ325015 |
| 110733 | AF (arthritis of the right ankle) | Tours, France | GQ325016 |
| 127598 | BB (osteomyelitis of the left calcaneus) | Tours, France | GQ325017 |
| 113899 | Abscess puncture (abscess of the left lateral sternum) | Tours, France | GQ325018 |
| 119951 | AF (arthritis of the left shoulder) | Tours, France | GQ325019 |
| 112222 | AF (arthritis of the right wrist) | Tours, France | GQ325020 |
| 112621 | BB (osteomyelitis of the astragalus) | Tours, France | GQ325021 |
| 112175 | AF (arthritis of the left ankle) | Tours, France | GQ325022 |
| 6307-2010 | BB (C2-C3 spondylodiskitis) | Toulouse, France | GQ325023 |
| 6079-2197 | AF (arthritis of the left knee) | Toulouse, France | GQ325024 |
| 6278-745 | AF (arthritis of the right knee) | Toulouse, France | GQ325025 |
| 6238-761 | Abscess puncture (sternum) | Toulouse, France | GQ325026 |
| 5077-535 | BB (osteomyelitis of the calcaneus) | Toulouse, France | GQ325027 |
| 508-1766 | BB (osteomyelitis of the sternum) | Toulouse, France | GQ325028 |
| 507-1756 | BB (osteomyelitis of the greater trochanter) | Toulouse, France | GQ325029 |
| 507-9849 | BB (osteomyelitis of the talus) | Toulouse, France | GQ324999 |
T, type strain. Strains 4177/66, 5530, 2941 and A2471 were previously described (13). A 1,198-bp rtxA sequence was determined for all samples.
Strains provided from the Collection of the Institut Pasteur (CIP), Paris, France.
NA, not available; AF, articular fluid; BB, bone biopsy specimen.
Other bacterial species from the Pasteur Institute collection (CIP) or isolated from clinical specimens, were used to determine the specificity of the PCR primers, especially Gram-negative bacteria (Neisseria meningitidis groups A, B, C, W135, and Y, Neisseria gonorrhoeae, Moraxella catarrhalis, Eikenella corrodens, Haemophilus influenzae, Haemophilus parainfluenzae, Escherichia coli, Pseudomonas aeruginosa, Bordetella pertussis strain Sato [CIP 81.32], Bordetella parapertussis strain 22651 L2 [CIP 63.2], Bordetella bronchiseptica type strain 452 [CIP 55.110], Bordetella holmesii type strain 5589 [CIP 104394], Mycoplasma pneumoniae, Mycoplasma genitalium, Ureaplasma urealyticum, Mycoplasma fermentans, Mycoplasma salivarium, Salmonella enterica serovar Enteritidis, Salmonella enterica serovar Typhimurium, Campylobacter jejuni, Chlamydia trachomatis, Chlamydia pneumoniae, Chlamydia psittaci, and Klebsiella pneumoniae) and Gram-positive bacteria (Staphylococcus aureus, Staphylococcus pettenkoferi, Streptococcus pyogenes, Streptococcus pneumoniae, and Corynebacterium species).
Clinical specimens.
Osteoarticular fluids and biopsy samples (n = 52) used in this study were provided by the Centre Hospitalier Universitaire de Bordeaux (n = 24), the Centre de Biologie Est, Hospices Civils de Lyon (n = 23), and the Groupe Hospitalier Necker-Enfants Malades (n = 5). The presence of K. kingae was previously detected in 20 of these samples by real-time PCR targeting the 16S rRNA and/or cpn60 gene (8, 19), of which two grew K. kingae and 18 did not grow any bacteria (Table 2). The following bacteria other than K. kingae were detected by culture in 12 of these 52 samples: S. aureus (n = 4), Staphylococcus epidermidis, Staphylococcus warneri, S. pyogenes, S. pneumoniae serotype 19A, Propionibacterium acnes, Salmonella enterica serovar Bredeney, Aggregatibacter aphrophilus, and Klebsiella oxytoca (16). Finally, 20 of these samples, which were negative for bacteria by both culture and universal 16S rRNA gene PCR, were used as negative controls.
Table 2.
Description of the Kingella kingae-positive specimens used in this study (n = 20) according to clinical diagnosis and origin
| Sample identificationa | Specimen, disease (site of infection)c | Patient age (yr) | Origin | GenBank accession no. for rtxA gene (∼1,159 bp) |
|---|---|---|---|---|
| BE08014199 | AF, arthritis (knee) | 3.1 | Lyon | GQ325031 |
| BE08083363 | AF, arthritis (shoulder) | 1.4 | Lyon | GQ325032 |
| BE08015084 | AF, arthritis (ankle) | 2.1 | Lyon | GQ325033 |
| BE07514129 | AF, arthritis (UN) | 1.1 | Lyon | GQ325034 |
| BE08037064 | AF, arthritis (hip) | 1.9 | Lyon | GQ325035 |
| BE07501417 | AF, arthritis (toe) | 1.1 | Lyon | GQ325036 |
| BE07504171 | AF, arthritis (knee) | 1 | Lyon | GQ325037 |
| BE07446172 | Bone biopsy specimen, osteomyelitis (trochanter) | 10.9 | Lyon | GQ325038 |
| BE08055440 | AF, arthritis (hip) | 1.8 | Lyon | GQ325039 |
| BE07457117 | AF, arthritis (shoulder) | 1.4 | Lyon | GQ325040 |
| BE07485320 | AF, arthritis (knee) | 1.1 | Lyon | GQ325041 |
| BE07501345 | AF, arthritis (shoulder) | 1.1 | Lyon | GQ325042 |
| BE07504020 | AF, arthritis (shoulder) | 1.4 | Lyon | GQ325043 |
| BE07497075 | AF, arthritis (knee) | 1.6 | Lyon | GQ325044 |
| 0706M290114b | AF, osteomyelitis (calcaneus) | 1.05 | Paris | GQ325045 |
| 0712M070328 | AF, arthritis (hand-wrist) | 1.74 | Paris | GQ325046 |
| 0711M080409 | AF, arthritis (hand-wrist) | 1.25 | Paris | GQ325047 |
| 0711M060013 | AF, arthritis (ankle) | 1.22 | Paris | GQ325048 |
| 0802M060005b | AF, arthritis (shoulder) | 1.05 | Paris | GQ325049 |
| 08/10-DESB | AF, arthritis (knee) | 1.3 | Bordeaux | GQ325030 |
All of these specimens were also bacteriologically documented by cpn60 PCR, and a 833-bp rtxA sequence was determined for all of these samples.
With regard to the phenotypic identification, only these articular fluids grew K. kingae. The other samples did not grow any bacteria.
AF, articular fluid; UN, unknown.
Genomic DNA extraction.
Genomic DNA was extracted by using the MagnaPure LC DNA isolation kit I and the MagnaPure LC isolation station (Roche Applied Science, Penzberg, Germany). DNA was stored at −20°C until required for analysis. Extracted DNA was tested both undiluted and diluted 1:10 and 1:100 using the PCRs described below.
Sequencing of the 5′ extremity of the rtxA gene in strains.
An 1,198-bp PCR product was amplified with the set of primers F2-seq-rtxC (5′-GCCGAATGGGAAGATTTCTG-3′) and R2-seq-rtxA (5′-GCATTCATAAACGCCAACG-3′) designed from nucleotide 366 of rtxC to nucleotide 962 of rtxA from the RTX gene locus sequence of K. kingae strain 269-492 (15), available in GenBank under accession number EF067866. PCR was performed with the Go Taq DNA polymerase (Promega, Madison, WI). Amplification parameters consisted of 1 cycle at 95°C for 5 min, followed by 45 cycles at 95°C for 30 s, 56°C for 30 s, and 72°C for 1 min, and finally 1 cycle at 72°C for 5 min. The amplicons were purified using MicroSpin S-400 HR columns (GE Healthcare, Saclay, France), and sequencing of these products was achieved on both strands using the initial set of PCR primers and internal primers F3-seq-rtxA (5′-GCTCGATTACAAGAAGATGCTAA-3′) and R3-seq-rtxA (5′-CACCAGTTTGAGTAAAGCTTAAAA-3′).
Sequencing of the rtxA gene in clinical specimens.
The PCR described above was first used. However, a nested PCR was required in some cases since several PCR products were observed on agarose gels. Extracted genomic DNA was initially amplified as described above, and the 1,198-bp amplicons were purified using MicroSpin S-400 HR columns (GE Healthcare) and diluted 1:10. The second PCR was then performed with the set of primers F1-seq-rtxA (5′-TGGATAGAACAGCTTGAATGG-3′) and R1-seq-rtxA (5′-AACCAGCTGAAATGCCTGAC-3′) under the PCR conditions described above, and the 833-bp PCR product was sequenced using the PCR primers and the internal primers described above.
Universal 16S rRNA PCR.
A real-time universal PCR targeting the 16S rRNA gene was used as a control to monitor the extraction and absence of PCR inhibitors (17). These two PCRs were performed simultaneously and under the same conditions as used for the search of the rtxA gene.
Design of the primers and probe for PCR amplification of the rtxA gene.
The available rtxA gene sequence of K. kingae strain 269-492 (GenBank accession number EF067866) and 31 additional rtxA gene sequences obtained for K. kingae strains were aligned using multiple-sequence alignment with hierarchical clustering (9) (http://multalin.toulouse.inra.fr/multalin/) and analyzed to identify conserved regions for the primer design. The primer set F2-KK-rtxA (5′-GCGCACAAGCAGGTGTACAA-3′) and R2-KK-rtxA (5′-ACCTGCTGCTACTGTACCTGTTTTAG-3′) and the TaqMan probe KK-rtxA FAM-TTGAACAAAGCTGGACACG-MGB-NFQ were designed on the rtxA gene using the Primer Express software package (Applied Biosystems, Foster City, CA) and were synthesized by Applied Biosystems (Cheshire, United Kingdom). A BLAST program search (2) (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi?PAGE=Nucleotides&PROGRAM=blastn&MEGABLAST=on&BLAST_PROGRAMS=megaBlast&PAGE_TYPE=BlastSearch&SHOW_DEFAULTS=on) performed with the 71-bp expected PCR product did not reveal any sequence homology with any organism other than K. kingae.
SYBR green real-time PCR and MCA analyses using different thermocyclers. (i) ABI PRISM 7000 instrument.
SYBR green real-time PCR amplification and melting curve analysis (MCA) were carried out with a final volume of 25 μl containing 12.5 μl of SYBR green PCR master mix (Applied Biosystems), 0.3 μM each primer, and 5 μl of purified DNA in an ABI PRISM 7000 thermocycler (Applied Biosystems). Amplification parameters consisted of 1 cycle at 95°C for 10 min, followed by 50 cycles at 95°C for 15 s and 60°C for 1 min. This was followed by use of the melting program of the ABI PRISM 7000 thermocycler, with continuous monitoring of the fluorescence (dissociation program).
(ii) LightCycler 2.0 instrument (Roche Applied Science, Meylan, France).
The 20-μl reaction mixture contained 4 μl of LightCycler FastStart DNA MasterPLUS SYBR green I (Roche Applied Science), 0.3 μM each primer, and 5 μl of purified DNA. Following an initial denaturation at 95°C for 10 min with a temperature transition rate of 20°C/s, amplification steps (95°C for 10 s, 62°C for 5 s, and 72°C for 10 s) were repeated for 50 cycles at a temperature transition rate of 20°C/s. Fluorescence was measured at 530 nm after each cycle. This was followed by a melting program (95°C for 0 s, 65°C for 60 s, and 95°C for 0 s) at a temperature transition rate of 20°C/s and then 95°C for 0 s at a rate of 0.1°C/s with continuous monitoring of the fluorescence. A final step consisted of cooling at 40°C with a 30-s hold.
(iii) SmartCycler II instrument (Cepheid, Sunnyvale, CA).
The 25-μl reaction mixture contained 12.5 μl of Premix Ex Taq (Takara Bio Inc., Otsu, Shiga, Japan), 2.5 μl of SYBR green (Sigma-Aldrich, St. Louis, MO), 0.3 μM each primer, and 5 μl of purified DNA. Following an initial denaturation at 95°C for 15 s with a temperature transition rate of 10°C/s, amplification steps (95°C for 10 s, 60°C for 10 s, and 72°C for 20 s) were repeated for 50 cycles at a temperature transition rate of 10°C/s. Fluorescence was measured at 530 nm after each cycle. This was followed by a melting program with continuous monitoring of the fluorescence.
TaqMan real-time PCR with the ABI PRISM 7000 instrument.
The TaqMan real-time PCR amplification and hybridization reactions were carried out in a final volume of 25 μl containing 12.5 μl of TaqMan Universal PCR master mix (Applied Biosystems), 0.3 μM each primer, 0.2 μM labeled probe KK-rtxA, and 5 μl of purified DNA in the ABI PRISM 7000 thermocycler. DNA was amplified using the following cycling parameters: heating at 95°C for 10 min, followed by 50 cycles of a two-stage temperature profile of 95°C for 15 s and 60°C for 1 min.
Nucleotide sequence accession numbers.
The GenBank accession numbers of the rtxA genes from the 31 K. kingae strains and the 20 OAI specimens used in this study are available in Tables 1 and 2, respectively.
RESULTS AND DISCUSSION
Several regions bearing point mutations were previously reported to be present in the cpn60 gene in K. kingae strains, “underlining the necessity of sequencing the target gene to optimize the probe design,” as cited by Ilharreborde et al. (14). In the present study, we first chose the gene of interest (i.e., the rtxA gene). We then sequenced the chosen region in 31 K. kingae strains prior to the primer and probe design to ensure the detection of all of the K. kingae strains.
Analysis of the RTX locus, sequencing of the rtxA gene, and PCR design.
The RTX locus from K. kingae strain 269-492 is comprised of the tolC, rtxA, rtxC, rtxD, and rtxB genes, which are necessary for the production and secretion of an active RTX toxin. The deduced proteins showed up to 81 and 83% sequence identity with those of Neisseria meningitidis and Moraxella bovis, respectively; the RtxA protein was the most distant from these species, with 72% identity with Moraxella bovis (15). A new protein blast (blastp) search indicated that the 956-bp residue RtxA sequence of K. kingae strain 269-492 had the highest sequence identity (72%) with its counterparts in Moraxella bovis, Moraxella bovoculi, and Moraxella ovis (GenBank accession numbers ABR28460, ABA39414, and ABA39419, respectively) in only a 919-residue overlap (see Fig. S1A in the supplemental material), revealing additional residues at the NH2-terminal extremity of RtxA of K. kingae. This original 38-amino-acid sequence corresponds to the 5′ end of the RtxA sequence. Thus, a PCR targeting this sequence corresponding to 114 bp of the rtxA gene was performed with the F2-seq-rtxC/R2-seq-rtxA set of primers on all the Kingella strains. The expected PCR product (approximate 1,198 bp) was obtained for all of the 31 K. kingae clinical strains, while no amplification was observed for K. oralis, K. denitrificans, and K. potus strains (not shown). Analysis of the 31 sequences showed that all of the K. kingae clinical strains possess the rtxA toxin gene, making this gene a relevant target to diagnose K. kingae infection by PCR (GenBank accession numbers are available in Table 1).
With regard to the nucleotide sequence of rtxA of strain 269-492 (accession no. EF067866), 18 polymorphisms were observed over the 943-bp sequenced region. In addition, an insertion was also present at position 67 according to the numbering of the rtxA gene from the start codon. This insertion occurs in six recently isolated strains (strains 0608-141565-SER, 12-05-04-MAR, 0801-260938-BOD, 508-1766, 507-1756, and 112222) and corresponds to a 33-nucleotide sequence leading to an 11-residue sequence that can be repeated twice (see Fig. S1B in the supplemental material). Most of the nucleotide polymorphisms observed in rtxA (12/18) are nonsynonymous, and six residue changes were observed in the 314-residue protein sequence (see Fig. S1B in the supplemental material). No correlation with the origin of the strain was observed when analyzing nucleotide and deduced amino acid sequences, particularly those from Norway (n = 4) (data not shown).
Validation of the PCR target choice and primer design.
Particular care was taken in order to (i) avoid mismatches in the primers and probe, since the 5′ end of the rtxA gene revealed several single-nucleotide polymorphisms (SNPs), and (ii) minimize the risk of primer dimers that could interfere with the interpretation of the results when using the SYBR green dye. Because of all these various constraints and especially those imposed by the software, the sequence from nucleotide 38 to 108 of the rtxA gene was selected to generate a 71-bp amplicon, and minor-groove binding was necessary to obtain a short probe. The specificity and sensitivity of the PCR assays were then tested by using the SYBR green and TaqMan chemistries on DNA extracted from these 31 isolates (Table 1) and from the 36 strains from other bacterial species (including the three other Kingella species).
With regard to the previously reported TaqMan-based PCR targeting the rtxA gene (from nucleotide 676 to 762) (6, 7), the set of primers (rtxA-F and rtxA-R) hybridized perfectly with all of these 31 sequences, while mismatches with the probe were observed for 27 of the 31 clinical strains used in the present study. These mismatches are located at positions 705 (G → A) and 707 (C → G). Such mismatches on a TaqMan probe would lead to the absence of detection for these strains. In fact, only 6 of the 31 clinical strains would be detected by this TaqMan-based PCR (strains A2471, 449-79, Clé, 19477, 553.85, and 116811).
Specificities of the SYBR green and TaqMan assays.
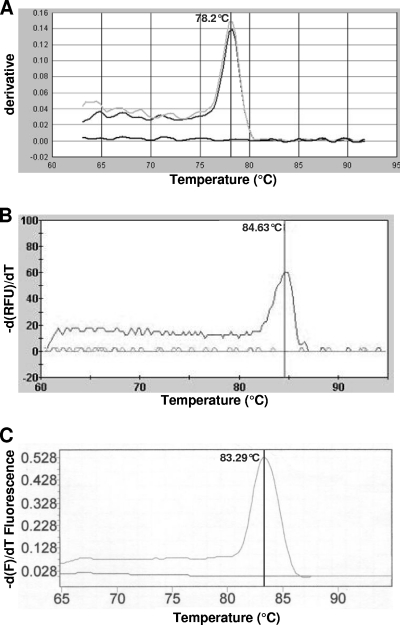
Real-time PCR assays and melting curve analysis (MCA) using SYBR green chemistry were performed using the ABI PRISM 7000, the SmartCycler, and the LightCycler systems. With regard to the F2-/R2-KK-rtxA primer set, only DNA extracted from K. kingae isolates (n = 31) yielded amplification products, whereas DNA extracted from other bacteria, including other Kingella species, did not. The universal 16S rRNA gene PCR yielded the expected amplification products for all of these isolates. SYBR green dye binds to any double-stranded DNA, including nonspecific double-stranded DNA sequences such as reaction products and/or primer dimers, which may generate false-positive signals. This was not the case in the present study even with 50 PCR cycles, which facilitates the interpretation of the results (Fig. 1). Similar results were obtained using the three PCR thermocyclers, demonstrating the usefulness and technical feasibility of the approach in different laboratory settings. However, the MCA of the 71-bp specific amplicons showed melting temperatures (Tm) ranging from 78.2°C to 84.63°C, depending on the mix and the thermocycler used (Fig. 1). These expected Tm variations are due to the salt and SYBR green concentration differences in the mixes used.
Fig. 1.
Melting curve analyses of the PCR products obtained from the amplification of the rtxA gene of Kingella kingae with the ABI PRISM 7000 (Applied Biosystems) (A), the SmartCycler (Cepheid) (B), and the LightCycler (Roche Diagnostics) (C). The temperature (°C) is indicated on the x axis, and the derivative of the fluorescence is indicated on the y axis. The peaks indicate the melting points of the respective amplicons.
The TaqMan assay was developed with the same set of primers in association with a probe to ensure a more specific assay. The specificity of the PCR was also confirmed with real-time PCR amplification using the TaqMan chemistry and hybridization reactions using the ABI PRISM 7000 and the LightCycler systems (not shown).
Thus, with regard to the specificity, no probes are required and the use of SYBR green reduces the assay setup and running costs. Moreover, the use of SYBR green allows the implementation of this assay easily using any real-time PCR apparatus.
Sensitivities of the SYBR green and TaqMan assays.
The analytical sensitivity of the PCR for the detection of K. kingae was then evaluated using 10-fold serial dilutions of a bacterial suspension (type strain 4177/66) in phosphate-buffered saline, which was quantified by culture on specific media and subsequent colony counts. PCRs were performed in an ABI PRISM 7000 thermocycler. SYBR green and TaqMan chemistries performed with the DNA extracted in a reaction volume of 25 μl allowed us to obtain regression curves with slopes of 3.3 and 3.5, respectively, i.e., very close to a slope of 3.32, which would correspond to the maximum efficiency. The regression curve was linear until 0.5 to 5 bacterial genomes for both assays, making rtxA an efficient target to diagnose K. kingae infection, as recently reported (6, 7). The SYBR green-based detection exhibited the highest sensitivity (with a shift in threshold cycle [CT] value of more than 2), which is probably due to multiple dyes (SYBR green) that can bind to a single amplified molecule and accumulate during PCR cycles, increasing sensitivity for detecting the amplicons (3), while TaqMan probes are hydrolyzed during the PCR by the 5′ nuclease activity of the Taq DNA polymerase. This lower sensitivity of the TaqMan probes can be compensated for by increasing the probe concentration in the mix and/or the cycle number. The analytical sensitivity of PCR targeting the rtxA and rtxB genes was 10-fold higher than that of PCR targeting the cpn60 gene previously reported by Chometon et al. (8).
Retrospective study on clinical specimens.
To validate the PCR, 52 OAI fluids or biopsy samples were tested. The expected peak corresponding to K. kingae was generated for all of the 20 positive samples, while no peak appeared for the 20 negative samples and for the 12 samples with other bacteria that were simultaneously detected by the real-time PCR targeting the 16S rRNA gene broadly.
However, it should be noted that in some cases more than 40 cycles or sample dilution was necessary to obtain a PCR amplification. The presence of K. kingae DNA in the 20 positive samples was also confirmed using the TaqMan chemistry (data not shown). Moreover, the sequencing of approximately 833 bp of the rtxA genes in these 20 positive samples revealed several SNPs that were different between the samples, suggesting the presence of different strains among these samples (GenBank accession numbers are given in Table 2).
In conclusion, both the TaqMan and SYBR green assays developed in the present study are specific and highly sensitive, making rtxA a pertinent target for the molecular diagnosis of K. kingae infection, as previously reported (6, 7). All 31 K. kingae strains used in this study harbored the rtxA gene, and this gene was also present in the 20 OAI specimens previously found positive for K. kingae in different laboratories by other PCR methods. These results suggest the constant presence of the toxin gene in pathogenic K. kingae strains and validate the interest in use of an rtx-based PCR in clinical diagnosis. Furthermore, the use of different chemistries and thermocyclers to perform this test allows a general utilization of this PCR. Alternatively, the nested PCR yielding a 833-bp amplicon of the rtxA gene would be useful for centers that are not equipped with a real-time PCR thermocycler.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Victoria Chuat, Elodie Sifré, and Jamilla Belbachir for technical assistance.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 19 January 2011.
REFERENCES
- 1. Abuamara S., et al. 2000. Kingella kingae osteoarticular infections in children. A report of a series of eight new cases. Arch. Pediatr. 7:927–932 [DOI] [PubMed] [Google Scholar]
- 2. Altschul S. F., et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Applied Biosystems 2010, posting date TaqMan® vs. SYBR® Green Chemistries. http://www.appliedbiosystems.com/absite/us/en/home/applications-technologies/real-time-pcr/taqman-and-sybr-green-chemistries.html
- 4. Baticle E., et al. 2008. Pediatric osteoarticular infections caused by Kingella kingae from 1995 to 2006 at CHRU de Tours. Ann. Biol. Clin. (Paris) 66:454–458 [DOI] [PubMed] [Google Scholar]
- 5. Bremell T., Abdelnour A., Tarkowski A. 1992. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect. Immun. 60:2976–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ceroni D., Cherkaoui A., Ferey S., Kaelin A., Schrenzel J. 2010. Kingella kingae osteoarticular infections in young children: clinical features and contribution of a new specific real-time PCR assay to the diagnosis. J. Pediatr. Orthop. 30:301–304 [DOI] [PubMed] [Google Scholar]
- 7. Cherkaoui A., Ceroni D., Emonet S., Lefevre Y., Schrenzel J. 2009. Molecular diagnosis of Kingella kingae osteoarticular infections by specific real-time PCR assay. J. Med. Microbiol. 58:65–68 [DOI] [PubMed] [Google Scholar]
- 8. Chometon S., et al. 2007. Specific real-time polymerase chain reaction places Kingella kingae as the most common cause of osteoarticular infections in young children. Pediatr. Infect. Dis. J. 26:377–381 [DOI] [PubMed] [Google Scholar]
- 9. Corpet F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881–10890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dubnov-Raz G., et al. 2010. Invasive pediatric Kingella kingae infections: a nationwide collaborative study. Pediatr. Infect. Dis. J. 29:639–643 [DOI] [PubMed] [Google Scholar]
- 11. Fenollar F., Levy P. Y., Raoult D. 2008. Usefulness of broad-range PCR for the diagnosis of osteoarticular infections. Curr. Opin. Rheumatol. 20:463–470 [DOI] [PubMed] [Google Scholar]
- 12. Ferroni A. 2007. Epidemiology and bacteriological diagnosis of paediatric acute osteoarticular infections. Arch. Pediatr. 14(Suppl. 2):S91–S96 [DOI] [PubMed] [Google Scholar]
- 13. Henriksen S. D., Bovre K. 1968. Moraxella kingii sp. nov., a haemolytic, saccharolytic species of the genus Moraxella. J. Gen. Microbiol. 51:377–385 [DOI] [PubMed] [Google Scholar]
- 14. Ilharreborde B., et al. 2009. New real-time PCR-based method for Kingella kingae DNA detection: application to samples collected from 89 children with acute arthritis. J. Clin. Microbiol. 47:1837–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kehl-Fie T. E., St. Geme J. W., III 2007. Identification and characterization of an RTX toxin in the emerging pathogen Kingella kingae. J. Bacteriol. 189:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ménard A., et al. 2010. First report of septic arthritis caused by Klebsiella oxytoca. J. Clin. Microbiol. 48:3021–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ménard A., et al. 2007. Development of a real-time PCR for the identification of Bordetella pertussis and Bordetella parapertussis. Clin. Microbiol. Infect. 13:419–423 [DOI] [PubMed] [Google Scholar]
- 18. Moumile K., Merckx J., Glorion C., Berche P., Ferroni A. 2003. Osteoarticular infections caused by Kingella kingae in children: contribution of polymerase chain reaction to the microbiologic diagnosis. Pediatr. Infect. Dis. J. 22:837–839 [DOI] [PubMed] [Google Scholar]
- 19. Rosey A. L., et al. 2007. Development of a broad-range 16S rDNA real-time PCR for the diagnosis of septic arthritis in children. J. Microbiol. Methods 68:88–93 [DOI] [PubMed] [Google Scholar]
- 20. Stahelin J., Goldenberger D., Gnehm H. E., Altwegg M. 1998. Polymerase chain reaction diagnosis of Kingella kingae arthritis in a young child. Clin. Infect. Dis. 27:1328–1329 [DOI] [PubMed] [Google Scholar]
- 21. Verdier I., et al. 2005. Contribution of a broad range polymerase chain reaction to the diagnosis of osteoarticular infections caused by Kingella kingae: description of twenty-four recent pediatric diagnoses. Pediatr. Infect. Dis. J. 24:692–696 [DOI] [PubMed] [Google Scholar]
- 22. Yagupsky P. 2004. Kingella kingae: from medical rarity to an emerging paediatric pathogen. Lancet Infect. Dis. 4:358–367 [DOI] [PubMed] [Google Scholar]
- 23. Yagupsky P. 2008. Use of blood culture vials and nucleic acid amplification for the diagnosis of pediatric septic arthritis. Clin. Infect. Dis. 46:1631–1632 [DOI] [PubMed] [Google Scholar]
- 24. Yagupsky P., Dagan R. 1997. Kingella kingae: an emerging cause of invasive infections in young children. Clin. Infect. Dis. 24:860–866 [DOI] [PubMed] [Google Scholar]
- 25. Yagupsky P., Dagan R. 2000. Population-based study of invasive Kingella kingae infections. Emerg. Infect. Dis. 6:85–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yagupsky P., et al. 1992. High prevalence of Kingella kingae in joint fluid from children with septic arthritis revealed by the BACTEC blood culture system. J. Clin. Microbiol. 30:1278–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.