Abstract
We genetically characterized pinworms obtained from 37 children from different regions of Germany and established new species-specific molecular diagnostic tools. No ribosomal DNA diversity was found; the phylogenetic position of Enterobius vermicularis within the Oxyurida order and its close relationship to the Ascaridida and Spirurida orders was confirmed.
Pinworms are the most common intestinal parasites in developed countries in temperate climates (1). Humans are the only natural host of Enterobius vermicularis, and children are the most often affected by this disease, which is spread from the anus to mouth. Although it is regarded as a harmless infection, severe disease like colitis, perianal abscess, ectopic infections in females, and appendicitis can occur (1, 3, 12). Whereas eradication is achieved by anthelmintics in most cases (12), recurrent or persistent oxyuriasis lasting for years despite several treatment courses is seen in some patients. Molecular tools might help to understand transmission routes and distinguish persistent from repeated infections. However, sequence information on E. vermicularis is limited (6, 7).
Pinworms were identified by examining feces from children with a magnifier, washed in tap water, and stored at −20°C. Then, pinworms from 37 children residing in different geographic regions of Germany were thawed and mechanically homogenized to extract DNA (Qiamp DNA minikit; Qiagen, Hilden, Germany). Enterobius vermicularis ribosomal DNA (rDNA) was amplified and sequenced as partially overlapping fragments using universal primers (AB28/TW81 and NEMF1/S3), degenerated nematode primers, and species-specific primers (Table 1). Amplification reaction mixtures (50 μl) consisted of 100 nM (each) primer, 50 μM (each) deoxynucleoside triphosphates (dNTPs), 2.5 mM MgCl2, 0.5 units polymerase, and 10 μl template. PCR amplification was performed as follows: (i) denaturation at 95°C for 5 min; (ii) 40 cycles, with 1 cycle consisting of 60 s at 94°C, 60 s at 50 to 60°C, and 2 min at 72°C for 2 min, and (iii) a final extension step at 72°C for 10 min. Amplification of 18S rDNA fragments for diagnostic purposes using Enterobius-specific primers Ev18S.F1 and Ev18S.R1 was performed at 55°C under otherwise identical conditions. Products were detected on ethidium bromide-stained agarose gels. PCR products were sequenced either directly or after gel extraction (QIAquick gel extraction kit; Qiagen, Hilden, Germany) and cloning (TOPO-TA; Invitrogen, Karlsruhe, Germany) using a BigDye terminator cycle sequencing kit and an ABI Prism 310 genetic analyzer (Applied Biosystems, Warrington, United Kingdom). Site polymorphisms were scored when alternative nucleotide peaks present were equal in height or when a minor peak significantly exceeded the background level and comprised ≥50% of the major peak. We amplified and sequenced the DNA of the small ribosomal subunit (18S rDNA, 1,716 bp), first and second internal transcribed spacer regions (ITS1 and ITS2, 1,073 bp) including the 5.8S rDNA (159 bp), and a 78-bp fragment of the large ribosomal subunit (28S). The complete 1,716-bp-long 18S rDNA sequence was obtained from only 27 worms, and all 37 ITS1-5.8S-ITS2 sequences were identical. Hence, our molecular analyses reveal no diversity of the genes examined to distinguish E. vermicularis isolates. Our result is in accordance with Iñiguez et al., who could not detect nucleotide differences within a 420-bp-long 5S rRNA sequence of seven pinworms from Brazil (6). The nonexistent genetic diversity might be due to excellent adaptation to the human host or low evolutionary pressure. However, analyses of less-conserved targets such as mitochondrial genes or the whole genome with for example randomly amplified polymorphic DNA (RAPD) using worldwide isolates are required to further elucidate the genetic diversity of E. vermicularis.
Table 1.
Primers used in PCR amplification and sequencing of Enterobius vermicularis ribosomal DNAa
| Primer | Position | Sequence (5′ to 3′) |
|---|---|---|
| TW81 | 3′ end of the 18S rDNA | GTT TCC GTA GGT GAA CCT GC |
| AB28 | 5′ end of the 28S rDNA (2812–2833) | ATA TGC TTA AGT TCA GCG GGT |
| NEMF1 | 376–393 | CGC AAA TTA CCC ACT CTC |
| S3 | 3′ end | AGT CAA ATT AAG CCG CAG |
| SSU_F_01 | 5′ end | AAC CTG GTT GAT CCT GCC AGT |
| SSU_R_26 | 927–907 | CAT TCT TGG CAA ATG CTT TCG |
| SSU_F_24 | 868–887 | AGR GGT GAA ATY CGT GGA CC |
| SSU_R_81 | 1817–1836 | TGA TCC WKC YGC AGG TTC AC |
| SSU_R_82 | 1817–1840 | TGA TCC TTC TGC AGG TTC ACC TAC |
| Ev18S.F1 | 1147–1166 | AAC ACG GGA AAA CTC ACC TG |
| Ev18S.R1 | 1341–1361 | GCA CTG ACG GTT AAG CCA AT |
| Ev18S.F2 | 445–464 | CCG GTT ATC GGA ATG AGT GG |
| Ev18S.R2 | 1024–1043 | TTC CGG AGA GCT ACC AGC TA |
| Ev18S.F3 | 963–982 | CGC CCT AGT TCT GAC CGT AA |
| Ev18S.R3 | 1539–1558 | GGA GGA TTT TCA GGG GGT TA |
The universal eukaryotic ITS primers TW81 and AB28 were used to amplify the ITS1-5.8S-ITS2 region; the nematode 18S consensus forward (F) primer NEMF1 was used with the universal eukaryotic reverse primer S3 (14). All small-subunit (SSU) primers were adapted from Nematode Phylogenetics Primers (http://www.nematodes.org/barcoding/sourhope/nemoprimers.html). All Ev18S primers were designed based on the 18S ribosomal DNA sequence obtained in this study. R, reverse.
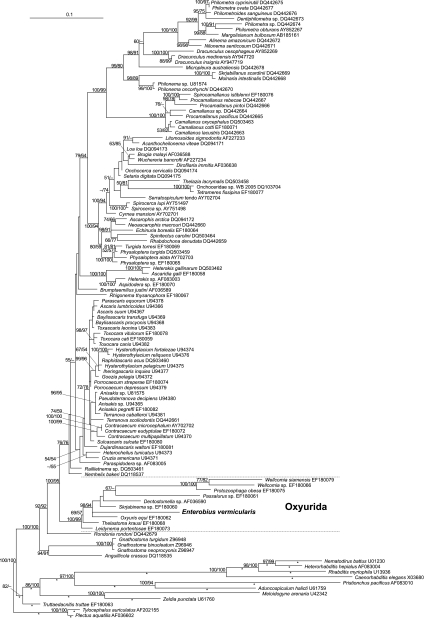
The small-subunit ribosomal DNA sequences were used to construct the molecular phylogeny of Nematoda (2, 9) where Enterobius vermicularis had not yet been included. Thus, we analyzed our 18S rDNA sequence together with other nematode sequences used by Nadler et al. (9) and published in GenBank (NCBI). This data set was aligned with DIALIGN (8). For phylogenetic reconstruction, we excluded those positions that received scores as low as 0 or 1 from the alignment. We ran maximum likelihood analyses using RaxML (11), involving rapid bootstrapping over 1,000 rounds and using every 5th bootstrap tree as a starting point for heuristic search; GTRMIX was used as a DNA substitution model. Additional branch support was calculated using maximum parsimony bootstrap in PAUP* (13); we ran 1,000 bootstrap replicates, with 100 rounds of heuristic search per replicate starting from trees that resulted from subsequent addition of sequences in random order, treating gaps in the alignment as missing data and the multitree option not in effect. The maximum likelihood tree was rooted with Plectus aquatilis (GenBank [NCBI] accession no. AF036602) and Tylocephalus auriculatus (GenBank [NCBI] accession no. AF202155) and compared with previous molecular phylogenetic hypotheses (2, 4, 5, 9). Our analyses (Fig. 1) assigned E. vermicularis to the Oxyurida and confirmed previous studies showing that nematodes from the orders Ascaridida, Oxyurida, Rhigonematida, and Spirurida belong to a monophyletic group, informally designated “clade III” (2, 4, 9). Oxyurida is a highly supported monophyletic group (the maximum likelihood [ML] and maximum parsimony [MP] bootstrap values are 100 and 99, respectively), which appears as the sister group to a clade that includes Ascaridida and Spirurida.
Fig. 1.
Phylogenetic placement of Enterobius vermicularis as derived from heuristic maximum likelihood analysis of an alignment of 18S rDNA sequences. The species is given first and then the GenBank (NCBI) accession number. The best tree found was rooted with Tylocephalus auriculatus and Plectus aquatilis. Branch lengths are given in terms of the estimated numbers of nucleotide substitutions per site. Asterisks mark branches that were scaled with factor 0.85 for graphical reasons. The numbers at the nodes are the maximum likelihood/maximum parsimony values. Branch support was calculated by maximum likelihood/maximum parsimony bootstrap from 1,000 replicates; values below 50% are designated by a − symbol or omitted. Bar, 0.1 nucleotide substitution per site.
Although not useful to distinguish isolates, the 18S rDNA appears conserved enough for diagnostic purposes. Using our primers Ev18S.F1 and Ev18S.R1 (Table 1), a single product of 215 bp was successfully amplified and sequenced from 35 pinworms examined. All Enterobius vermicularis sequences were identical, showing 73.9% identity to 18S rDNA of Trypanoxyuris sciuri (Oxyurida), 71% identity to Oxyuris equi (Oxyurida), and 73% identity to Anisakis sp. (Ascaridida) when applied to NCBI Blast Search (15). No amplification was observed when DNA from adult Ascaris lumbricoides, Acanthocheilonema sp., or human DNA was examined.
This PCR assay was used to examine 60 pieces of Scotch tape each fixed to a glass slide and sent to our lab for diagnosis. Typical pinworm eggs were identified by microscopy for only 40. Then, pieces of the tape (1 to 1.5 cm by 1 cm) were cut and scraped off the glass slide using a sterile scalpel to avoid carryover contamination, and the eggs were transferred into tubes for DNA extraction as described above. DNA was amplified from all 40 microscopically positive samples and from three negative samples. No PCR product was obtained from the remaining 17 samples. Two of the three unexpected PCR-positive samples originated from samples from two children that gave positive results by microscopy and PCR in a second sample. The third false-positive sample was obtained from a household member of an infected child. It was concluded that the diagnostic PCR assay is at least as sensitive as microscopy (10). Pinworm eggs are usually deposited on the perianal skin and detected by the Scotch tape technique. Thus, they are very rarely found in fecal samples usually sent for parasitological diagnostics. At this time, we are organizing a comparative diagnostic Enterobius PCR study using DNA isolated from fecal samples and Scotch tape.
In conclusion, we did not find any ribosomal DNA diversity of Enterobius vermicularis in Germany, we confirmed its phylogenetic position within Oxyurida, and developed a new species-specific diagnostic PCR which might identify more pinworm carriers than conventional tests.
Nucleotide sequence accession number.
The 2,867-bp sequence was deposited in GenBank (NCBI) under accession number HQ646164.
Acknowledgments
The technical assistance of Ulrike Müller-Pinau and Andrea Dlugosch is highly appreciated. We thank two anonymous reviewers for very helpful suggestions and astute comments.
Footnotes
Published ahead of print on 19 January 2011.
REFERENCES
- 1. Ariyarathenam A. V., et al. 2010. Enterobius vermicularis infestation of the appendix and management at the time of laparoscopic appendectomy: case series and literature review. Int. J. Surg. 8:466–469 [DOI] [PubMed] [Google Scholar]
- 2. Blaxter M. L., et al. 1998. A molecular evolutionary framework for the phylum Nematoda. Nature 392:71–75 [DOI] [PubMed] [Google Scholar]
- 3. Craggs B., et al. 2009. Enterobius vermicularis infection with tuboovarian abscess and peritonitis occuring during pregnancy. Surg. Infect. 10:545–547 [DOI] [PubMed] [Google Scholar]
- 4. De Ley P., Blaxter M. L. 2002. Systematic position and phylogeny, p. 1–30 In Lee D. L. (ed.), The biology of nematodes. Taylor and Francis, London, United Kingdom [Google Scholar]
- 5. Feldman S. H., Bowman S. G. 2007. Molecular phylogeny of the pinworms of mice, rats and rabbits, and its use to develop molecular beacon assays for the detection of pinworms in mice. Lab Anim. (New York) 36:43–50 [DOI] [PubMed] [Google Scholar]
- 6. Iñiguez A. M., Vicente A. C., Araúj A., Ferreira L. F., Reinhard K. J. 2002. Enterobius vermicularis: specific detection by amplification of an internal region of 5S ribosomal RNA intergenic spacer and trans-splicing leader RNA analysis. E. vermicularis: specific detection by PCR and SL1 RNA analysis. Exp. Parasitol. 102:218–222 [DOI] [PubMed] [Google Scholar]
- 7. Kang S., et al. 2009. The mitochondrial genome sequence of Enterobius vermicularis (Nematoda: Oxyurida)–an idiosyncratic gene order and phylogenetic information for chromadorean nematodes. Gene 429:87–97 [DOI] [PubMed] [Google Scholar]
- 8. Morgenstern B. 1999. Dialign 2: improvement of the segment-to-segment-approach to multiple sequence alignment. Bioinformatics 15:211–218 [DOI] [PubMed] [Google Scholar]
- 9. Nadler S. A., et al. 2007. Molecular phylogeny of clade III nematodes reveals multiple origins of tissue parasitism. Parasitology 134:1421–1442 [DOI] [PubMed] [Google Scholar]
- 10. Parija S. C., Sheeladevi C., Shivaprakash M. R., Biswal N. 2001. Evaluation of lactophenol cotton blue stain for detection of eggs of Enterobius vermicularis in perianal surface samples. Trop. Doct. 31:214–215 [DOI] [PubMed] [Google Scholar]
- 11. Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690 [DOI] [PubMed] [Google Scholar]
- 12. St. Georgiev V. 2001. Chemotherapy of enterobiasis (oxyuriasis). Expert Opin. Pharmacother. 2:267–275 [DOI] [PubMed] [Google Scholar]
- 13. Swofford D. L. 2002. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods), version 4b10. Sinauer Associates, Sunderland, MA [Google Scholar]
- 14. Waite I. S., et al. 2003. Design and evaluation of nematode 18S rDNA primers for PCR and denaturing gradient gel electrophoresis (DGGE) of soil community DNA. Soil Biol. Biochem. 35:1165–1173 [Google Scholar]
- 15. Zhang Z., Schwartz S., Wagner L., Miller W. 2000. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 7:203–214 [DOI] [PubMed] [Google Scholar]