Rapid antigen tests (RAT) are used to screen patients with suspected influenza virus infection and provide results in a timely manner. RAT can also help to reduce unnecessary diagnostic testing, to facilitate antiviral treatment, and to decrease inappropriate use of antibiotics (4). However, the clinical sensitivity of RAT was poor for 2009 H1N1 influenza virus, showing an accuracy from 11.1% to 51% (2–5). Drexler et al. have suggested that the viral concentrations in clinical samples influence the outcome of RAT (2). Thus, the collection time of the samples may be an important factor for the accuracy of RAT.
Retrospectively, we tested 637 clinical samples from 637 different patients. Samples were collected during the pandemic 2009 H1N1 influenza season by nasopharyngeal swab and were kept frozen at −80°C until use. The 120 controls were taken from H1N1-negative febrile subjects.
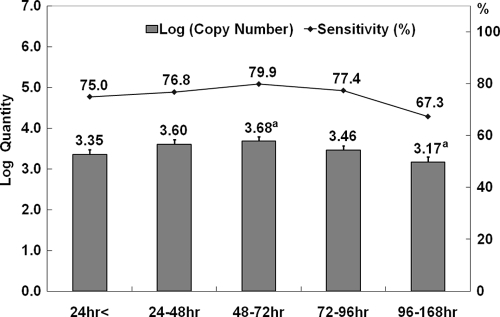
The 2009 H1N1 influenza virus was confirmed by real-time reverse transcription-PCR. A standard curve of control RNA transcripts was constructed in parallel with the detection of viral M segment RNA in clinical samples. Using this standard curve, we calculated the log10 viral copy number from the cycle threshold (CT). The cutoff value of CT was set at 37 for H1N1 influenza diagnosis. All processes were conducted by following the Centers for Disease Control protocol for H1N1. The RAT was done by using the SD Bioline influenza A/B/A(H1N1) pandemic test kit (Standard Diagnostics, Yongin, South Korea). The RAT has four lines, for the detection of 2009 H1N1, influenza A, influenza B, and controls, and distinguishes between seasonal influenza virus and 2009 H1N1 influenza virus (1). Samples were classified when they were collected by the number of hours elapsed after the first symptoms appeared. They were classified into ≤24 h (D1), 24 to 48 h (D2), 48 to 72 h (D3), 72 to 96 h (D4), and 96 to ≤168 h (D5). We calculated the sensitivity and specificity of the RAT. Data analysis was performed using SPSS, version 16.0. The analysis of variance (ANOVA) test and Tukey's post hoc test were used to compare the mean log10 viral copy numbers. The study protocol was approved by the Institutional Review Board of the Chonbuk National University Hospital. The mean age of the subjects was 23.4 ± 12.81 (median, 18.0; range, 13 to 82; male, 51.0%). The control patients had a mean age of 34.6 ± 20.8 (median, 27.0; range, 8 to 82; male, 54.3%). The overall sensitivity and specificity of the RAT were 75.6% and 99.3%, respectively. The sensitivity of the RAT at D1, D2, D3, D4, and D5 was 75.0%, 76.8%, 79.9%, 77.4%, and 67.3%, respectively (Fig. 1). The log quantity of virus copy numbers at D1, D2, D3, D4, and D5 was 3.35, 3.60, 3.68, 3.46, and 3.17, respectively (P = 0.025). Only D3 and D5 showed a significant difference by Tukey's post hoc test (P = 0.026). The log10 virus copy number was increased until D3 and then decreased.
Fig. 1.
2009 H1N1 influenza viral loads and time-dependent sensitivity of RAT in clinical samples. The viral loads are presented as log10 of M segment copy number/1 ml of viral transport medium. An ANOVA test was used to compare the mean log10 viral copy numbers in specimens from patients with different collection times (P = 0.025). “a” indicates a significant difference of the viral load between 48 to 72 h versus 96 to 168 h by using Tukey's post hoc test (P = 0.026). The sensitivity of the RAT is presented as a percentage.
The RAT is known as a point-of-care test because it can provide results within 30 min or less in every facility. However, the poor sensitivity of the RAT for H1N1 virus was a major problem. In this study, our results revealed that the most appropriate time frame of sample collection for the detection of influenza virus with RAT was 48 to 72 h after the first clinical symptoms appeared. The sensitivity value of RAT in our study was similar to the values reported by Choi et al. (1), but it was higher than those reported by others (2–5). Our results may help to raise the sensitivity of RAT to be used during the influenza seasons.
Footnotes
Published ahead of print on 19 January 2011.
Contributor Information
Chang-Seop Lee, Department of Internal Medicine, Chonbuk National University Medical School, San 2-20, Geumam-Dong, Deokjin-Gu, Jeonju, Jeonbuk, South Korea.
Ju-Hyung Lee, Department of Preventive Medicine, Chonbuk National University Medical School, San 2-20, Geumam-Dong, Deokjin-Gu, Jeonju, Jeonbuk, South Korea.
Cheon-Hyeon Kim, Division of Zoonoses, Jeollabukdo Institute of Health & Environmental Research, Jeonbuk, South Korea.
REFERENCES
- 1. Choi Y. J., et al. 2010. Evaluation of new rapid antigen test for detection of pandemic influenza A/H1N1 2009 virus. J. Clin. Microbiol. 48:2260–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drexler J. F., et al. 2009. Poor clinical sensitivity of rapid antigen test for influenza A pandemic (H1N1) 2009 virus. Emerg. Infect. Dis. 15:1662–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Faix D. J., Sherman S. S., Waterman S. H. 2009. Rapid-test sensitivity for novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 361:728–729 [DOI] [PubMed] [Google Scholar]
- 4. Uyeki T. M., et al. 2009. Low sensitivity of rapid diagnostic test for influenza. Clin. Infect. Dis. 48:e89–e92 [DOI] [PubMed] [Google Scholar]
- 5. Vasoo S., Stevens J., Singh K. 2009. Rapid antigen tests for diagnosis of pandemic (swine) influenza A/H1N1. Clin. Infect. Dis. 49:1090–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]