Abstract
Hyperglycemia that does not satisfy the diagnostic criteria for diabetes mellitus (DM) is generally called prediabetes (preDM). The global prevalence of preDM has been increasing progressively in the past few decades, and it has been established that preDM status is a strong risk factor for DM and cardiovascular disease. Currently, preDM status is classified into two subtypes: impaired fasting glucose and impaired glucose tolerance. Currently, preDM is not regarded as an independent clinical entity, but only as a risk factor for others. In this article, we review various clinical aspects of preDM in terms of the working definition, changes in criteria over the years, epidemiology, and pathophysiological characteristics, and its clinical significance in current medicine.
Keywords: Diabetes mellitus, type 2; Insulin resistance; Korea; Prediabetic state
INTRODUCTION
Non-diabetic hyperglycemia that does not satisfy the diagnostic criteria for diabetes mellitus (DM) is generally known as prediabetes (preDM). It is generally agreed that all forms of diabetes pass through this prediabetic state before escalating into full-blown diabetes [1]. Similar to DM, fasting plasma glucose (FPG) and oral glucose tolerance tests are both used independently as defining criteria for preDM [1,2]. Recently, hemoglobin A1c (HbA1c) has also been used in the diagnosis of preDM [1,3].
Because it is well-established that the risks of type 2 DM (T2DM) and cardiovascular disease are significantly increased in preDM subjects, life style modifications have been recommended for this population as a method to control preDM [2,4]. The benefits of this have been confirmed in many prospective, randomized studies where life style modification and pharmacological intervention have been shown to significantly improve clinical markers related to the risk of T2DM and cardiovascular disease [5-7]. However, current preDM medical guidelines treat this condition as a risk factor for diabetes and cardiovascular disease, rather than a separate clinical entity. Additionally, the clinical significance of preDM has not been clearly established [1,2,4]. Studies suggest that preDM has specific clinical significance separate from type 2 diabetes. Because of this, it is thought that more active discussions are needed on the topic. Here, we review various clinical aspects of preDM with respect to the working definition, changes in criteria over time, epidemiology, pathophysiological characteristics, and its clinical significance in current medicine.
DEFINITION OF PREDIABETES AND DIAGNOSTIC CRITERIA
According to the most recent clinical practice recommendations published in 2010 by the American Diabetes Association (ADA), preDM is defined as 1) impaired fasting glucose (IFG) with fasting plasma glucose levels of 100 to 125 mg/dL (5.6 to 6.9 mmol/L), 2) impaired glucose tolerance (IGT) with plasma glucose levels of 140 to 199 mg/dL (7.8 to 11.0 mmol/L) 2-hour postprandial, or 3) an HbA1c of 5.7 to 6.4% [1]. These criteria have evolved over the years and discussions on the topic continue.
Changes in preDM diagnostic criteria
In the 1979 American National Diabetes Data Group (NDDG) report, the first consensus-based, systematic criteria for DM were proposed. Specifically, non-diabetic hyperglycemia was defined as being in the high-risk group for the progression of DM and the presence of IGT [8]. The diagnostic criteria for IGT required satisfaction of the following three conditions: 1) FPG <140 mg/dL (7.8 mmol/L), 2) FPG ≥200 mg/dL (11.1 mmol/L) 0.5, 1, and 1.5 hours after a 75-g oral glucose tolerance test, 3) and FPG between 140 and 200 mg/dL (7.8 to 11.1 mmol/L) 2 hours after a 75-g oral glucose tolerance test [8]. However, the diagnostic criteria for IGT based on the NDDG reports are somewhat restricting, and there were difficulties including some subjects during the screening period [9].
The diagnostic criteria for IGT that were published by the World Health Organization (WHO) in 1980 were simpler than the NDDG classification criteria. IGT was defined by a 2-hour plasma glucose level of 140 to 200 mg/dL (8.0 to 11.0 mmol/L) after a 75-g oral glucose tolerance test [10]. However, WHO criteria excluded the fasting criteria in the IGT diagnosis and only emphasized post-challenge glucose. This is because confirmation of fasting during screening is not easily obtained [10].
Eighteen years after the NDDG report, ADA proposed new diagnosis criteria for DM in 1997 [11]. When considering the cost, time, and reproducibility of oral glucose tolerance tests, it was recommended that diabetes screening and diagnostic tests primarily use the FPG test. Based on macrovascular complication risks, the threshold for the diagnostic criterion changed from 140 to 126 mg/dL [11]. Thus, according to these criteria, a fasting glucose of 110 to 126 mg/dL in preDM (6.1 to 7.0 mmol/L) is classified as IFG, while a 2-hour post-challenge plasma glucose concentration between 140 and 200 mg/dL (7.8 to 11.1 mmol/L) is now classified as IGT. The WHO diagnostic criteria that were released in 1999 were very similar to the ADA diagnostic criteria [12,13].
To prevent similarity between IGT and IFG, in the ADA Expert Committee report, the IFG criteria was changed from 110 mg/dL (6.1 mmol/L) to 100 mg/dL in 2003 (5.6 mmol/L) [14,15]. Recently, the International Expert Committee recommended that HbA1c be added to the diagnosis of DM; the 2010 ADA clinical practice recommendation defining HbA1c levels of over 6.5% as DM, and an HbA1c between 5.7 and 6.4% as preDM [1,3].
Major issues in diagnostic criteria changes for preDM
The diagnostic criteria for diabetes and preDM have been changed several times, and the use of A1c and others tests as diagnostic criteria for DM have been actively discussed. However, there does not appear to be a complete consensus regarding this matter.
Issues regarding IFG criteria
To determine the cut-off values for preDM, criteria that sharply delineate increases in the risk of adverse clinical or metabolic outcomes based on changes in blood glucose are needed. These questions are important for both IFG and IGT. However, unlike the widely accepted IGT criteria, controversy remains regarding diagnostic criteria for IFG.
In 2003, the ADA recommended that the criterion for IFG be lowered from 110 mg/dL (6.1 mmol/L) to 100 mg/dL (5.6 mmol/L) [14]. This change was based on receiver operating characteristic (ROC) analysis results for fasting blood glucose levels. Specifically, these criteria were able to maximize sensitivity and specificity in detecting DM over a period of 5 years from Pima Indian, Mauritius, San Antonio, and Hoorn Study data [14,16-18]. The cut-off value for maximum sensitivity and specificity for detecting DM was set at 97 to 99 mg/dL (5.4 to 5.5 mmol/L) [14]. Additionally, by lowering the IFG cut-off value, IFG prevalence became similar to IGT prevalence, and the percentages of people who were screened for FPG and postprandial blood glucose were similarly matched [14].
However, the WHO advised that the cut-off value for IFG should be maintained at 110 mg/dL, because IFG prevalence would be increased significantly by changing the cut-off criterion. Although this could have important personal or social impacts, there is not a major reduction in adverse outcomes or the prevention of DM progression as a result of this change [19].
The actual risk of developing DM is known to be associated with an increase in FPG concentrations from "normal" conditions. In studies conducted in young men, the risk of developing diabetes gradually increased when fasting blood glucose levels rose from under 81 mg/dL (4.5 mmol/L) to over 86 mg/dL (4.8 mmol/L) [20]. This result suggests that there is no exact cut-off value for FPG levels that predicts adverse outcomes. Additionally, in the Atherosclerosis Risk in Communities (ARIC) study, when FPG levels were over 106 mg/dL (5.9 mmol/L), it was predicted with 50% sensitivity that subjects would develop diabetes, whereas when FPG was over 100 mg/dL (5.6 mmol/L), it was predicted with 70% sensitivity; thus, higher-risk groups could be screened for. However, the percentage of the population that can be screened as a high-risk group increases by more than double the normal value with this kind of increase in sensitivity [21]. Additionally, when the ADA diagnostic criterion cut-off value was lowered, new IFG patients had more favorable cardiovascular risk profiles and a lower risk of developing diabetes compared with patients who were selected by the WHO criteria [22-25]. For these reasons, many diabetes experts did not completely agree with the ADA's IFG diagnostic criteria [19,22,26].
HbA1c criteria issues
A1c had been widely used as a test to estimate the degree of glycemic control. Because it can be measured regardless of food intake, A1c is simpler than FPG or oral glucose tolerance tests. Additionally, because it reflects long-term glucose concentrations, versus frequently changing glucose levels, it is more closely related to the chronic complications of diabetes [27-29]. Because the current DM diagnostic criteria are based on an increased risk of developing DM-related chronic complications, using A1c for the diagnosis of DM is a rational approach. However, the cost of A1c compared with simple glucose tests is high. Additionally, because measurement standards have not been established, its use as a diagnostic criterion for diabetes has been limited. Measurement methods are continuously improving, and according to the efforts of professionals from the American National Glycohemoglobin Standardization Program, European International Federation of Clinical Chemistry, Japanese Diabetes Society/Japanese Society of Clinical Chemistry, and other organizations for measurement standardization, A1c is now actively being considered as a diagnostic criterion for diabetes [27,30,31].
In several studies that have examined the diagnostic value of A1c, compared with FPG, A1c has been shown to have lower variability and higher specificity [32]. Based on several population-based studies, the A1c cut-off value has been set at 5.9 to 6.2% for estimated diabetes diagnoses [33-36]. Specifically, in the National Health and Nutrition Examination Survey (NHANES) III, where the mean value of the A1c cut-off was set at over 6.5%, diabetes diagnoses had a 99.6% specificity and a 43 to 44% sensitivity [37]. Based on the results of these studies, the International Expert Committee proposed that the new standard A1c value for the diagnosis of DM be changed to above 6.5%. Since this time, the ADA has adopted it as a new official diagnostic criterion [1,3].
For preDM, the International Expert Committee also decided that A1c values between 6 and 6.5% are considered to be in the high-risk category for diabetes [3]. In an actual population-based study, participants who had A1c levels between 6 and 6.5% had a 10-fold increase in the occurrence of diabetes compared with participants with A1c levels below 6% [38,39]. However, over 5 years, participants with A1c levels of 5.5 to 6.0% had cumulative occurrence percentages between 12 and 25%, which was 3 to 8 times higher than the control group. Based on data from NHANES study subjects, the closest A1c level for current IFG and IGT criteria was 5.5 to 6.0%; however, in the linear regression analysis, a fasting glucose level of 100 mg/dL corresponded with an A1c of 5.4%, and a fasting glucose level of 110 mg/dL corresponded with an A1c of 5.6% [1]. When the A1c cut-off value for preDM was set at 5.7%, sensitivity was 39 to 45% and specificity was 81 to 91% for IFG and IGT predictions [1]. Based on large-scale prospective studies, when the preDM cut-off value was set at 5.7%, the sensitivity was 66% and specificity was 88% for predicting the risk of DM risk over the next 6 years [36]. Consistent with these results, an HbA1c of 5.7 to 6.4% was presented by the ADA as a preDM criterion [1].
However, based on the current criteria, diagnosis of subjects screened using the A1c 5.7 to 6.4% criterion are not completely consistent with IFG and IGT methods. With respect to the fasting blood glucose cut-off values proposed by the ADA, it is expected that that it will take some time until the aforementioned diagnostic criteria are widely accepted.
EPIDEMIOLOGY OF PREDM
According to several epidemiological studies, the worldwide prevalence of preDM exceeds that of true diabetes. The prevalence of preDM is expected to increase, and factors related to race, age, and various characteristics related to gender are known to exist.
Prevalence
According to the IDF Diabetes Atlas, currently, the number of cases of IGT (2010) worldwide is estimated to be approximately 340 million [40,41]. North America has the highest prevalence of IGT in the world, with 10.4%. For Europe and the Middle East, the values are 8.9% and 8.2%, respectively, which is also relatively high versus other parts of the world [41]. In Southeast Asia and the Western Pacific Region, the prevalences are 6.2% and 7.7%, respectively [41]. However, according to the absolute differences in population size, the Western Pacific Region is estimated to have the largest number of IGT subjects, approximately 120 million people [41]. When compared with DM, the prevalence of IGT is generally similar; however, in North America, the prevalence of IGT is lower than that of DM. In Africa and the Western Pacific Region, IGT prevalence is slightly higher than that of diabetes [41]. By 2030, the global prevalence of IGT is estimated to reach 8.4%, which will be approximately 462 million people [41].
There are no additional comprehensive global estimates of IGF prevalence; however, in most parts of the world, it is known that the prevalence is above 5% [2]. In 2002, an IGT/IFG consensus statement published by the International Diabetes Federation (IDF) stated that based on surveys conducted in many parts of the world, IGF prevalence was 2.0 to 17.3% [2]. Generally, the prevalence of IGT is known to be higher than that of IFG; however, these data were mostly based on the previous ADA/WHO criteria. According to the new ADA criteria, when the IFG cut-off value is adjusted to 100 mg/dL, IFG prevalence increases dramatically. In this case, the increase in IFG prevalence is greater than that of IGT [26,42]. For example, by changing the IFG diagnostic criteria, the Danish IFG prevalence increased from 11.8% to 37.6% [43]. Further, by changing the diagnostic criteria in DETECT-2 study subjects, IFG prevalence increased from 12.7% to 28.7% in Chinese subjects, from 11.0% to 38.6% in Asian Indians, from 16.3% to 45.7% in French subjects, and from 12.1% to 32.0% in the Unites States [44]. According to data from a survey on NHANES subjects, the IFG prevalence of Americans over the age of 20 was reported as 25.7% and IGT was reported as 13.8% [45].
Overlap of IFG and IGT
The issue with the IFG and IGT concept began with whether screening for diabetic high-risk groups should be performed using fasting blood glucose or postprandial blood glucose tests. However, subjects selected through IFG and IGT criteria were not identical.
Generally, among subjects screened using IGT, only 20 to 25% of individuals have FPG levels ≥110 mg/dL. In subjects screened with IFG, <50% have a postprandial 2-hour glucose level ≥140 mg/dL [2]. In the DECODE studies, which examined European IFG subjects that were defined exclusively by FPG levels between 6.1 and 6.9 mmol/L, 64.8% of subjects had 'isolated' IFG, 28.6% showed combined IFG with IGT, and 6.6% were diagnosed with diabetes [2,46]. In the DECODA studies, 45.9% of Asian IFG subjects that were defined exclusively by FPG levels had 'isolated' IFG, 35.2% showed IFG values that were associated with IGT, and 18.9% had diabetes [47]. Even in the analyses of NHANES subjects, only some of the preDM subjects showed overlap between IGT and IFG. Using the ADA IFG criteria, 60% of all IGT subjects were screened; however, among subjects classified as IFG, only 18.5% of IGT subjects could be screened.
These data suggest that screening for all high-risk diabetes groups is not possible through the simple methods of either fasting glucose or postprandial blood glucose. Additionally, these results show that across physiological conditions, there can be a significant difference between IFG and IGT.
Characteristics of age and gender
Differences in age and gender between IFG and IGT are known to exist depending on the populations and region investigated. Generally, the prevalence of IFG and IGT increases according to age. Additionally, while IFG prevalence is relatively high in men, it is lower in women [2]. In the DECODE studies, the prevalence of isolated IFG in men increased gradually with age. In the 50 to 59 age group, IFG decreased gradually after reaching a plateau of 10.1%. Additionally, when the over 70 group was excluded, prevalence in men was significantly higher [2,46,48]. The prevalence of isolated IGT increased continuously regardless of gender, and excluding the over 70 group, the prevalence in women of all ages was significantly higher [2,46]. In the DECODA studies in Asians, excluding Indian cohorts, isolated IFG and isolated IGT prevalence both tended to increase with age, but not gender [2,47]. Differences by gender were not significant when compared with Europeans. Further, the isolated IFG prevalence of women in the same age group was much higher than in men, and women had higher incidences of isolated IGT [2,47]. Even in NHANES subjects, the prevalence of IFG and IGT increased proportionally with age [45]. However, IFG prevalence in men was 32.1%, compared with 19.8% in women. There was no significant difference in IGT based on gender; IGT prevalence in men was 14.6% and 13.1% in women [45]. There was also no observed difference in the prevalence of preDM in NHANES subjects based on ethnic backgrounds [45].
There are differences between target populations in the above large-scale epidemiological survey results, and an exact comparison between each is very difficult. Estimating incidence is not possible using a cross-sectional design. Furthermore, with the rapidly changing lifestyle habits observed in many Asian countries, there is a problem applying epidemiological characteristics longitudinally.
The clinical course of preDM
The risk for DM in preDM subjects is much higher compared with those with normal glucose tolerance (NGT). When several prospective epidemiological studies were compiled, the incidence of T2DM in isolated IFG and IGT subjects was estimated at 4 to 6% per year, and this value was significantly higher than NGT subjects (<0.5% per year) [5-7,13,16,49]. In the subjects who were diagnosed with IFG and IGT in combination, the annual percentage for the risk of developing T2DM increased by 10%. This suggests that IFG and IGT have additive roles in predicting the risk of developing T2DM. However, some preDM subjects also experienced complete recovery to normal glucose tolerance levels. In an 11-year follow-up study on Mauritius adults with IGT, 46% progressed into overt DM, 28% retained their condition, 4% changed to IFG, and 24% showed normalized glucose tolerance [16]. For patients with IFG, 38% developed overt DM, 7% experienced no change, 17% progressed to IGT, and 38% developed normalized glucose tolerance [16].
The occurrence of cardiovascular disease and associated mortality in preDM subjects compared with normal glucose tolerance are known to be significantly elevated [25,50,51]. In several studies, the risk of cardiovascular disease in preDM patients compared with NGT increased by over 50%, and this risk was greater in young adults [25]. However, in each study, there were differences in the risks of developing cardiovascular disease between IFG and IGT, and when confounding variables were compensated for, the risk of developing cardiovascular disease was lowered [25]. As previously mentioned, in patients screened as having IFG based on the new ADA diagnostic criteria, and using the WHO criteria as a comparison, the cardiovascular risk profile is a little more favorable, and the risk of developing diabetes is known to be lower. There is much room for debate regarding IFG diagnostic criteria and the risk of developing cardiovascular disease [22-25].
PATHOPHYSIOLOGY OF PREDM
Epidemiological studies examining a large number of subjects who were independently screened for both IFG and IGT suggest that the pathophysiological characteristics of IFG and IGT are significantly different [2]. The most important factors that may explain the pathophysiology of T2DM are increased insulin resistance and decreased insulin secretion. Studies analyzing insulin resistance and insulin secretion in isolated IFG and IGT subjects suggest that there are distinguishable characteristics between the subtypes [52-57]. However, differences in pathophysiology between each preDM subtypes still do not clarify whether these commonly shared phenomena occur in various regions or ethnicities around the world. Additionally, their significance in clinical practice is unknown.
Perspectives on insulin resistance
During glucose stimulation, pancreatic insulin secretion physiologically suppresses hepatic glucose production in the liver; however, glucose utilization is promoted in the peripheral tissues, including muscle and adipose tissue. Insulin resistance refers to a dysfunctional physiological response to insulin secretion in vivo. Despite normal or higher insulin levels, hepatic glucose production is not adequately suppressed, or a reduction in glucose utilization in peripheral tissue causes increased plasma glucose concentrations. Compared with NGT subjects, there is a significantly higher tendency for insulin resistance to increase in preDM subjects. Additionally, important differences in the mechanisms underlying insulin resistance are known to exist between preDM subtypes [42,52-55,57].
Hepatic insulin resistance in IFG subjects is significantly higher than in NGT subjects. In contrast, peripheral insulin resistance is known to be significantly higher in IGT subjects [42,52,53,57]. Fasting blood glucose levels are significantly higher in basal hepatic glucose production in response to insulin in IFG subjects, despite hyperinsulinemia, compared with NGT subjects. These results suggest that proper suppression does not occur; however, hepatic insulin resistance eventually increases in IFG subjects [42,52,57]. In IGT subjects, hepatic insulin resistance is significantly lower than in IFG subjects; however, there is no significant difference compared with NGT [42,52,57]. In studies that use the euglycemic hyperinsulinemic clamp, the gold-standard for assessing insulin resistance, total body glucose disposal in isolated IGT subjects is significantly reduced compared with NGT, while in isolated IFG, it is similar to NGT [58,59]. In isolated IGT subjects, this significantly increases insulin resistance in peripheral tissues. In contrast, peripheral insulin resistance is not significantly different between isolated IFG and NGT. While IFG is associated with IGT, both hepatic and peripheral insulin resistance are significantly higher than NGT [42,52,55,57].
Perspectives on insulin secretion
The progressive failure of beta cells is the major factor underlying the development and progression of T2DM. Physiological pancreatic insulin secretion occurs biphasically. Generally, sharply increasing early phase secretion and late phase secretion can be divided by post-continuous glucose stimulation during initial glucose loading. Even if the same amount of glucose is loaded, differences in in vivo insulin secretion exist during the course of administration. When glucose is administered orally, insulin secretion promotion occurs via the additional effects of incretin; however, when glucose is delivered intravenously, this effect is not observed [55]. It is well-known that a reduction in insulin secretion due to beta cell failure is an early step in NGT and preDM stages. In addition to simple reduction in insulin secretion, however, differences in beta cell dysfunction patterns are known to exist between preDM subtypes [56,57,60].
According to studies based on oral glucose tolerance tests, early phase insulin secretion in IFG subjects is significantly reduced compared with NGT, while late-phase insulin secretion is normal. Further, supranormal conditions can be observed [52,57]. On the other hand, early phase insulin secretion was relatively intact in IGT subjects, while late-phase insulin secretion was severely decreased [52,57]. In other studies, first- and second-phase insulin secretion in IGT subjects decreased in response to glucose infusion, while in IFG subjects, first-phase secretion decreased and second-phase insulin secretion remained relatively intact [54,55]. For both oral and intravenous glucose loads, symptoms of insulin secretion disorders in preDM subjects do not appear to be the result of incretin, but, instead, are caused by direct beta cell dysfunction. When IFG is accompanied by IGT, both early and late-phase insulin secretion were extremely reduced [52,57].
Racial differences in the pathophysiological characteristics of preDM
Based on the results discussed above, it seems clear that there is a difference between insulin resistance and insulin secretion between IFG and IGT subjects; however, most studies have targeted Caucasians, and there are not many studies that include physiological characteristics of other races, including Asians.
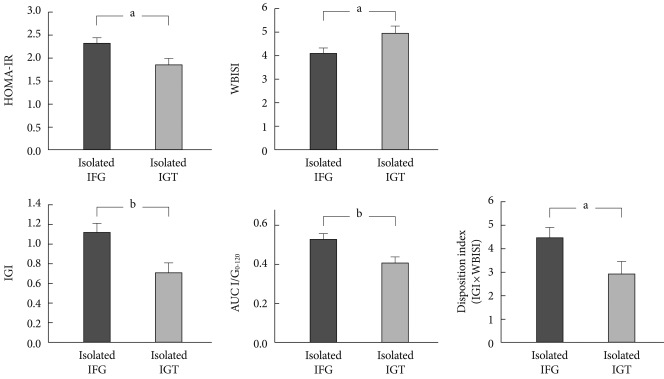
Using a 75-g oral glucose tolerance test, differences between insulin secretion and insulin resistance have recently been compared according to preDM subtypes in 307 Korean subjects diagnosed with drug-naïve preDM [56]. 75-g oral glucose tolerance test results were classified according to ADA diagnostic criteria: 87 subjects had isolated IFG, 75 had isolated IGT, and 145 had both. Within the subjects, homeostasis model assessment of insulin resistance (HOMA-IR) represents hepatic insulin resistance, whole body insulin sensitivity index (WBISI; Matsuda index) represents peripheral insulin resistance, the insulinogenic index (IGI) represents early phase insulin secretion, AUC I/G0-120 represents late-phase insulin secretion, and insulin resistance effects are compensated for. To assess beta cell function, the disposition index (DI, IGI×WBISI), was measured [56]. After adjusting for confounding variables, it was found that HOMA-IR, IGI, AUC I/G0-120, and DI were high in isolated IFG subjects, while only WBISI was high in isolated IGT subjects (Fig. 1) [56]. Although the gold-standard was being used in this study, preDM pathophysiology increased insulin resistance in IFG and reduced insulin secretion in IGT. Additionally, there was an apparent difference among study results, which suggests that something else is playing a relatively important role [56]. In future, additional studies are required between each preDM subtype to determine the physiological characteristics in various regions around the world to confirm the most common racial characteristics.
Fig. 1.
Comparison of insulin resistance and secretory indices between Korean prediabetic subjects after adjusting for age, gender, body mass index, and abdominal circumference [56]. Data are presented as mean±standard error. HOMA-IR, homeostasis model assessment of insulin resistance; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; WBISI, whole body insulin sensitivity index; IGI, insulinogenic index. aP<0.05, bP<0.01.
Do pathophysiological characteristics between preDM subtypes have clinical significance?
It remains unclear as to how clinically important heterogeneous pathophysiological features are between the observed subtypes. In many of the previous prospective, randomized trials that used interventions such as lifestyle modification and pharmacological agents, the risk of developing T2DM decreased in preDM subjects and it is known that several cardiovascular risk factors can be significantly improved upon [5-7]. In previous studies, however, interventions according to preDM subtypes have considered distinct pathophysiological characteristics, which could not be tested in subjects. Although it is only a hypothesis, it is suspected that when medical interventions are made during preDM and the early phase of T2DM according to hepatic/peripheral insulin resistance-dominant phenotype recognition or beta cell dysfunction-dominant phenotype recognition, more positive clinical outcomes may be obtained. For example, metformin and TZDs are used as insulin sensitizers for hepatic/peripheral insulin resistance phenotypes. In contrast, for beta cell dysfunction phenotypes, GLP-1 antagonists are thought to be effective. Additional studies will be necessary to determine whether this hypothesis is accurate.
CONCLUSIONS
PreDM is an obvious risk factor for both T2DM and cardiovascular disease. However, there are almost no symptoms of preDM and the clinical course is variable. Because there is not a worldwide consensus on diagnostic criteria, there are inadequacies in defining it as a single disease. However, we feel that the prevalence of preDM is higher than that of T2DM, and there are many epidemiological characteristics involved in different regions and races. Compared with observable symptoms, physiologically dynamic changes are occurring. Through proper management, recovery of normal glucose tolerance is possible. Additionally, because complications can be prevented, the clinical approach is more aggressive and more time is required to develop a more systematic approach. This suggests that individual management is required given the observed significant pathophysiological heterogeneity between preDM subtypes for the management of preDM subjects. Through future research, the pathophysiology of preDM and early stage T2DM is expected to become much more widely understood.
ACKNOWLEDGMENT
This article was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (Grant No. A050463).
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19:708–723. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 3.International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Standards of medical care in diabetes--2010. Diabetes Care. 2010;33(Suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 6.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 8.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 9.Massari V, Eschwege E, Valleron AJ. Imprecision of new criteria for the oral glucose tolerance test. Diabetologia. 1983;24:100–106. doi: 10.1007/BF00297390. [DOI] [PubMed] [Google Scholar]
- 10.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 11.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 12.Puavilai G, Chanprasertyotin S, Sriphrapradaeng A. Diagnostic criteria for diabetes mellitus and other categories of glucose intolerance: 1997 criteria by the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (ADA), 1998 WHO consultation criteria, and 1985 WHO criteria. World Health Organization. Diabetes Res Clin Pract. 1999;44:21–26. doi: 10.1016/s0168-8227(99)00008-x. [DOI] [PubMed] [Google Scholar]
- 13.Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, Knowler WC. The 1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care. 2000;23:1108–1112. doi: 10.2337/diacare.23.8.1108. [DOI] [PubMed] [Google Scholar]
- 14.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 15.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 16.Shaw JE, Zimmet PZ, de Courten M, Dowse GK, Chitson P, Gareeboo H, Hemraj F, Fareed D, Tuomilehto J, Alberti KG. Impaired fasting glucose or impaired glucose tolerance. What best predicts future diabetes in Mauritius? Diabetes Care. 1999;22:399–402. doi: 10.2337/diacare.22.3.399. [DOI] [PubMed] [Google Scholar]
- 17.Hanley AJ, Williams K, Gonzalez C, D'Agostino RB, Jr, Wagenknecht LE, Stern MP, Haffner SM San Antonio Heart Study; Mexico City Diabetes Study; Insulin Resistance Atherosclerosis Study. Prediction of type 2 diabetes using simple measures of insulin resistance: combined results from the San Antonio Heart Study, the Mexico City Diabetes Study, and the Insulin Resistance Atherosclerosis Study. Diabetes. 2003;52:463–469. doi: 10.2337/diabetes.52.2.463. [DOI] [PubMed] [Google Scholar]
- 18.de Vegt F, Dekker JM, Jager A, Hienkens E, Kostense PJ, Stehouwer CD, Nijpels G, Bouter LM, Heine RJ. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: The Hoorn Study. JAMA. 2001;285:2109–2113. doi: 10.1001/jama.285.16.2109. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. Geneva: World Health Organization; 2006. [Google Scholar]
- 20.Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, Kochba I, Rudich A Israeli Diabetes Research Group. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med. 2005;353:1454–1462. doi: 10.1056/NEJMoa050080. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt MI, Duncan BB, Bang H, Pankow JS, Ballantyne CM, Golden SH, Folsom AR, Chambless LE Atherosclerosis Risk in Communities Investigators. Identifying individuals at high risk for diabetes: The Atherosclerosis Risk in Communities study. Diabetes Care. 2005;28:2013–2018. doi: 10.2337/diacare.28.8.2013. [DOI] [PubMed] [Google Scholar]
- 22.Forouhi NG, Balkau B, Borch-Johnsen K, Dekker J, Glumer C, Qiao Q, Spijkerman A, Stolk R, Tabac A, Wareham NJ EDEG. The threshold for diagnosing impaired fasting glucose: a position statement by the European Diabetes Epidemiology Group. Diabetologia. 2006;49:822–827. doi: 10.1007/s00125-006-0189-4. [DOI] [PubMed] [Google Scholar]
- 23.Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004;164:2147–2155. doi: 10.1001/archinte.164.19.2147. [DOI] [PubMed] [Google Scholar]
- 24.DECODE Study Group, European Diabetes Epidemiology Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care. 2003;26:688–696. doi: 10.2337/diacare.26.3.688. [DOI] [PubMed] [Google Scholar]
- 25.Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55:1310–1317. doi: 10.1016/j.jacc.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 26.Davidson MB, Landsman PB, Alexander CM. Lowering the criterion for impaired fasting glucose will not provide clinical benefit. Diabetes Care. 2003;26:3329–3330. doi: 10.2337/diacare.26.12.3329. [DOI] [PubMed] [Google Scholar]
- 27.Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab. 2008;93:2447–2453. doi: 10.1210/jc.2007-2174. [DOI] [PubMed] [Google Scholar]
- 28.Wong TY, Liew G, Tapp RJ, Schmidt MI, Wang JJ, Mitchell P, Klein R, Klein BE, Zimmet P, Shaw J. Relation between fasting glucose and retinopathy for diagnosis of diabetes: three population-based cross-sectional studies. Lancet. 2008;371:736–743. doi: 10.1016/S0140-6736(08)60343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabanayagam C, Liew G, Tai ES, Shankar A, Lim SC, Subramaniam T, Wong TY. Relationship between glycated haemoglobin and microvascular complications: is there a natural cut-off point for the diagnosis of diabetes? Diabetologia. 2009;52:1279–1289. doi: 10.1007/s00125-009-1360-5. [DOI] [PubMed] [Google Scholar]
- 30.Weykamp C, John WG, Mosca A, Hoshino T, Little R, Jeppsson JO, Goodall I, Miedema K, Myers G, Reinauer H, Sacks DB, Slingerland R, Siebelder C. The IFCC Reference Measurement System for HbA1c: a 6-year progress report. Clin Chem. 2008;54:240–248. doi: 10.1373/clinchem.2007.097402. [DOI] [PubMed] [Google Scholar]
- 31.Tominaga M, Makino H, Yoshino G, Kuwa K, Takei I, Aono Y, Hoshino T, Umemoto M, Shimatsu A, Sanke T, Kuwashima M, Taminato T, Ono J. Japanese standard reference material for JDS Lot 2 haemoglobin A1c. I: comparison of Japan Diabetes Society-assigned values to those obtained by the Japanese and USA domestic standardization programmes and by the International Federation of Clinical Chemistry reference laboratories. Ann Clin Biochem. 2005;42(Pt 1):41–46. doi: 10.1258/0004563053026835. [DOI] [PubMed] [Google Scholar]
- 32.Bennett CM, Guo M, Dharmage SC. HbA(1c) as a screening tool for detection of type 2 diabetes: a systematic review. Diabet Med. 2007;24:333–343. doi: 10.1111/j.1464-5491.2007.02106.x. [DOI] [PubMed] [Google Scholar]
- 33.Nakagami T, Tominaga M, Nishimura R, Yoshiike N, Daimon M, Oizumi T, Tajima N. Is the measurement of glycated hemoglobin A1c alone an efficient screening test for undiagnosed diabetes? Japan National Diabetes Survey. Diabetes Res Clin Pract. 2007;76:251–256. doi: 10.1016/j.diabres.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 34.Herman WH, Engelgau MM, Zhang Y, Brown MB. Use of GHb (HbA(1c)) to screen for undiagnosed diabetes in the US population. Diabetes Care. 2000;23:1207–1208. doi: 10.2337/diacare.23.8.1207. [DOI] [PubMed] [Google Scholar]
- 35.Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25:275–278. doi: 10.2337/diacare.25.2.275. [DOI] [PubMed] [Google Scholar]
- 36.Droumaguet C, Balkau B, Simon D, Caces E, Tichet J, Charles MA, Eschwege E DESIR Study Group. Use of HbA1c in predicting progression to diabetes in French men and women: data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR) Diabetes Care. 2006;29:1619–1625. doi: 10.2337/dc05-2525. [DOI] [PubMed] [Google Scholar]
- 37.Buell C, Kermah D, Davidson MB. Utility of A1C for diabetes screening in the 1999 2004 NHANES population. Diabetes Care. 2007;30:2233–2235. doi: 10.2337/dc07-0585. [DOI] [PubMed] [Google Scholar]
- 38.Pradhan AD, Rifai N, Buring JE, Ridker PM. Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. Am J Med. 2007;120:720–727. doi: 10.1016/j.amjmed.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato KK, Hayashi T, Harita N, Yoneda T, Nakamura Y, Endo G, Kambe H. Combined measurement of fasting plasma glucose and A1C is effective for the prediction of type 2 diabetes: the Kansai Healthcare Study. Diabetes Care. 2009;32:644–646. doi: 10.2337/dc08-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Unwin N, Gan D, Whiting D. The IDF Diabetes Atlas: providing evidence, raising awareness and promoting action. Diabetes Res Clin Pract. 2010;87:2–3. doi: 10.1016/j.diabres.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 41.International Diabetes Federation (IDF) IDF diabetes atlas. 4th ed. Brussels: IDF Executive Office; 2010. [PubMed] [Google Scholar]
- 42.Abdul-Ghani MA, DeFronzo RA. Pathophysiology of prediabetes. Curr Diab Rep. 2009;9:193–199. doi: 10.1007/s11892-009-0032-7. [DOI] [PubMed] [Google Scholar]
- 43.Glumer C, Jorgensen T, Borch-Johnsen K Inter99 study. Prevalences of diabetes and impaired glucose regulation in a Danish population: the Inter99 study. Diabetes Care. 2003;26:2335–2340. doi: 10.2337/diacare.26.8.2335. [DOI] [PubMed] [Google Scholar]
- 44.Borch-Johnsen K, Colagiuri S, Balkau B, Glumer C, Carstensen B, Ramachandran A, Dong Y, Gao W. Creating a pandemic of prediabetes: the proposed new diagnostic criteria for impaired fasting glycaemia. Diabetologia. 2004;47:1396–1402. doi: 10.1007/s00125-004-1468-6. [DOI] [PubMed] [Google Scholar]
- 45.Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, Geiss LS. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DECODE Study Group on behalf of the European Diabetes Epidemiology Study Group. Will new diagnostic criteria for diabetes mellitus change phenotype of patients with diabetes? Reanalysis of European epidemiological data. BMJ. 1998;317:371–375. doi: 10.1136/bmj.317.7155.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiao Q, Hu G, Tuomilehto J, Nakagami T, Balkau B, Borch-Johnsen K, Ramachandran A, Mohan V, Iyer SR, Tominaga M, Kiyohara Y, Kato I, Okubo K, Nagai M, Shibazaki S, Yang Z, Tong Z, Fan Q, Wang B, Chew SK, Tan BY, Heng D, Emmanuel S, Tajima N, Iwamoto Y, Snehalatha C, Vijay V, Kapur A, Dong Y, Nan H, Gao W, Shi H, Fu F DECODA Study Group. Age- and sex-specific prevalence of diabetes and impaired glucose regulation in 11 Asian cohorts. Diabetes Care. 2003;26:1770–1780. doi: 10.2337/diacare.26.6.1770. [DOI] [PubMed] [Google Scholar]
- 48.The DECODE study group, European Diabetes Epidemiology Group, Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Lancet. 1999;354:617–621. [PubMed] [Google Scholar]
- 49.de Vegt F, Dekker JM, Stehouwer CD, Nijpels G, Bouter LM, Heine RJ. The 1997 American Diabetes Association criteria versus the 1985 World Health Organization criteria for the diagnosis of abnormal glucose tolerance: poor agreement in the Hoorn Study. Diabetes Care. 1998;21:1686–1690. doi: 10.2337/diacare.21.10.1686. [DOI] [PubMed] [Google Scholar]
- 50.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 51.Saydah SH, Miret M, Sung J, Varas C, Gause D, Brancati FL. Postchallenge hyperglycemia and mortality in a national sample of U.S. adults. Diabetes Care. 2001;24:1397–1402. doi: 10.2337/diacare.24.8.1397. [DOI] [PubMed] [Google Scholar]
- 52.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes. 2006;55:1430–1435. doi: 10.2337/db05-1200. [DOI] [PubMed] [Google Scholar]
- 53.Meyer C, Pimenta W, Woerle HJ, Van Haeften T, Szoke E, Mitrakou A, Gerich J. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care. 2006;29:1909–1914. doi: 10.2337/dc06-0438. [DOI] [PubMed] [Google Scholar]
- 54.Perreault L, Bergman BC, Playdon MC, Dalla Man C, Cobelli C, Eckel RH. Impaired fasting glucose with or without impaired glucose tolerance: progressive or parallel states of prediabetes? Am J Physiol Endocrinol Metab. 2008;295:E428–E435. doi: 10.1152/ajpendo.90354.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faerch K, Vaag A, Holst JJ, Glumer C, Pedersen O, Borch-Johnsen K. Impaired fasting glycaemia vs impaired glucose tolerance: similar impairment of pancreatic alpha and beta cell function but differential roles of incretin hormones and insulin action. Diabetologia. 2008;51:853–861. doi: 10.1007/s00125-008-0951-x. [DOI] [PubMed] [Google Scholar]
- 56.Rhee SY, Woo JT, Chon S, Hwang YC, Oh S, Ahn KJ, Chung HY, Kim SW, Kim JW, Kim YS. Characteristics of insulin resistance and insulin secretory capacity in Korean subjects with IFG and IGT. Diabetes Res Clin Pract. 2010;89:250–255. doi: 10.1016/j.diabres.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Faerch K, Vaag A, Holst JJ, Hansen T, Jorgensen T, Borch-Johnsen K. Natural history of insulin sensitivity and insulin secretion in the progression from normal glucose tolerance to impaired fasting glycemia and impaired glucose tolerance: the Inter99 study. Diabetes Care. 2009;32:439–444. doi: 10.2337/dc08-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weyer C, Bogardus C, Pratley RE. Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 1999;48:2197–2203. doi: 10.2337/diabetes.48.11.2197. [DOI] [PubMed] [Google Scholar]
- 59.Wasada T, Kuroki H, Katsumori K, Arii H, Sato A, Aoki K. Who are more insulin resistant, people with IFG or people with IGT? Diabetologia. 2004;47:758–759. doi: 10.1007/s00125-004-1339-1. [DOI] [PubMed] [Google Scholar]
- 60.Rhee SY, Kim JY, Chon S, Hwang YC, Jeong IK, Oh S, Ahn KJ, Chung HY, Woo J, Kim SW, Kim JW, Kim YS. The changes in early phase insulin secretion in newly diagnosed, drug naïve Korean prediabetes subjects. Korean Diabetes J. 2010;34:157–165. doi: 10.4093/kdj.2010.34.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]