Abstract
Objectives
Much of our understanding about the effect of concurrent sexual partnerships on the spread of HIV derives from mathematical models, but the empirical evidence is limited. In this contribution, we focus on polygyny, a common and institutionalized form of concurrency for which data are available, and study its relationship with HIV prevalence at the ecological level.
Methods
First, we describe country-level variation in the prevalence of polygyny and HIV. Second, we test the relationship between HIV and polygyny at the sub-national level using country fixed-effects regression models with data from nineteen Demographic and Health Surveys.
Results
The ecological association between polygyny and HIV prevalence is negative at the country as well as sub-national level: HIV prevalence is lower in countries where the practice of polygyny is common, and within countries it is lower in areas with higher levels of polygyny. Proposed explanations for the protective effect of polygyny include the distinctive structure of sexual networks produced by polygyny, the disproportionate recruitment of HIV positive women into marriages with a polygynous husband, and the lower coital frequency in conjugal dyads of polygynous marriages.
Conclusion
Existing mathematical models of concurrency are not sufficiently specific to account for the relatively benign effect of polygyny on the spread of HIV, and require refinements before they are used to inform HIV prevention policies.
Introduction
The practice of concurrent sexual partnerships is now considered to be a critical driver of the HIV/AIDS epidemic in high prevalence countries in sub-Saharan Africa (SSA) [1–3]. The upsurge of interest in concurrency is based on insight from persuasive mathematical models demonstrating that overlapping sexual partnerships are more efficient loci for the spread of infectious diseases [4, 5]. Under serial monogamy, the spread of the virus beyond the couple is interrupted until the relationship dissolves and a new partnership is formed. In contrast, a salient feature of concurrency is that it inflates the number of individuals who are directly or indirectly connected at any point in time. As a result the virus can be transmitted relatively quickly through the sexual network. In addition, there is biological evidence that high levels of viral load in acute infection increase the probability of transmitting HIV [6, 7], which becomes particularly pertinent if individuals have overlapping partnerships. The conviction that concurrency is conducive to the spread of HIV has gained support from the observation that there is both more concurrency and higher HIV prevalence in SSA than elsewhere [1, 8, 9].
These arguments are suggestive indeed, but the weak link in the case for a concurrency effect is the lack of direct empirical evidence linking concurrency to the faster and more pervasive spread of HIV [10, 11]. A few studies failed to identify a consistent concurrency effect [12–14], but these often focused on the relationship between the respondent’s (concurrent) partnerships and his or her own HIV status, whereas the concurrency model stresses the importance of overlap in the respondent’s partnerships for transmitting HIV to someone else. In other words, these studies largely assessed the role of concurrency as a risk factor for HIV acquisition, but failed to test the key hypothesis that concurrency has a measurable effect on the transmission of HIV. Sexual network studies based on partner-tracing designs (i.e., designs whereby the partners of an index case are traced and tested for HIV) should in principle facilitate more rigorous tests of transmission, but populations of substantial size need to be covered to produce convincing statistical evidence [15].
Our point of entry into this debate is to focus on a special form of concurrency, namely polygyny, and to examine its association with HIV prevalence. Polygyny is an institutionalized form of concurrency that is probably more accurately (although not perfectly [16]) reported than informal partnerships. Moreover, polygynous marriages are likely to be less transient than informal partnerships, and they account for a substantial share of all concurrent partnerships in SSA [12, 17]. In a survey conducted in Zambia in 1998, for example, 17.8% of rural Zambian men aged 25–49 reported more than one ongoing relationship; when polygynous marriages were excluded, the percentage of concurrent relationships dropped to 9.1% [17].
We thus propose that an analysis of the association between polygyny and HIV provides a valuable empirical contribution to understanding the relationship between overlapping sexual partnerships and HIV transmission. We focus on the ecological relation between polygyny and HIV because (1) most readily available datasets are ego- or respondent-centered, and these are by design not useful for demonstrating the effect of concurrency on HIV transmission (cfr. supra), and (2) HIV prevalence as a measure of epidemic severity is an attribute of aggregates rather than individuals.
Data and methods
We first present country-level associations between HIV prevalence and polygyny using secondary data sources. Second, we seek to confirm our findings at a lower level of aggregation (the survey cluster) using data from nineteen African Demographic and Health Surveys (DHS) and HIV/AIDS Indicator Surveys (AIS) with individually-linked survey and HIV serostatus data. The DHS and AIS use a two-stage randomized cluster sample design. Survey clusters are the smallest area units, comparable to enumeration areas in a national census. A cluster contains about 100 households, and there are on average 379 (standard deviation = 80) clusters per survey in the surveys used here. In the second sampling stage, a predetermined number of households are selected from each cluster. In each household, all women aged 15–49 are eligible for an interview. The age ranges are a little broader for men (usually 15–54 or 15–59), but the number of households selected for male interviews is often substantially smaller. Between 20 and 40 women are typically interviewed per survey cluster [18]. DHS and AIS data, survey instruments, and other documentation can be retrieved from the Measure DHS website (http://www.measuredhs.com). An important disadvantage of the DHS and AIS for this analysis is the lack of detail on marriage and partnerships (e.g., no full marriage histories) and, sometimes, the lack of standardized questions (e.g., the wording of the question about current marital status has changed slightly over time). The DHS and AIS are nonetheless important resources for studying concurrency because they constitute the largest collection of comparable datasets from African countries with partnership information linked to HIV serostatus. In our analysis we combine consensual and formal unions, and the definition of the polygynous character of the union is based on a question about the number of (co-)wives. We refer to a study by Timæus and Reynar [16] for a discussion of the correspondence between husbands and wives in survey responses to these questions.
We use DHS and AIS data in conjunction with a count-response model with a negative binomial distribution to model the number of prevalent infections per survey cluster with the logarithm of the total number tested per cluster as an exposure or offset parameter. A negative binomial distribution is a generalization of the Poisson distribution with an additional parameter that accounts for over-dispersion in the data [19]. One source of over-dispersion in count models is event-dependency. In this case, event-dependency is produced by the infectiousness of HIV, and –to the extent that partnership markets are geographically bound– the ensuing correlation between an individual’s likelihood of being HIV positive and the HIV prevalence in the survey-cluster to which (s)he belongs. Empirically, over-dispersion can be diagnosed when the variance of the count distribution is larger than its mean.
We start with an analysis of pooled –but sex disaggregated– datasets for the nineteen countries, and include dummies for all but one country to obtain unconditional fixed-effects estimates. We also summarize the results from country-wise regression analyses.
The regression framework allows us to control for selected characteristics that have previously been identified as risk factors of HIV infection. The most plausible are age, the type of place of residence (urban/rural) [20], male circumcision [21–23], the interval between the age at sexual debut and first marriage [24], the prevalence of other sexually transmitted infections (STIs) [25, 26], religion [27], mobility or migration [28], and the prevalence of extra-marital sex. Age is measured as the sex-specific median age in each survey cluster. Our measures of first marriage and sexual initiation are survey-cluster life table values of the median age at these events (values are imputed at the age of the oldest individual plus one for clusters where over 50% of respondents are self-reported virgins or single). The other indices are operationalized as percentages. The measure of religion distinguishes between Islam and other religions, and the prevalence of STIs is based on self-reports of genital sores, ulcers or discharge, categorized (0%, 0–10% and >10%) because its distribution is highly skewed. Mobility is measured as the percentage of interviewed men who spent more than one month away from their home (the information is not available for women). The measure of (non- or) extra-marital sex is based on self-reports of married respondents. The prevalence of STIs, male mobility and extra-marital sex all pertain to the year before the survey.
In the regression models, we define the prevalence of polygyny as the average of the percentage of married men and the percentage of married women in polygynous unions. We average male and female-centered definitions of polygyny because it smoothes some of the measurement error and random variation, and because it accounts for two aspects of polygyny, namely the incidence (the proportion of men with more than one wife), and the intensity of polygyny (the average number of wives per polygynyst) [29].
Results
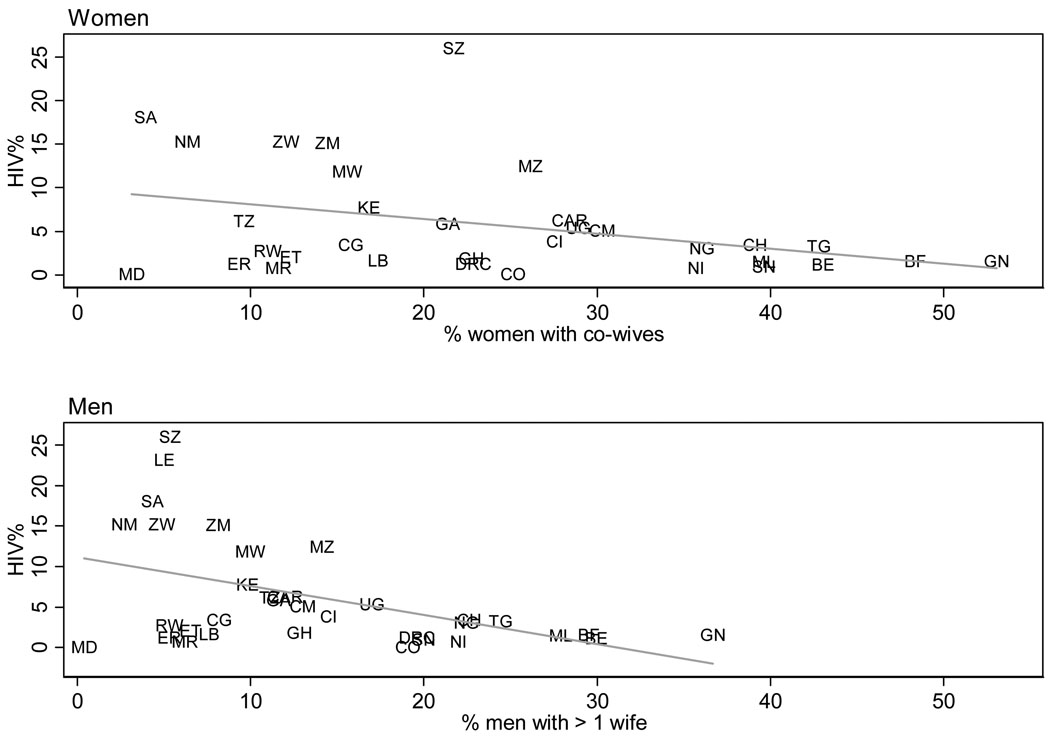
The scatterplots in Figure 1 suggest that polygyny and HIV prevalence are negatively correlated: western and central African countries have generally higher levels of polygyny and lower HIV prevalence than populations of eastern and southern Africa, and vice versa. The relationship holds for female-centered definitions of polygyny (the percentage of women with one or more co-wives) as well as male-centered definitions (the percentage of men with more than one wife).
Figure 1. Prevalence of HIV (age 15–49, both sexes) and polygyny (by sex), Sub-Saharan Africa.
Notes: Estimates of adult HIV prevalence (both sexes combined) are UNAIDS estimates for 2007[48], and the estimates of prevalence of polygyny come from a recent DHS survey. The year of the survey is listed following the country label used on the graph: Benin (BE, 2006), Burkina Faso (BF, 2003), Cameroon (CM,2004), Central African Republic (CAR, 1994–95), Chad (CH, 2004), Comoros (CO, 1996), Congo (CG, 2005), Ivory Coast (CI, 2005), Democratic Republic of Congo (DRC, 2007), Eritrea (women only, ER, 2002), Ethiopia (ET, 2005), Gabon (GA, 2000), Ghana (GH, 2003), Guinea (GN, 2005), Kenya (KE, 2003), Lesotho (LE, 2004, men only), Liberia (LB, 2007), Madagascar (MD, 2003–04), Malawi (MW, 2004), Mali (ML, 2006), Mauritania (MR, 2000–01), Mozambique (MZ, 2003), Namibia (NM, 2006–07), Niger (NI, 2006), Nigeria (NG, 2003), Rwanda (RW, 2005), Senegal (SN, 2005), South Africa (SA, 2003), Swaziland (SZ, 2006–07), Tanzania (TZ, 2004–05), Togo (TG, 1998), Uganda (UG, 2006), Zambia (ZM, 2007), Zimbabwe (ZW, 2005–06).
This country-level analysis is, however, a weak test of the polygyny-HIV association because there are several possibly confounding factors that are unaccounted for. In Table 1, we thus present results from regression models that aim to reduce the omitted variable bias. In these analyses, we change the level of aggregation from the country to the survey cluster-level, and regress the sex-specific count of HIV positives in each survey-cluster on the prevalence of polygyny. We first present crude associations, followed by coefficients that are adjusted for the statistical controls described earlier. Figure 2 summarizes the distribution of most of these variables. We present the regression parameters in exponentiated form, as incidence rate ratios (IRR), followed by their z-statistic. Control variables are only included in the full (adjusted) models if they are significant for at least one of the sexes.
Table 1.
Unconditional country fixed-effects negative binomial regression models predicting the count of HIV positives per survey cluster (pooled analysis for 19 African countries)
| Women | Men | |||
|---|---|---|---|---|
| Variables | Unadjusted IRR | Adjusted IRR | Unadjusted IRR | Adjusted IRR |
| % Polygynous | 0.993*** | 0.995*** | 0.992*** | 0.995*** |
| (−6.201) | (−3.908) | (−4.789) | (−3.313) | |
| Age a | 1.084 | 1.018*** | 1.216*** | 1.152*** |
| (1.565) | (4.041) | (6.638) | (4.837) | |
| Age – squared a | 0.999 | n.s. | 0.997*** | 0.998*** |
| (−1.473) | (−5.679) | (−3.807) | ||
| Urban (versus rural) | 1.619*** | 1.610*** | 1.584*** | 1.511*** |
| (15.92) | (14.10) | (11.52) | (9.403) | |
| % Circumcised (men) | 1.000 | 0.998*** | 0.998** | 0.996*** |
| (−0.409) | (−3.354) | (−2.125) | (−4.625) | |
| % Muslim | 0.998** | n.s. | 0.998* | n.s. |
| (−2.230) | (−1.858) | |||
| Mobility (% of men >1 month elsewhere) | 1.000 | n.s. | 0.999 | n.s. |
| (0.0405) | (−0.595) | |||
| Median age 1st marriage | ||||
| Women | 1.030*** | 0.925*** | 1.016** | 0.944*** |
| (6.683) | (−6.316) | (2.514) | (−3.586) | |
| Men | 1.029*** | n.s. | 1.015*** | n.s. |
| (7.300) | (2.811) | |||
| Difference median age 1st marriage – | ||||
| Women | 1.037*** | 1.095*** | 1.021*** | 1.058*** |
| (7.231) | (6.864) | (2.909) | (3.285) | |
| Men | 1.034*** | 1.023*** | 1.023*** | 1.019*** |
| (9.160) | (6.011) | (4.637) | (3.680) | |
| % Reporting STI symptoms (versus 0%) | ||||
| Women: ≤10% | 1.226*** | 1.189*** | 1.260*** | 1.228*** |
| (5.027) | (4.302) | (4.259) | (3.836) | |
| >10% | 1.345*** | 1.314*** | 1.612*** | 1.522*** |
| (6.116) | (5.651) | (7.526) | (6.717) | |
| Men: ≤10% | 1.084** | n.s. | 1.106** | n.s. |
| (2.197) | (2.114) | |||
| >10% | 1.123*** | n.s. | 1.328*** | n.s. |
| (3.015) | (5.708) | |||
| % Reporting extra-marital sex | ||||
| Women | 2.393*** | 1.588*** | 2.410*** | 1.444* |
| (6.040) | (3.146) | (4.531) | (1.888) | |
| Men | 1.543*** | 1.278*** | 1.776*** | 1.496*** |
| (5.971) | (3.318) | (5.925) | (4.106) | |
| N (missing) | 6867(300) | 6812(299) | ||
Notes:
p<0.01,
p<0.05,
p<0.1,
z-statistics (based on the sum of the outer product of the gradient vectors (OPG) variance estimator) in parentheses, n.s.: predictor is not significant in the adjusted regression models for either sex and omitted.
Age is defined as the survey-cluster median age of male respondents in the models for men, and median age of female respondents in the models for women. Male respondents’ median age was not a significant predictor of the count of HIV positives in the model for women and vice versa. See text for variable definitions. All models included a set of dummy variables for all but one of the countries (coefficients not shown). See Figure 2 for a list of countries included in the analysis. For Lesotho, the % in a polygynous union is entirely based on responses for men (the question was not asked to women).
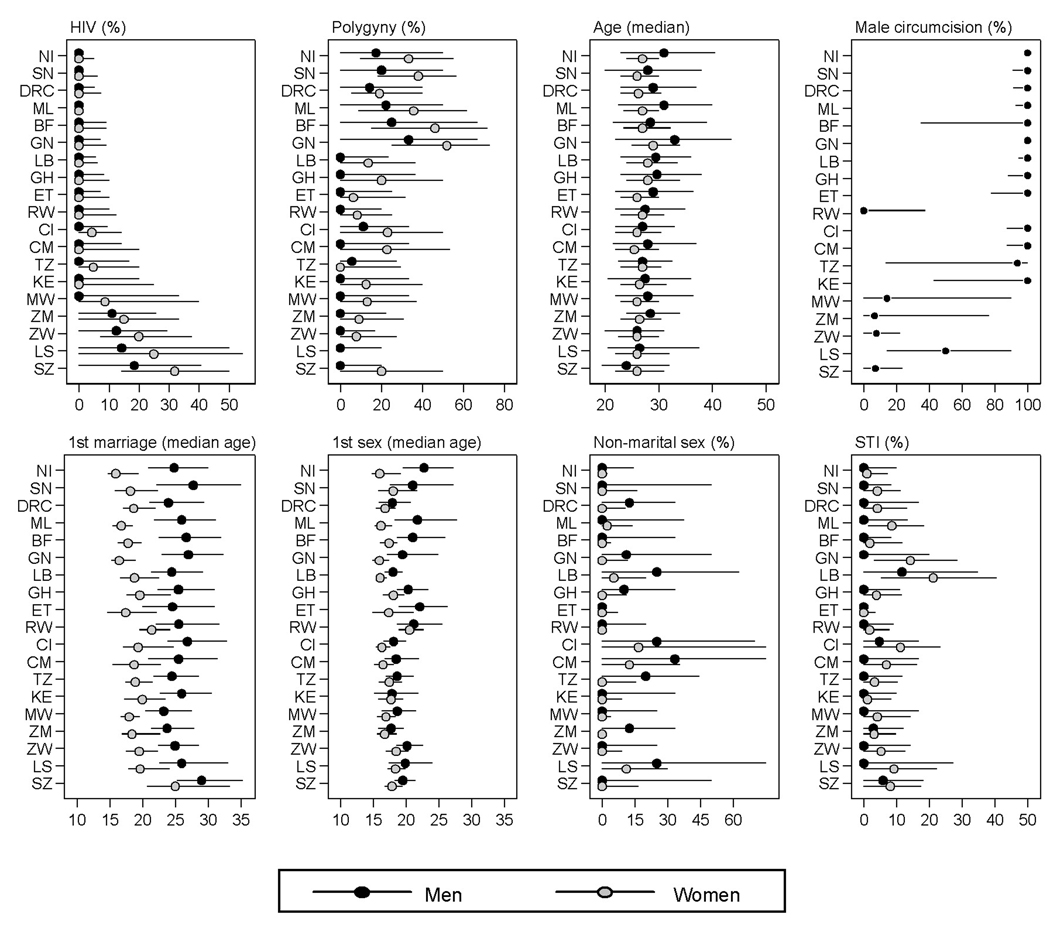
Figure 2. Sample descriptive statistics (10–90th percentiles range and median) by country and sex.
Notes: The unit of analysis is a survey cluster. The range between the 10th and 90th percentile is depicted by the horizontal lines; the dots represent the median for men and women, respectively. Countries are sorted along the Y-axis according to national HIV prevalence rates for both sexes combined [48]. Countries included: Burkina Faso (BF, 2003), Democratic Republic of Congo (DRC, 2007), Ivory Coast (CI, 2005), Cameroon (CM, 2004), Ghana (GH, 2003), Guinea (GN, 2005), Kenya (KE, 2003), Liberia (LB, 2007), Lesotho (LS, 2004), Mali (ML, 2006), Malawi (MW, 2004), Niger (NI, 2006), Rwanda (RW, 2005), Senegal (SN, 2005), Swaziland (SW, 2006–07), Tanzania (TZ, 2004–05), Zambia (ZM, 2007), Zimbabwe (ZW, 2005–06). The question about the number of co-wives was not included in the female questionnaire for Lesotho. The distribution for the type of place of residence is not shown because survey clusters are either entirely urban or rural, and the 10–90th percentile range spans both values for all countries.
The results in Table 1 corroborate the negative association between polygyny and HIV prevalence. In the presence of other statistical controls, the IRR is 0.995 (95% confidence interval: 0.991–0.998) for men and 0.995 (95% confidence interval: 0.993–0.998) for women. This translates into a 0.5% decrease in HIV prevalence for each one percentage point increase in the prevalence of polygyny. This is a considerable association given the heterogeneity in the prevalence of polygyny within countries: the smallest 10–90th percentile range for the prevalence of polygyny across clusters is 19.1 percentage points (Rwanda), and the largest value is 57.2 points (Burkina Faso)(see also Figure 2).
The associations with the other covariates largely operate in the expected direction. The coefficient for age is positive, indicating that clusters with older respondents have a higher HIV prevalence (the association is curvilinear for men). HIV prevalence is generally higher in urban areas, and male circumcision has a protective effect. Although the percentage of Muslims tends to be negatively correlated with HIV prevalence, the coefficient is not significant after adjusting for other controls. A more thorough evaluation of religion would include a measure of religious involvement [30], but that is not available in the DHS. The coefficient of the measure of male mobility is not significant either. Late marriage is protective (particularly for women). Its coefficient should, however, be interpreted in conjunction with the parameter estimate for the duration between first sex and first marriage: this suggests that late marriage will only contain the spread of HIV if it is not accompanied by pre-marital sexual activity. As could be expected, the latter is positively correlated with HIV prevalence, as is the percentage of married men and women reporting an extra-marital sex partner. STI symptoms are another important covariate of HIV prevalence, with coefficients that appear stronger for women than for men (this could be due to sex differences in the presence or the reporting of STI symptoms). Importantly, none of these controls eliminate the apparent protective effect of polygyny at the population (survey-cluster)-level.
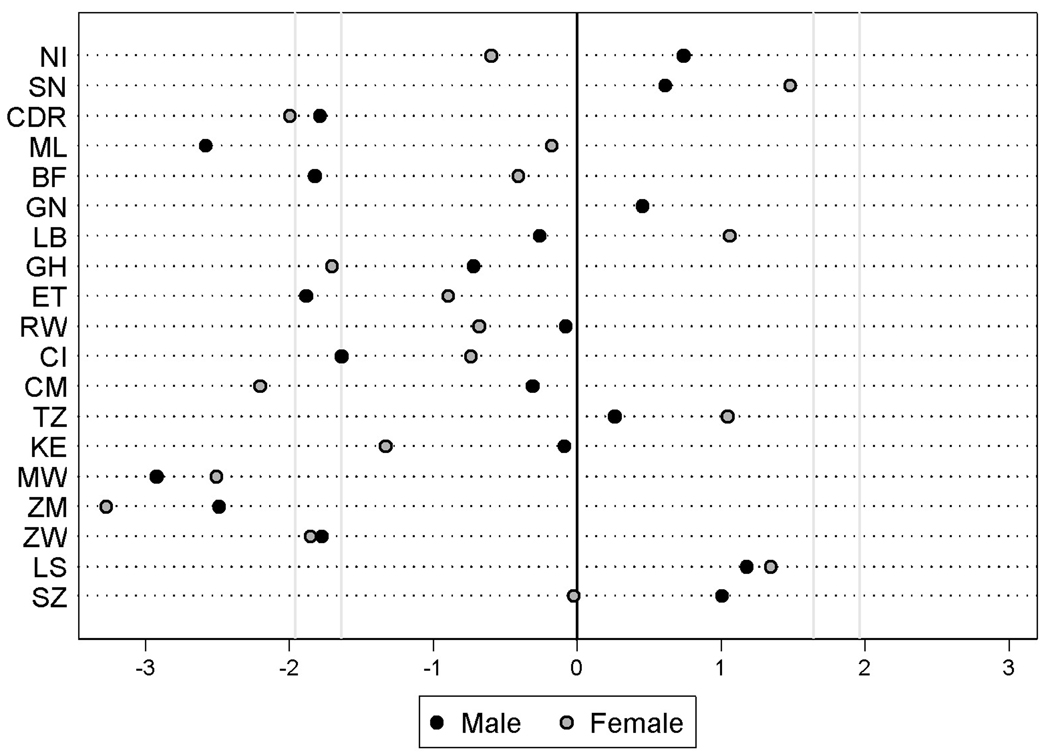
We extended the analyses in two respects. First, we estimated the model with all statistical controls for each country and sex separately, and plotted the z-statistic for the coefficient of polygyny in these regressions in Figure 3. Despite the relatively small number of clusters per country, seven of the parameter estimates are negative and significant at the 10% level (z-score ≤ −1.64), and another seven are significant at the 5% level (z-score ≤ −1.96). None of the parameter estimates are positive and statistically significant, further supporting the results from the pooled analysis. Second, we fitted models with self-reports of STI symptoms instead of HIV as the outcome (pooled analysis for all countries). In these models, the coefficient of polygyny is insignificant, and it is probably worth revisiting the association between polygyny and STIs other than HIV with more objective data STI prevalence.
Figure 3. The association (z-score) between the prevalence of polygyny and the count of HIV positives per survey cluster, by country and sex.
Notes:
See Figure 2 for the list of countries and their abbreviations. Countries are sorted along the Y-axis according to national HIV prevalence rates for both sexes combined [48]. z-scores are based on country and sex specific negative binomial regression models with all (significant) controls from the adjusted model in Table 1. z-scores ≥ 1.64 or ≤ −1.64 are significant at the 10%-level, z-scores ≥ 1.96 or ≤ −1.96 are significant at the 5%-level. Negative z-scores are indicative of a negative association between polygyny and HIV prevalence. For Lesotho, the % in a polygynous union is entirely based on responses for men (the question was not asked to women).
Discussion
Our analyses establish a negative ecological relationship between polygyny and HIV prevalence at two levels of aggregation. At the country-level, the association is most obvious, but it is confirmed by analyses that exploit within-country variation only (Kuate et al. report a similar result for Cameroon [31]), and control for other factors that are suspected to affect the spread of HIV (e.g., the prevalence of STIs, male circumcision, and the length of the pre-marital sexually active period). These findings are perhaps unexpected, and it is worth reflecting on the conditions that would render them consistent with predictions of the concurrency model.
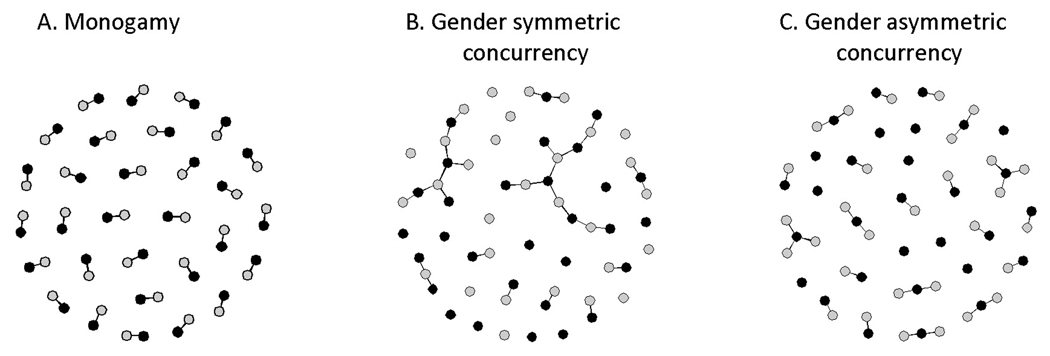
We begin by acknowledging that concurrency could be driving our results if survey clusters with lower levels of polygyny are characterized by higher levels of informal concurrency and vice versa. Whereas we included statistical controls for the prevalence of pre-marital and extra-marital sex, we cannot rule out that these measures are affected by reporting bias. Prior analyses of the relationship between polygyny and extra-marital sex are not conclusive either [32, 33]. A more interesting point relates to the sexual network structure produced by polygyny. On the one hand, polygyny generates a pattern of disassortative mixing whereby high-degree nodes (men who already have one or more partners) are paired with low degree nodes (women). As Morris and Kretzschmar have shown [34], disassortative mixing is likely to increase the final epidemic size compared to a random mixing model. This would not bring us closer to reconciling our findings with predictions from their concurrency model, were it not for another respect in which polygyny constitutes a special case. Barring extra-marital relationships, polygyny produces a system of gender asymmetric disassortative mixing: this implies that the size of the largest temporally connected sexual network component will not exceed the maximum number of wives of any of the individual men in the population (see also [35]). Thus polygyny, in effect, creates small isolates of concurrent partnerships in which the virus is trapped until one or more of the (infected) spouses start a new relationship (Figure 4C). In contrast, gender symmetric concurrency produces larger temporally connected network components (Figure 4B), thus facilitating a more rapid and pervasive spread of the virus.
Figure 4. Simulated sexual network structure (cross-section) under monogamy, gender symmetric concurrency, and gender asymmetric concurrency (i.e., polygyny).
Notes:
Each network consists of 30 men ( ), 30 women (
), 30 women ( ), and 30 –given the constraints on partnership concurrency– randomly assigned heterosexual partnerships (
), and 30 –given the constraints on partnership concurrency– randomly assigned heterosexual partnerships ( ). Graphs generated with the networksis package in R [49].
). Graphs generated with the networksis package in R [49].
The arguments presented so far imply that polygyny hinders epidemic growth compared to the scenario of gender symmetric concurrency in the Morris-Kretzchmar models. It is unlikely, however, that the sexual network structure alone can fully account for the negative statistical relationship between polygyny and HIV observed in this study. For a number of plausible complementary effects, we refer to other analyses of individual-level data that suggest that there are two other features of polygyny that influence the spread of HIV over and above the structural network effect [32, 36]. First, polygynous marriage systems are in large part sustained by the rapid remarriage of divorcees and widows (often as second or third wives of polygynous men, and sometimes via the practice widow inheritance) [37, 38]. Because higher marriage order and widowhood are positively correlated with HIV status [39–41], the addition of new wives is likely to introduce HIV into what might have been an HIV-free monogamous marriage. This would, of course, fuel the epidemic, were it not for a second, and counterbalancing characteristic of polygynous marriages that delays the spread of the virus. We conveniently label it a coital dilution effect [42, 43]: compared to a monogamous man, a polygynous husband divides his time between two or more wives, which inevitably leads to a reduction in the coital frequency with each wife. Just as coital dilution is claimed to affect fertility in polygynous marriages [44–46], it could reduce HIV incidence in serodiscordant couples within a polygynous union. The reduction in coital frequency not only arises from the resource constraints on a polygynous husband’s coital budget, it may also result from the relatively old age of husbands in polygynous unions, and, more interestingly, from a conscious decision to reduce the risk of transmitting HIV (e.g., the new husband of an inherited spouse may be aware of the cause of death of his wife’s previous husband). Together, these two mechanisms produce a mixing pattern whereby HIV positive women are disproportionately recruited into polygynous marriages where coital frequency is lower. As a result, seronegative individuals in polygynous marriages may face greater exposure to HIV than those in a monogamous marriage, but the population level effects of polygyny on the spread of HIV are beneficial on average. The policy implications of this finding will depend on whether individuals or the population as a whole ought to be the primary beneficiaries of public health policy interventions.
Other avenues through which polygyny may affect the spread of HIV deserve more careful consideration than we have been able to provide. Polygynous marriage systems may, for example, exert greater control over female sexuality or restrict younger men’s access to women, and, as a result, not only reduce the coital frequency in conjugal dyads of polygynous marriages, but also among those who are single. Given that the polygyny effect persists after accounting for the duration of the pre-marital sexual interval and the prevalence of extra-marital sex, this mechanism is, however, unlikely to fully account for the polygyny effect observed in this study. We also recognize that polygyny could have a different effect at different stages of the epidemic, just as it has been demonstrated that disassortative mixing on the number of existing partners can have different effects on the take-off and the leveling of a simulated epidemic [5]. Similarly, we have not explored whether the mediating effect of polygyny is dependent on the distribution of wives per polygynous husband (i.e., whether it is also dependent on the intensity of polygyny).
Because of the cross-sectional nature of the DHS and AIS, we are limited to a contemporaneous measure of polygyny (and most other control variables) whereas prevalent HIV infection is the cumulative result of past exposure. We tested the hypothesis that the prevalence of polygyny has changed over time such that the association between polygyny and HIV prevalence would have reversed entirely by analyzing the correlation between changes in the practice of polygyny and HIV prevalence. Of the countries with more than one DHS, the proportion of married women in polygynous marriages declined in all but one (Eritrea). The proportion of men in polygynous unions increased only in Guinea. The annual rate of change in the prevalence of polygyny between the first and the last DHS with information on polygyny is, however, not significantly correlated with HIV prevalence: r=0.09 (p=0.85) and r=0.26 (p=0.21) for men and women, respectively.
Despite these open questions and limitations, it is clear from the rather benign relationship between polygyny and HIV that refinements to the description of concurrency effects on the spread of HIV are in order, particularly in settings where a substantial proportion of concurrent partnerships are polygynous marriages. Polygyny produces a specific pattern of sexual mixing with outcomes that are not accommodated by existing simulation models. We believe that future models of concurrency need to incorporate (1) variability in sexual mixing patterns and how these correlate with concurrency and HIV status, and (2) the heterogeneity across union types in coital frequency. More realistic models of concurrency also need to account for (3) the variable infectiousness by duration since infection. Such models might also help us understand whether, and if so why, the relationship between polygyny and HIV differs from its relationship with other STIs (as our analyses suggest).
We have described polygyny as a special type of concurrency, but it is not an uncommon form of concurrency in SSA. Public health policies that target concurrency in a generic fashion are likely to be as culturally insensitive as early missionary efforts to ban polygyny. In addition, the negative association identified in this study suggests that they may have counterproductive public health implications. We conclude by invoking the practice of widow inheritance as a concrete illustration. Whereas the re-entry of widows into the marriage or partnership market implies a non-negligible risk of transmitting HIV, an important social function of widow inheritance is to provide a safety net for the surviving spouse, who may or may not have been previously infected [47]. Women in populations where the practice is common are embedded in the lineage of their husband and their livelihood is, at least in principle, independent of their husband’s survival. If a widow remarries her former husband’s relative, she is likely to become a second or third wife in a polygynous marriage, which, as we have argued above, implies a reduction in her sexual activity level. In the absence of widow inheritance (and adult male children), a widow may need to seek an alliance with another man to secure her own and possibly her children’s livelihoods. That process may induce an even greater risk of transmitting HIV if she has indeed been infected by her deceased spouse.
Acknowledgements
We wish to thank Jimi Adams, Ron Brookmeyer, Jeffrey Eaton, Stephane Helleringer, Jenny Higgins, Matthew Salganik, Rania Tfaily, Erik Vickstrom and several other colleagues for useful comments and suggestions. We thank Measure DHS for granting us access to the data.
References
- 1.Halperin DT, Epstein H. Concurrent sexual partnerships help to explain Africa's high HIV prevalence: implications for prevention. Lancet. 2004;364:4–6. doi: 10.1016/S0140-6736(04)16606-3. [DOI] [PubMed] [Google Scholar]
- 2.Shelton JD. Ten myths and one truth about generalised HIV epidemics. Lancet. 2007;370:1809–1811. doi: 10.1016/S0140-6736(07)61755-3. [DOI] [PubMed] [Google Scholar]
- 3.Epstein H. New York: Farrar, Straus and Giroux; 2007. The invisible cure. [Google Scholar]
- 4.Watts CH, May RM. The influence of concurrent partnerships on the dynamics of HIV/AIDS. Math Biosci. 1992;108:89–104. doi: 10.1016/0025-5564(92)90006-i. [DOI] [PubMed] [Google Scholar]
- 5.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS. 1997;11:641–648. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J. Infect. Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 7.Wilson DP, Law MG, Grulich AE, Cooper DA, Kaldor JM. Relation between HIV viral load and infectiousness: a model-based analysis. Lancet. 2008;372:314–320. doi: 10.1016/S0140-6736(08)61115-0. [DOI] [PubMed] [Google Scholar]
- 8.Mah TL, Halperin DT. Concurrent sexual partnerships and the HIV epidemics in Africa: Evidence to move forward. AIDS Behav. 2008 doi: 10.1007/s10461-008-9433-x. (epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 9.Carael M. Sexual behaviour. In: Cleland J, Ferry B, editors. Sexual behaviour and AIDS in the developing world. Findings from a multisite study. London: Taylor and Francis; 1995. pp. 75–123. [Google Scholar]
- 10.Lurie MN, Rosenthal S. Concurrent partnerships as the driver of the HIV epidemic in sub-Saharan Africa? The evidence is limited. AIDS Behav. 2009 doi: 10.1007/s10461-009-9583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UNAIDS. Geneva: UNAIDS Reference Group on Estimates, Modeling and Projections; 2009. Consultation on concurrent sexual partnerships. Recommendations from a meeting of the UNAIDS Reference Group on Estimates, Modeling and Projections held in Nairobi, Kenya, April 20–21. [Google Scholar]
- 12.Mishra V, Bignami-Van Assche S. DHS Working Papers No 62. Calverton, MD: Macro International Inc; 2009. Concurrent sexual partnerships and HIV infection: evidence from national population-based surveys. [Google Scholar]
- 13.Lagarde E, Auvert B, Carael M, Laourou M, Ferry B, Akam E, et al. Concurrent sexual partnerships and HIV prevalence in five urban communities of Sub-Saharan Africa. AIDS. 2001;15:877–884. doi: 10.1097/00002030-200105040-00008. [DOI] [PubMed] [Google Scholar]
- 14.Kelly RJ. PhD Thesis. Baltimore: Johns Hopkins University; 2001. Determinants of HIV transmission in rural Uganda: Circumcision, concurrency and age differences in sexual partners. [Google Scholar]
- 15.Helleringer S, Kohler HP, Kalilani-Phiri L. The association of HIV serodiscordance and partnership concurrency in Likoma Island (Malawi) AIDS. 2009;23:1285–1287. doi: 10.1097/QAD.0b013e32832aa85c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Timæus IM, Reynar A. Polygynists and their wives in sub-Saharan Africa: An analysis of five Demographic and Health Surveys. Popul Stud. 1998;52:145–162. [Google Scholar]
- 17.Sandoy IF, Dzekedzeke K, Fylkesnes K. Prevalence and correlates of concurrent sexual partnerships in Zambia. AIDS Behav. 2008 doi: 10.1007/s10461-008-9472-3. (epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 18.Macro International. Calverton, MD: Macro International Inc.; 1996. DHS-III basic documentation: Sampling manual. [Google Scholar]
- 19.Hilbe J. New York: Cambridge University Press; 2007. Negative binomial regression. [Google Scholar]
- 20.Carael M. Urban-rural differentials in HIV / STDs and sexual behaviour. In: Herdt G, editor. Sexual cultures and migration in the era of AIDS: Anthropological and demographic perspectives. Oxford: Oxford University Press; 1997. [Google Scholar]
- 21.Moses S, Bradley JE, Nagelkerke NJ, Ronald AR, Ndinya-Achola JO, Plummer FA. Geographical patterns of male circumcision practices in Africa: Association with HIV seroprevalence. Int J Epidemiol. 1990;19:693–697. doi: 10.1093/ije/19.3.693. [DOI] [PubMed] [Google Scholar]
- 22.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: The ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 24.Bongaarts J. Late marriage and the HIV epidemic in sub-Saharan Africa. Popul Stud. 2007;61:73–83. doi: 10.1080/00324720601048343. [DOI] [PubMed] [Google Scholar]
- 25.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Over M, Piot P. Population, Health and Nutrition Division, Population and Human Resources Department, World Bank; 1991. HIV infection and sexually transmitted diseases. [Google Scholar]
- 27.Gray PB. HIV and Islam: is HIV prevalence lower among Muslims? Soc Sci Med. 2004;58:1751–1756. doi: 10.1016/S0277-9536(03)00367-8. [DOI] [PubMed] [Google Scholar]
- 28.Decosas J, Kane F, Anarfi JK, Sodji KD, Wagner HU. Migration and AIDS. Lancet. 1995;346:826–828. doi: 10.1016/s0140-6736(95)91631-8. [DOI] [PubMed] [Google Scholar]
- 29.Van de Walle E. Marriage in African censuses and inquiries. In: Brass W, Coale A, Demeney P, et al., editors. The demography of tropical Africa. Princeton: Princeton University Press; 1968. pp. 183–238. [Google Scholar]
- 30.Trinitapoli J, Regnerus MD. Religion and HIV risk behaviors among married men: Initial results from a study in rural sub-Saharan Africa. J Sci Study Relig. 2006;45:505–528. [Google Scholar]
- 31.Kuate S, Mikolajczyk RT, Forgwei GW, Tih PM, Welty TK, Kretzschmar M. Time Trends and Regional Differences in the Prevalence of HIV Infection Among Women Attending Antenatal Clinics in 2 Provinces in Cameroon. J Acquir Immune Defic Syndr. 2009 doi: 10.1097/QAI.0b013e3181ab6d2e. [DOI] [PubMed] [Google Scholar]
- 32.Reniers G, Tfaily R. Polygyny and HIV in Malawi. Demographic Res. 2008;19:1811–1830. doi: 10.4054/DemRes.2008.19.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitsunaga TM, Powell AM, Heard NJ, Larsen UM. Extramarital sex among Nigerian men: polygyny and other risk factors. J Acquir Immune Defic Syndr. 2005;39:478–488. doi: 10.1097/01.qai.0000152396.60014.69. [DOI] [PubMed] [Google Scholar]
- 34.Morris M, Kretzschmar M. Concurrent partnerships and transmission dynamics in networks. Soc Networks. 1995;17:299–318. [Google Scholar]
- 35.Morris M, Kretzschmar M. A microsimulation study of the effect of concurrent partnerships on the spread of HIV in Uganda. Math Popul Stud. 2000;8:109–133. [Google Scholar]
- 36.Reniers G, Tfaily R. Polygyny and the spread of HIV in sub-Saharan Africa; Population Association of America Annual Conference; New Orleans, LA. 2008. [Google Scholar]
- 37.Goldman N, Pebley A, Lesthaeghe RJ. The demography of polygyny in Sub-Saharan Africa. In: Lesthaeghe R, editor. Reproduction and social organization in Sub-Saharan Africa. Berkeley: University of California Press; 1989. pp. 212–237. [Google Scholar]
- 38.Pison G. La démographie de la polygamie. Population. 1986;41:93–122. [Google Scholar]
- 39.Reniers G. Marital strategies for regulating exposure to HIV. Demography. 2008;45:417–438. doi: 10.1353/dem.0.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopman BA, Nyamukapa C, Hallett TB, Mushati P, Spark-du Preez N, Kurwa F, et al. Role of widows in the heterosexual transmission of HIV in Manicaland, Zimbabwe, 1998–2003. Sex Transm Infect. 2009;85 Suppl 1:i41–i48. doi: 10.1136/sti.2008.033043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quigley M, Munguti K, Grosskurth H, Todd J, Mosha F, Senkoro K, et al. Sexual behaviour patterns and other risk factors for HIV infection in rural Tanzania: a case-control study. AIDS. 1997;11:237–248. doi: 10.1097/00002030-199702000-00015. [DOI] [PubMed] [Google Scholar]
- 42.Blower SM, Boe C. Sex acts, sex partners, and sex budgets: implications for risk factor analysis and estimation of HIV transmission probabilities. J Acquir Immune Defic Syndr. 1993;6:1347–1352. [PubMed] [Google Scholar]
- 43.Josephson SC. Does polygyny reduce fertility? Am J Hum Biol. 2002;14:222–232. doi: 10.1002/ajhb.10045. [DOI] [PubMed] [Google Scholar]
- 44.Musham HV. Fertility of polygamous marriages. Popul Stud. 1956;10:3–16. [Google Scholar]
- 45.Barrett JC. Fecundability and coital frequency. Popul Stud. 1971;25:309–313. doi: 10.1080/00324728.1971.10405806. [DOI] [PubMed] [Google Scholar]
- 46.Pebley AR, Mbugua W. Polygyny and fertility in sub-Saharan Africa. In: Lesthaeghe R, editor. Reproduction and social organization in sub-Saharan Africa. Berkeley: University of California Press; 1989. pp. 338–364. [Google Scholar]
- 47.Palmore E. Cross-cultural perspectives on widowhood. J Cross Cult Gerontol. 1987;2:93–105. doi: 10.1007/BF00117178. [DOI] [PubMed] [Google Scholar]
- 48.UNAIDS. Geneva: UNAIDS; 2008. Report on the global AIDS epidemic. [Google Scholar]
- 49.Admiraal R, Handcock MS. networksis: a package to simulate bipartite graphs with fixed marginals through sequential importance sampling. JSS. 2008;24 doi: 10.18637/jss.v024.i08. [DOI] [PMC free article] [PubMed] [Google Scholar]