Abstract
Introduction
Chagas disease is the highest impact human infectious disease in Latin America, and the leading worldwide cause of myocarditis. Despite the availability of several compounds that have demonstrated efficacy in limiting the effects of T. cruzi, these compounds are rarely used due to their variable efficacy, substantial side effects and the lack of methodologies for confirming their effectiveness. Furthermore, the development of more efficacious compounds is challenged by limitations of systems for assessing drug efficacy in vitro and in vivo.
Areas covered
Herein, the authors review the development of Chagas disease drug discovery methodology, focusing on recent developments in high throughput screening, in vivo testing methods and assessments of efficacy in humans. Particularly, this review documents the significant progress that has taken place over the last 5 years that have paved the way for both target-focused and high-throughput screens of compound libraries.
Expert opinion
The tools for in vitro and in vivo screening of anti-T. cruzi compounds have improved dramatically in the last few years and there are now a number of excellent in vivo testing models available; this somewhat alleviates the bottleneck issue of quickly and definitively demonstrating in vivo efficacy in a relevant host animal system. These advances emphasize the potential for additional progress resulting in new treatments for Chagas disease in the coming years. That being said, national and international agencies must improve the coordination of research and development efforts in addition to cultivating the funding sources for the development of these new treatments.
Keywords: Chagas disease, high-throughput screening, reporter genes, surrogate markers, Trypanosoma cruzi
1. Introduction
Chagas disease, an endemic infection caused by the protozoan parasite Trypanosoma cruzi is a major public health problem in Latin America. Estimates of the prevalence of Chagas vary widely but it is likely that between 10 and 20 million people are infected and between 25,000 and 50,000 die per year as a result. Transmitted to humans primarily by reduviid insects, the infection generally has modest acute impact (swelling near the infection site, fever, fatigue, and enlarged lymphatic organs) and is well-controlled by the host immune response but appears to rarely be completely eliminated. Thus, individuals often do not know they are infected until decades later, and in many cases, not until clinical symptoms (primarily cardiac insufficiency or failure and/or gut dysfunction) are evident 1–2.
The T. cruzi life-cycle includes four distinct stages: the non-replicative bloodstream trypomastigotes and the replicative intracellular amastigotes in mammalian hosts and epimastigotes and mammalian-infective metacyclic trypomastigotes in the triatomine vector. The two drugs available for the treatment of T. cruzi infection, benznidazole (BZ, N-benzyl-2- nitroimidazolylacetamide) and Nifurtimox (NFX, (R,S)-3-methyl-N(italic)-[(1E)-(5-nitro-2- furyl)methylene]thiomorpholin-4-amine-1,1-dioxide) have substantial potential side effects and variable efficacy that is also difficult to measure 3–5. These attributes have combined to limit the use of these drugs, particularly in treating those with chronic infections. Thus, there is a crucial need to identify new and more effective compounds to treat T. cruzi infection. However, one important factor in the search for better therapies is the scarcity of efficient in vitro and in vivo systems to determine compound efficacy. Recently, there have been several improvements in this regard that are reviewed here.
2. In vitro screening for anti-T. cruzi compounds
Manual microscopic counting of parasite growth has long been a important tool for the measurement of the inhibitory effects of compounds on T. cruzi 6–8. This approach is suitable for assaying a small number of compounds but is clearly unacceptable for screening large compound libraries. Both colorimetric 9–13 and fluorometric 14–15 methods have been employed to increase throughput in these assays. Although these latter approaches are more rapid and have greater objectivity than manual counting by microscopy, they are limited in that they are only useful for monitoring inhibitory effects on epimastigotes but not for measuring the growth of intracellular amastigotes, the forms that are the replicating stage of T. cruzi in the mammalian hosts. Automated, high content microscopy has recently been applied to this challenge. By combining high resolution microscopy with image analysis and often times, robotic sample handling, this methodology allows the analysis of images of cells for drug discovery 16. Engle and co-workers used an imaged cell-based high throughput screening (HTS) assay to simultaneously measure the anti-T.cruzi efficacy and host cell toxicity of >900 compounds, identifying 55 new hits 17. A larger, high content HTS assay of a 4,000 compound library against T. cruzi Y and Dm28c strains in host cells was also recently reported by Freitas- Junior and colleagues (Freitas-Junior et al 2010 Int. Congress of Parasitol. Melbourne, Australia). An advantage of the high content imaging approach is that potentially any of the hundreds of T. cruzi lines currently available in laboratories (or freshly isolated from infected hosts) can be used in HTS assays without the need of incorporating a reporter molecule.
More than a decade ago, Buckner, et al developed transgenic lines of the T. cruzi , CL and Tulahuen strain, that express the reporter enzyme β-galactosidase (β-gal), from Escherichia coli, also known as LacZ 18. These strains allow detection of parasite growth by measuring the β-gal activity, thus facilitating the facile enumeration of both extracellular and intracellular parasite growth. Parasites expressing β-gal have also been used for screening of compounds for activity against other parasitic pathogens, including Toxoplasma gondii, Leishmania mexicana and Trypanosoma brucei 18–20. Colorometric substrates (chlorophenol-red- β-D-galactopyranoside (CPRG) and o-nitrophenyl-β-D-galactoside (ONPG)) have classically been used for quantifying β-gal activity but the increased sensitivity needed for scale-up to 384 well and higher density plates is provided by chemoluminescent substrates based on 1,2-dioxetanes 21. T. cruzi-β-gal parasites were successfully used to screen compounds for activity against epimastigotes and in a HTS against T. cruzi amastigotes 22. Using this approach, investigators at NYU and the Broad Institute recently completed a HTS of the ~300,000 compounds in the NIH compound collection, providing nearly 4,000 conclusive hits and approximately 1,600 of these confirmed hits showed an IC50<1.2 μM and a 100 fold selectivity 23.
T. cruzi lines expressing other reporters such as firefly luciferase have also been used to screen for trypanocidal compounds 24. This system uses the substrate luciferin that is oxidized by the luciferase yielding light 25. One disadvantage of both high content microscopy screening 6 methods and screens employing β-gal or luciferase is the need for a processing step at the end of each assay – the fixation and staining with a DNA stain and/or antibodies in the case of high content microscopy and substrate addition in the case of β-gal or luciferase. This end-point processing step also means that only a single time point for the measurement of parasite growth is possible in these assays. This is not a problem in the case of true HTS assays, where only a single point for assessing growth is needed, but is an issue when an evaluation of the kinetics of growth (and thus insights into the mechanisms of action of active compounds) is desired.
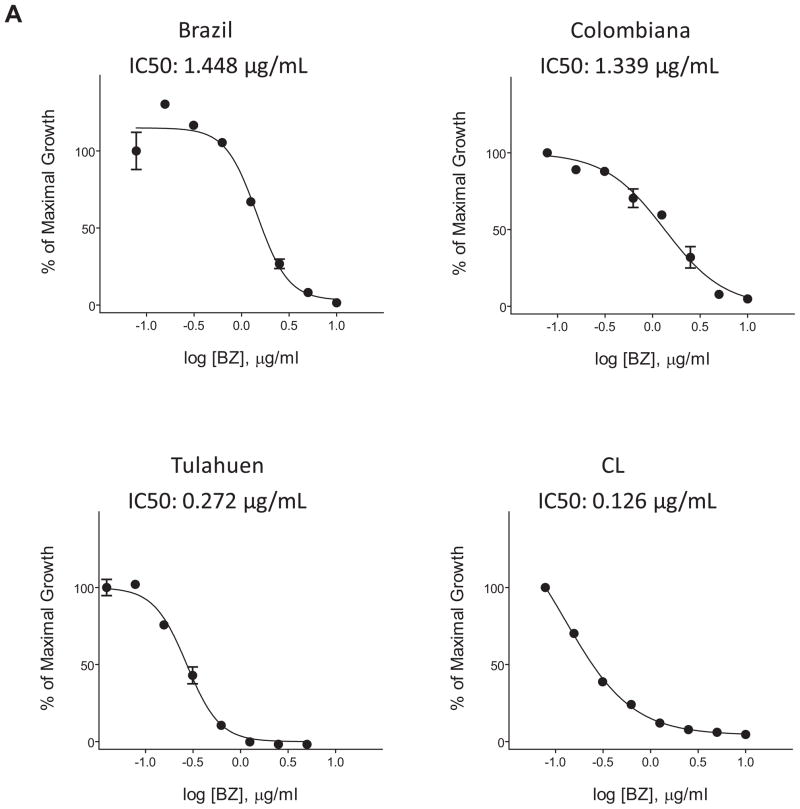
For the latter purpose, parasite lines expressing reporter molecules that do not require substrates for their detection are useful. A wide range of naturally fluorescent proteins are now available and a number have been expressed in T. cruzi for vector-biology as well as for in vitro and in vivo infectivity studies, including analysis of tissue tropism, mechanisms of cell invasion, and genetic exchange among parasites 26–29. We have recently used T. cruzi lines constitutively expressing the tandem tomato fluorescence protein (tdTomato) for the screening of potential anti-T. cruzi compounds (Figure 1; 30. Among the advantages of these particular parasite lines for drug screening, in addition to the elimination of the need for fixation or cell permeabilization, is that the fluorescence signal is sufficiently bright to be able to quantified with a fluorimeter (measuring the entire fluorescence of individual wells) in addition to high content imaging systems (that can quantify parasite growth at the individual host cell level but with much increased time required). In addition, the use of these parasites allows for the continuous measurement of parasite replication or growth inhibition by drugs over time with a high intra- and inter-assay consistency that could be easily scaled up to a 384 well format, allowing for the development of HTS. Multiple parasite strains differing in susceptibility to existing anti-T. cruzi compounds have also been produced (Figure 2; 30.
Figure 1.
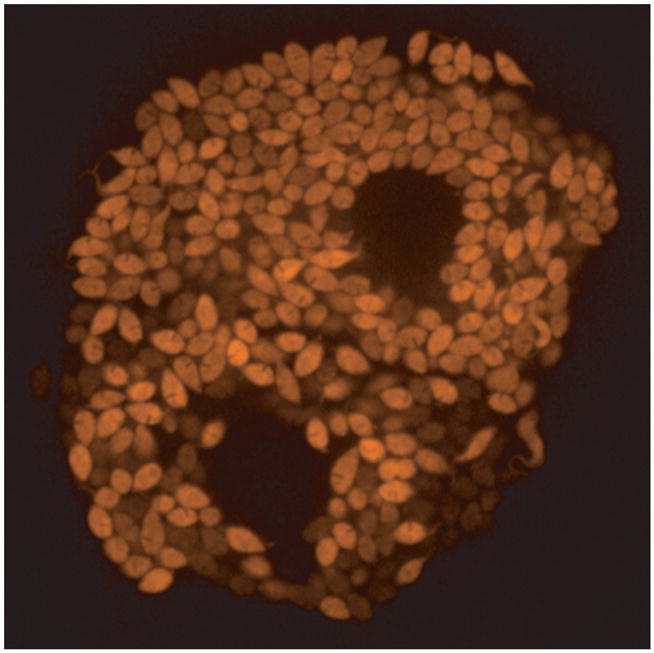
Image of tdTomato-expressing amastigotes of T. cruzi replicating in vitro within host cells.
Figure 2.
Monitoring effect of benznidazole on growth of the indicated strains of T. cruzi amastigotes in Vero cells by the expression of the tdTomato protein. IC50 were calculated at 3 days of treatment.
3. In vivo drug screening and testing
T. cruzi naturally infects a large number of mammals in addition to humans, and mice have been the model of choice for the in vivo testing of anti-T. cruzi compounds. The standard measures that have been used to assess in vivo drug efficacy in this model are the suppression of parasitemia early in the acute infection and/or the quantification of survival rates following a normally lethal infection 31–33. Although both of these measures are indicative of anti-T. cruzi activity, neither inform on the ability of compounds to bring about parasitological cure. Many compounds suppress parasitemia and prevent or delay death following an otherwise lethal infection but fail to completely clear the infection 30. Additionally, since T. cruzi is very challenging to detect in hosts during the chronic phase of the infection – even with the use of amplification techniques such as PCR - drugs are virtually never tested for efficacy in this stage. So neither existing drugs nor newly discovered ones are tested for efficacy in the task that is most needed of a drug for use in T. cruzi infection – the ability to completely eradicate the chronic infection.
With this problem in mind, we recently used immune suppression as a tool to determine if BZ could achieve parasitological cure in mice. This approach is based upon the premise that the loss of immune control will reveal infection that is otherwise not easily or consistently detectable, particularly in a well-established infection. Immunosuppression has been shown to intensify infection in experimental models of T. cruzi infection 30, 34 and as well in human infections as illustrated with HIV-infected subjects who exhibit exacerbated parasitemia and tissue parasite load 35–37. In the initial study in mice, a 40 day course of BZ treatment during either the acute or chronic phases of the infection was found to completely eliminate T. cruzi infection as indicated by the inability of cyclophosphamide-induced immunosuppression to reveal T. cruzi or T. cruzi DNA using multiple highly sensitive methods 34. In contrast, non-treated mice with infections of varying lengths (up to 460 days) showed rapid reoccurrence of parasitemia after cyclophosphamide treatment. The conclusion that mice were parasite-free following BZ treatment was further supported by the conversion in parasite-specific T cells to a central memory phenotype, indicative of the absence of continuous antigenic stimulation. We went on to show that other compounds that could quickly suppress parasitemia within a few days of the initiation of treatment were rarely effective in attaining complete parasitological cure 30, further establishing that suppression of parasitemia is a poor indicator of in vivo efficacy. These results create a new standard for assessing the anti-T. cruzi activity of compounds in vivo. The assay for cure requires no specific parasite lines or reagents (other than a method of immunosuppression) nor special equipment. Also, BZ can be used as a standard of efficacy against which other compounds can be compared.
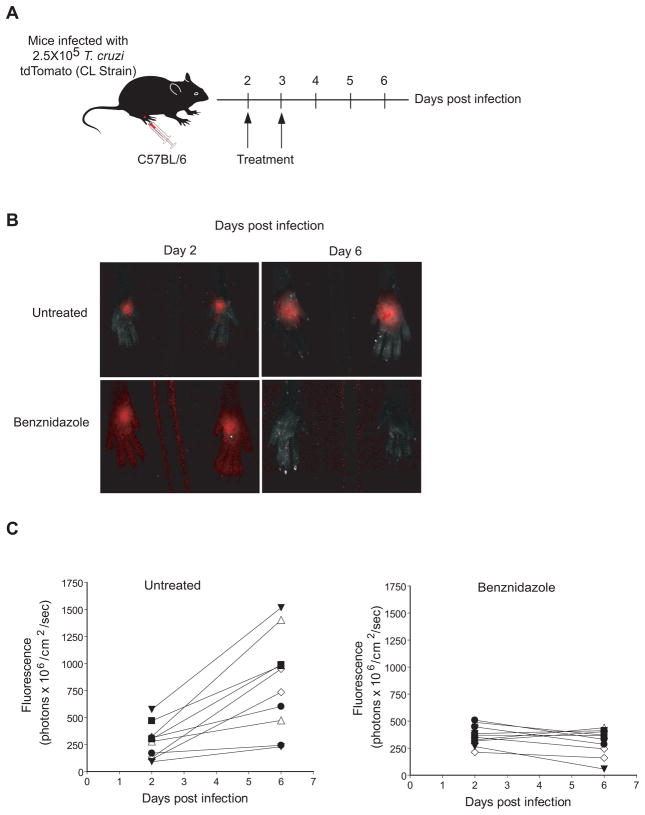
Although this treatment/suppression system is a powerful and sensitive method to address in vivo drug efficacy in T. cruzi infection, the limitation of this protocol is the long course of the experiment, requiring approximately 80 days for the specific treatment and the subsequent immunosuppression. Library screens such as those reported by the Broad/NYU group are identifying 1000s of compounds that are active in vitro and that need to be tested in vivo. For 9 this purpose, a more rapid in vivo screening assay would be very useful. To address this issue, we have developed an assay that measures the ability of compounds to alter in vivo parasite growth using whole animal imaging (Figure 3; 30. Previous investigators have used T. cruzi lines expressing luciferase 38 or betagalactosidase 39 to track parasite growth and persistence in T. cruzi infection. To determine if reporter gene-expressing parasites could be used to more quickly evaluate treatment efficacy, T. cruzi lines expressing luciferase or tdTomato protein were injected into the footpads of mice, and parasite growth was monitored in this site with or without treatment. In the original assay, mice were submitted to treatment between day 6 and 11 post infection, and the change in fluorescence or bioluminescence intensity before, during and after completion of treatment was used as a surrogate of parasite load 30. Since the measurement is non-invasive, the kinetics of parasite growth can be determined by periodic (daily if desired) measurements in the same animals. And as parasite replication only occurs intracellularly in mammals, by division of amastigote, the assay measures the ability of compounds to reach parasites inside of host cells – where they spend the majority of their time in this phase of the life cycle.
Figure 3.
(A) Schematic of the short in vivo assay used to screen anti-T. cruzi compounds in 1 week. Mice are infected in the hind foot pads with 2.5 × 105 T. cruzi-tdTomato trypomastigotes of the CL strain, and the specific treatment (Benznidazole) was given at days 2 and 3 postinfection. B) Parasites are imaged at day 2 and 6 post-infection. C) Quantification of the fluorescent signal from the individual mice at day 2 and 6 post infection. The efficiency factor (EF) was calculated using the following formula: [Fluorescence of Treated mice day 6 - Fluorescence of Treated mice day 2)/(Fluorescence of Untreated mice day 6 - Fluorescence of Untreated mice day 2)] X 100.
More recent modifications of the assay have reduced the length to less than 1 week – parasites are injected on day 0 and the first imaging and first drug treatment is given at day 2 (Figure 3). Only 1 treatment dose is needed for highly effective compounds such as BZ, but our standard protocol is to treat twice (days 2 and 3). A second imaging is done at day 6 and the relative change in parasite growth is determined by comparing pre- (day 2) and post- (day 6) treatment images. Both luminescent and fluorescent parasite lines work well for these assays Figure 3 and 30; fluorescent parasites have the added advantage of not requiring injection of substrate in order to image.
A particularly important aspect of this short term assay is that compounds that are effective at achieving cure in the long-term treatment followed by immunosuppression protocol (e.g. BZ and posaconazole (POS)) are also highly effective at controlling parasite growth in the short-term assay 30. Alternatively, those compounds that fail to cure mice in the long-term, including ones that strongly suppress epimastigote and amastigote growth in vitro, and suppress the level of parasites in the blood of acutely infected mice, also fail to suppress parasite replication in the tissues in the short-term assay. Thus this short-term assay appears to faithfully evaluate the characteristics that make an anti-T. cruzi compound highly effective and capable to achieving parasitological cure. Using this system we have screened more than 150 compounds in a few months and have identified some promising leads which are under a more extensive in vivo screening.
In addition to assessing compound effectiveness versus T. cruzi, the short-term assay yields at the same time data on compound toxicity in vivo (a number of compounds show toxicity (death) in mice in as few as 1–2 doses). It should also be noted that this rapid assay is a screening tool and will miss compounds which, by virtue of their structure, have poor bioavailability or in vivo stability. Thus, negative results in this assay should not be used to rule-out otherwise promising compounds (which might be improved by other modifications). However positive results indicate not only good anti-T. cruzi activity but also relatively low in vivo toxicity and good distribution.
Both the long and short-term assays are also providing insights into optimization for treatment protocols using existing drugs. The toxicity of benznidazole and nifurtimox, is thought to be cumulative 5 so any regiment that reduces the total drug dose should reduce toxicity. Preliminary results using the long-term treatment protocol suggest that parasitological cure in mice can be achieved using a much reduced frequency of doses (Bustamante, in preparation). Also being explored in the long-term treatment model are regimens that combine promising new drugs having good safety profiles with doses of BZ or NFX that are below toxicity thresholds.
4. Surrogate markers for treatment efficacy in Chagas disease
Monitoring treatment efficacy in human T. cruzi infection remains not only a major challenge but also a huge impediment to the development of new therapies. The improved methods for drug discovery noted above are useless if these drugs cannot be tested and proven effective in humans. The problem here is actually a direct result of the efficiency of immune responses in controlling T. cruzi infection to the point of making infection detection very difficult. This is especially problematic in those with chronic infection – the vast majority of cases requiring treatment. Detection of T. cruzi in chronically infected subjects always requires an amplification step – either of the parasite themselves using blood cultures or the insect vectors themselves as a “culture vessel” – a practice known as xenodiagnosis – or of parasite DNA by polymerase chain reaction (PCR). And even with amplification, direct confirmation of T. cruzi infection is possible in only a fraction of individuals – sometimes as few as 10% but rarely greater than 50%. Thus, using even the best technology, failure to detect T. cruzi is more the norm than the exception. This conclusion also means detection of T. cruzi after drug treatment is indicative of treatment failure but the lack of detection is largely meaningless, even in those who were positive prior to treatment.
Two surrogates of parasitological cure have been used to assess treatment effectiveness in humans – negativization of conventional diagnostic tests 40–41 and the prevention of progression of symptomatic disease 42–44 Unfortunately, changes in both of these parameters requires years if not decades of follow-up in those treated many years after the initial infection 45, making the methods unacceptable for the evaluation of new treatments. Recent developments in serological tests may offer some improvements in this regard. By measuring changes in antibody titers to individual recombinant proteins among a set of ~15 used in a multiplex format, it is possible to detect consistent deceases in antibody titers as early 12 months post-treatment 46–47. Similar changes are more difficult, or impossible to detect over the same time period using conventional serological tests, which mostly use whole parasite preparations, or pools or fusions of a few proteins. Thus, the ability to monitor the responses to individual parasite proteins seems to make a significant difference when using serology as a marker for treatment efficacy.
In mice – the only model in which definitive cure following drug treatment has been demonstrated 34 – immunosuppression is used to determine if treatment has been effective (see above). Although this protocol cannot be routinely used humans, in a single chronic human Chagas disease case where immunosuppression was required to treat systemic lupus 13 erythematosus, experimental use of POS was found to resolve the infection when BZ treatment had failed 48. Mice cured of chronic T. cruzi infection via BZ treatment exhibit a lower frequency of T. cruzi-specific CD8+ T cells and an increase in the number of a subpopulation of memory T cells known as central memory T cells (TCM), as compared to their untreated counterparts 34. TCM cells, in contrast to T effector memory cells (TEM), express the L-selectin receptor (CD62L) as well as IL-7Rα (CD127), molecules involved in T cells entry into lymph nodes and Peyer’s patches and homeostatic maintenance of memory T cells, respectively 49–50. A shift in the T. cruzi-specific CD8+ T cells from the predominantly TEM phenotype (CD62Llo, CD127lo) characteristic of the persistent infection to a majority TCM cell population (CD62Lhi, CD127hi) is also seen when cure is obtained using POS or the nitrotriazole derivative NTLA-1 (Bustamante, et al., in preparation). Thus, T cell phenotypic markers have potential as surrogates for the assessment of drug efficacy and cure.
In human infections, the tracking and phenotyping T. cruzi –specific T cells is complicated by their low frequency and largely unknown antigen specificity 51–52. Nevertheless, alterations in T. cruzi–specific T cell responses can be detected in a substantial proportion of BZ-treated subjects and these changes correlate with changes in antibody titers detected by multiplex tests 47. Similar alterations in T cell responses were not evident either prior to treatment in the same individuals or in untreated subjects over the same observation time. These are technically more challenging tests to perform, as compared to serology, and so are not likely to be easily implemented in non-research laboratories. Nevertheless, they may be useful as an adjunct, especially in this situation where other quality benchmarks are limiting. 14
5. Conclusions
The tools for in vitro and in vivo screening of anti-T. cruzi compounds have improved dramatically in the last few years. Very high throughput (millions of compounds) in vitro screening assays are now possible. There is also substantial interest in the screening of these large compound libraries by entities with access to them (e.g. big Pharma and public-private partnerships like Drugs for Neglected Diseases initiative (DNDi)). So expectations are high that a substantial number of hits and leads will emerge from these screens in the coming years. The in vivo follow-up screening assays recently developed are among the fastest and most definitive in the infectious diseases area, leaving few impediments to quickly determining the in vivo efficacy of compounds. Useful surrogate markers for treatment efficacy in humans are also emerging although additional investigation is needed to validate these.
6. Expert Opinion
Drug development for Chagas disease has been crippled by two exasperating situations: 1) the persistent view that the disease is not treatable as an infectious disease because of a putative autoimmune etiology, and 2) the failure to appreciate the benefits of treatment irrespective of the stage of infection (e.g. acute or chronic). In short, most infected patients are untreated because the data on the cause of the disease and the benefits of treatment are limited or misinterpreted. Added to this is the conundrum that proving treatment efficacy in a persistent, low-level infection such as T. cruzi is very challenging and the fact that research in Chagas disease is grossly underfunded, even compared to other neglected diseases 53. The end result is a situation where there is little impetus for the development of new drugs – because they cannot be easily tested to validate their efficacy and will not be widely used even if proven efficacious. Fortunately, this situation is changing. It is now widely appreciated that Chagas disease is, in fact, a persistent parasitic infection that requires treatment and parasitological cure in order to prevent progressive clinical disease. Data on the efficacy of current drugs in achieving cure and reducing disease severity is growing, despite the fact that these compounds are not optimal and are under-utilized. The combination of these advances with the scientific innovations reviewed above provides the opportunity for rapid progress in drug discovery for Chagas disease. Several additional high-throughput screens for in vitro-active compounds are likely to be completed in the coming years and the tools for more informative in vivo evaluation of hits and leads from these screens are now in place. Barriers to the realization of efficient clinical trials for promising compounds remain: In the absence of a method to reliably detect persisting infection, surrogates of cure will have to be used. Just as we rely on the detection of immune responses to certify the presence of infection, we will very likely have to depend on the immune system to indicate when treatment has worked. Several promising options for surrogate markers exist and the proposed clinical trials for the licensed anti-fungal posaconazole by Merck and the ergosterol biosynthesis inhibitor ravuconazole pro-drug E1224 by DNDi and Eisai in the near future will provide opportunities for more fully evaluating these and other biomarkers of cure in T. cruzi infection and Chagas disease. As importantly, national and international agencies have to do a much better job of coordinating research and development efforts and cultivating the funding sources for all of these efforts with the potential for significant advancement in drug development for Chagas disease.
Acknowledgments
The authors thank the members of the Tarleton lab and Member of the Chagas Drug Discovery Consortium for their contributions to the research and ideas discussed in this review. Special thanks to Hea Jin Park and Bharath Bolla for assistance with the production of Figures 2 and 3.
Footnotes
Declaration of interest
RL Tarleton and JM Bustamante are supported by grants R01AI-22070, R01AI-33106 and R01AI- 082542 from the U.S. National Institutes of Health. The authors state no other conflict of interest and have received no other payment in preparation of this manuscript.
References
- 1.Reithinger R, Tarleton RL, Urbina JA, et al. Eliminating Chagas disease: challenges and a roadmap. BMJ. 2009;338:b1283. doi: 10.1136/bmj.b1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarleton RL, Reithinger R, Urbina JA, et al. The challenges of Chagas Disease-- grim outlook or glimmer of hope. PLoS medicine. 2007 Dec;4(12):e332. doi: 10.1371/journal.pmed.0040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriques Coura J, de Castro SL. A critical review on Chagas disease chemotherapy. Memorias do Instituto Oswaldo Cruz. 2002 Jan;97(1):3–24. doi: 10.1590/s0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- 4.Urbina JA. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta tropica. 2010 Jul–Aug;115(1–2):55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Viotti R, Vigliano C, Lococo B, et al. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert review of anti-infective therapy. 2009 Mar;7(2):157–63. doi: 10.1586/14787210.7.2.157. [DOI] [PubMed] [Google Scholar]
- 6.Berens RL, Marr JJ, Steele da Cruz FS, et al. Effect of allopurinol on Trypanosoma cruzi: metabolism and biological activity in intracellular and bloodstream forms. Antimicrob Agents Chemother. 1982 Oct;22(4):657–61. doi: 10.1128/aac.22.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiari E, de Oliveira AB, Raslan DS, et al. Screening in vitro of natural products against blood forms of Trypanosoma cruzi. Trans R Soc Trop Med Hyg. 1991 May–Jun;85(3):372–4. doi: 10.1016/0035-9203(91)90296-b. [DOI] [PubMed] [Google Scholar]
- 8.Gómez-Barrio AM-DRAJ, Escario JA, Diego C, Avendaño C. New derivatives of gentian violet as trypanocides: in vitro and in vivo assays on Trypanosoma cruzi. Res Rev Parasitol. 1997;57:25–31. [Google Scholar]
- 9.Muelas-Serrano S, Nogal-Ruiz JJ, Gomez-Barrio A. Setting of a colorimetric method to determine the viability of Trypanosoma cruzi epimastigotes. Parasitol Res. 2000 Dec;86(12):999–1002. doi: 10.1007/pl00008532. [DOI] [PubMed] [Google Scholar]
- 10.Rolon M, Vega C, Escario JA, et al. Development of resazurin microtiter assay for drug sensibility testing of Trypanosoma cruzi epimastigotes. Parasitol Res. 2006 Jul;99(2):103–7. doi: 10.1007/s00436-006-0126-y. [DOI] [PubMed] [Google Scholar]
- 11.Saraiva J, Vega C, Rolon M, et al. In vitro and in vivo activity of lignan lactones derivatives against Trypanosoma cruzi. Parasitol Res. 2007 Mar;100(4):791–5. doi: 10.1007/s00436-006-0327-4. [DOI] [PubMed] [Google Scholar]
- 12.Vega C, Rolon M, Martinez-Fernandez AR, et al. A new pharmacological screening assay with Trypanosoma cruzi epimastigotes expressing beta-galactosidase. Parasitol Res. 2005 Mar;95(4):296–8. doi: 10.1007/s00436-005-1300-3. [DOI] [PubMed] [Google Scholar]
- 13.Kouznetsov VV, Castro JR, Puentes CO, et al. Synthesis and antiparasitic properties of new 4-N-benzylamino-4-hetarylbut-1-enes. Arch Pharm (Weinheim) 2005 Jan;338(1):32–7. doi: 10.1002/ardp.200400909. [DOI] [PubMed] [Google Scholar]
- 14.Raz B, Iten M, Grether-Buhler Y, et al. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta tropica. 1997 Nov;68(2):139–47. doi: 10.1016/s0001-706x(97)00079-x. [DOI] [PubMed] [Google Scholar]
- 15.Neres J, Brewer ML, Ratier L, et al. Discovery of novel inhibitors of Trypanosoma cruzi trans-sialidase from in silico screening. Bioorganic & medicinal chemistry letters. 2009 Feb 1;19(3):589–96. doi: 10.1016/j.bmcl.2008.12.065. [DOI] [PubMed] [Google Scholar]
- 16.Abraham VC, Taylor DL, Haskins JR. High content screening applied to large-scale cell biology. Trends Biotechnol. 2004 Jan;22(1):15–22. doi: 10.1016/j.tibtech.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Engel JC, Ang KK, Chen S, et al. Image-based high-throughput drug screening targeting the intracellular stage of Trypanosoma cruzi, the agent of Chagas' disease. Antimicrob Agents Chemother. 2010 Aug;54(8):3326–34. doi: 10.1128/AAC.01777-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckner FS, Verlinde CL, La Flamme AC, et al. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob Agents Chemother. 1996 Nov;40(11):2592–7. doi: 10.1128/aac.40.11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McFadden DC, Seeber F, Boothroyd JC. Use of Toxoplasma gondii expressing betagalactosidase for colorimetric assessment of drug activity in vitro. Antimicrob Agents Chemother. 1997 Sep;41(9):1849–53. doi: 10.1128/aac.41.9.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seeber F, Boothroyd JC. Escherichia coli beta-galactosidase as an in vitro and in vivo reporter enzyme and stable transfection marker in the intracellular protozoan parasite Toxoplasma gondii. Gene. 1996 Feb 22;169(1):39–45. doi: 10.1016/0378-1119(95)00786-5. [DOI] [PubMed] [Google Scholar]
- 21.Bronstein I, Edwards B, Voyta JC. 1,2-dioxetanes: novel chemiluminescent enzyme substrates. Applications to immunoassays. J Biolumin Chemilumin. 1989 Jul;4(1):99–111. doi: 10.1002/bio.1170040116. [DOI] [PubMed] [Google Scholar]
- 22.Bettiol E, Samanovic M, Murkin AS, et al. Identification of three classes of heteroaromatic compounds with activity against intracellular Trypanosoma cruzi by chemical library screening. PLoS neglected tropical diseases. 2009;3(2):e384. doi: 10.1371/journal.pntd.0000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=1885.
- 24.Bot C, Hall BS, Bashir N, et al. Trypanocidal activity of aziridinyl nitrobenzamide prodrugs. Antimicrob Agents Chemother. 2010 Oct;54(10):4246–52. doi: 10.1128/AAC.00800-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan F, Wood KV. Bioluminescent assays for high-throughput screening. Assay Drug Dev Technol. 2007 Feb;5(1):127–36. doi: 10.1089/adt.2006.053. [DOI] [PubMed] [Google Scholar]
- 26.Guevara P, Dias M, Rojas A, et al. Expression of fluorescent genes in Trypanosoma cruzi and Trypanosoma rangeli (Kinetoplastida: Trypanosomatidae): its application to parasite-vector biology. J Med Entomol. 2005 Jan;42(1):48–56. doi: 10.1093/jmedent/42.1.48. [DOI] [PubMed] [Google Scholar]
- 27.Florencio-Martinez L, Marquez-Duenas C, Ballesteros-Rodea G, et al. Cellular analysis of host cell infection by different developmental stages of Trypanosoma cruzi. Exp Parasitol. 2010 Nov;126(3):332–6. doi: 10.1016/j.exppara.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Dube A, Gupta R, Singh N. Reporter genes facilitating discovery of drugs targeting protozoan parasites. Trends in parasitology. 2009 Sep;25(9):432–9. doi: 10.1016/j.pt.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Pires SF, DaRocha WD, Freitas JM, et al. Cell culture and animal infection with distinct Trypanosoma cruzi strains expressing red and green fluorescent proteins. International journal for parasitology. 2008 Mar;38(3–4):289–97. doi: 10.1016/j.ijpara.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Canavaci AM, Bustamante JM, Padilla AM, et al. In vitro and in vivo high-throughput assays for the testing of anti-Trypanosoma cruzi compounds. PLoS neglected tropical diseases. 2010;4(7):e740. doi: 10.1371/journal.pntd.0000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engel JC, Doyle PS, Hsieh I, et al. Cysteine protease inhibitors cure an experimental Trypanosoma cruzi infection. The Journal of experimental medicine. 1998 Aug 17;188(4):725–34. doi: 10.1084/jem.188.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urbina JA, Payares G, Sanoja C, et al. Parasitological cure of acute and chronic experimental Chagas disease using the long-acting experimental triazole TAK-187. Activity against drug-resistant Trypanosoma cruzi strains. Int J Antimicrob Agents. 2003 Jan;21(1):39–48. doi: 10.1016/s0924-8579(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 33.Romanha AJ, Alves RO, Murta SM, et al. Experimental chemotherapy against Trypanosoma cruzi infection: essential role of endogenous interferon-gamma in mediating parasitologic cure. The Journal of infectious diseases. 2002 Sep 15;186(6):823–8. doi: 10.1086/342415. [DOI] [PubMed] [Google Scholar]
- 34.Bustamante JM, Bixby LM, Tarleton RL. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat Med. 2008 May;14(5):542–50. doi: 10.1038/nm1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosemberg S, Chaves CJ, Higuchi ML, et al. Fatal meningoencephalitis caused by reactivation of Trypanosoma cruzi infection in a patient with AIDS. Neurology. 1992;42:640–2. doi: 10.1212/wnl.42.3.640. [DOI] [PubMed] [Google Scholar]
- 36.Sartori AM, Shikanai-Yasuda MA, Amato Neto V, et al. Follow-up of 18 patients with human immunodeficiency virus infection and chronic Chagas' disease, with reactivation of Chagas' disease causing cardiac disease in three patients. Clin Infect Dis. 1998 Jan;26(1):177–9. doi: 10.1086/516257. [DOI] [PubMed] [Google Scholar]
- 37.Vaidian AK, Weiss LM, Tanowitz HB. Chagas' disease and AIDS. Kinetoplastid Biol Dis. 2004 May 13;3(1):2. doi: 10.1186/1475-9292-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyland KV, Asfaw SH, Olson CL, et al. Bioluminescent imaging of Trypanosoma cruzi infection. International journal for parasitology. 2008 Oct;38(12):1391–400. doi: 10.1016/j.ijpara.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esperandim VR, da Silva Ferreira D, Toldo MP, et al. New method for quantification of Trypanosoma cruzi in animal's tissue in the chronic phase of experimental Chagas' disease. Parasitol Res. 2010 May;106(6):1471–3. doi: 10.1007/s00436-010-1780-7. [DOI] [PubMed] [Google Scholar]
- 40.Sosa Estani S, Segura EL, Ruiz AM, et al. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas' disease. The American journal of tropical medicine and hygiene. 1998 Oct;59(4):526–9. doi: 10.4269/ajtmh.1998.59.526. [DOI] [PubMed] [Google Scholar]
- 41.Andrade AL, Zicker F, de Oliveira RM, et al. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet. 1996;348(9039):1407–13. doi: 10.1016/s0140-6736(96)04128-1. [DOI] [PubMed] [Google Scholar]
- 42.Viotti R, Vigliano C. Etiological treatment of chronic Chagas disease: neglected 'evidence' by evidence-based medicine. Expert review of anti-infective therapy. 2007 Aug;5(4):717–26. doi: 10.1586/14787210.5.4.717. [DOI] [PubMed] [Google Scholar]
- 43.Viotti R, Vigliano C, Armenti H, et al. Treatment of chronic Chagas' disease with benznidazole: clinical and serologic evolution of patients with long-term follow-up. Am Heart J. 1994 Jan;127(1):151–62. doi: 10.1016/0002-8703(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 44.Viotti R, Vigliano C, Lococo B, et al. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Annals of internal medicine. 2006 May 16;144(10):724–34. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]
- 45.Pinto Dias JC. The treatment of Chagas disease (South American trypanosomiasis) Annals of internal medicine. 2006 May 16;144(10):772–4. doi: 10.7326/0003-4819-144-10-200605160-00012. [DOI] [PubMed] [Google Scholar]
- 46.Cooley G, Etheridge RD, Boehlke C, et al. High Throughput Selection of Effective Serodiagnostics for Trypanosoma cruzi infection. PLoS neglected tropical diseases. 2008;2(10):e316. doi: 10.1371/journal.pntd.0000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laucella SA, Perez Mazliah D, Bertocchi G, et al. Changes in Trypanosoma cruzi-specific immune responses following treatment: surrogate markers of treatment efficacy. Clinical Infectious Diseases. 2009;49(11):1675–84. doi: 10.1086/648072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinazo MJ, Espinosa G, Gallego M, et al. Successful treatment with posaconazole of a patient with chronic Chagas disease and systemic lupus erythematosus. The American journal of tropical medicine and hygiene. 2010 Apr;82(4):583–7. doi: 10.4269/ajtmh.2010.09-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 50.Sallusto F, Lenig D, Forster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999 Oct 14;401(6754):708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 51.Alvarez MG, Postan M, Weatherly DB, et al. HLA Class I-T Cell Epitopes from trans- Sialidase Proteins Reveal Functionally Distinct Subsets of CD8 T Cells in Chronic Chagas Disease. PLoS neglected tropical diseases. 2008;2(9):e288. doi: 10.1371/journal.pntd.0000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laucella SA, Postan M, Martin D, Fralish BH, Albareda MC, Alvarez MG, Lococo B, Barbeieri G, Viotti RJ, Tarleton RL. Frequency of Interferon-gamma-producing T cells specific for Trypanosoma cruzi inversely correlates with disease severity in chronic human Chagas disease. J Infectious Diseases. 2004;189(5):909–18. doi: 10.1086/381682. [DOI] [PubMed] [Google Scholar]
- 53.Moran M, Guzman J, Ropars AL, et al. Neglected disease research and development: how much are we really spending? PLoS medicine. 2009 Feb 3;6(2):e30. doi: 10.1371/journal.pmed.1000030. [DOI] [PMC free article] [PubMed] [Google Scholar]