Abstract
Objective
The purpose of this study was to evaluate contrast-enhanced ultrasound and neural network data classification for determining the breast cancer response to bevacizumab therapy in a murine model.
Methods
An ultrasound scanner operating in the harmonic mode was used to measure ultrasound contrast agent (UCA) time-intensity curves in vivo. Twenty-five nude athymic mice with orthotopic breast cancers received a 30-µL tail vein bolus of a perflutren microsphere UCA, and baseline tumor imaging was performed using microbubble destruction-replenishment techniques. Subsequently, 15 animals received a 0.2-mg injection of bevacizumab, whereas 10 control animals received an equivalent dose of saline. Animals were reimaged on days 1, 2, 3, and 6 before euthanasia. Histologic assessment of excised tumor sections was performed. Time-intensity curve analysis for a given region of interest was conducted using customized software. Tumor perfusion metrics on days 1, 2, 3, and 6 were modeled using neural network data classification schemes (60% learning and 40% testing) to predict the breast cancer response to therapy.
Results
The breast cancer response to a single dose of bevacizumab in a murine model was immediate and transient. Permutations of input to the neural network data classification scheme revealed that tumor perfusion data within 3 days of bevacizumab dosing was sufficient to minimize the prediction error to 10%, whereas measurements of physical tumor size alone did not appear adequate to assess the therapeutic response.
Conclusions
Contrast-enhanced ultrasound may be a useful tool for determining the response to bevacizumab therapy and monitoring the subsequent restoration of blood flow to breast cancer.
Keywords: bevacizumab, breast cancer, contrast agent, neural networks, ultrasound
Vascular endothelial growth factor (VEGF) is a receptor ligand that promotes neovascularization,1,2 allowing tumor growth3 and metastatic dissemination. Tumor neovasculature is structurally and functionally abnormal when compared with normal vasculature.4 This tortuous, dilated, and poorly organized tumor vascularity is hyperpermeable, which in turn raises tumor interstitial fluid pressure5 and compromises delivery of both ultrasound contrast agents (UCAs) and chemotherapeutic agents. Because VEGF-driven angiogenesis is a process critical in the early stages of tumor development, anti-VEGF treatments are ideally administered early in cancer therapy to improve progression-free survival.6 In addition to preventing angiogenesis, inhibition of the VEGF signaling pathway increases effectiveness of combinational therapies likely through normalization of the tumor vasculature network and reduction of tumor interstitial pressure,4,7 thereby allowing improved delivery of chemotherapeutic drugs.8
Bevacizumab is a recombinant humanized monoclonal antibody to VEGF and has emerged as a promising treatment option for the management of breast cancer and other cancer types.6,9–12 Unfortunately, not all patients respond to bevacizumab therapy.13 It has been proposed that bevacizumab drug resistance and continued tumor growth may be due to the advanced state of tumor development whereby compensatory mechanisms for angiogenesis have emerged.1,14 Selection of only those patients likely to benefit from bevacizumab therapy would be ideal, but no proven predictive factors for VEGF-targeted therapy have been identified to date.
Traditional gray scale ultrasound imaging is one of the most frequently used clinical imaging modalities. The primary advantages of this imaging method are minimal health risks associated with patient exposure to ultrasonic energy, real-time capability, and the relatively low cost associated with both examinations and system maintenance. A recent advance in ultrasound imaging has been the development of UCAs. These microbubbles are subcapillary sized gas-filled spheres. The use of a low-diffusivity gas and a thin stabilizing shell (lipid or protein based) permits a circulatory lifetime on the order of tens of minutes. Because of a large acoustical impedance difference between intravascular UCAs and the surrounding blood, they are excellent ultrasound scatterers. Ultrasound contrast agents, therefore, improve ultrasound sensitivity to slow blood flow in small vessels such as those associated with tumor neovasculature.
In response to an ultrasonic field, UCAs undergo an oscillatory movement (or resonate), a process called stable cavitation. Under sufficiently high ultrasonic pressures (50–200 kPa), this can lead to nonlinear scattering and generation of echoes with frequency components at multiples of the transmit frequency.15 These harmonic signals can be selectively isolated from the ultrasonic signals using various signal-processing and pulsing techniques, forming the basis of many contrast-enhanced ultrasound (CEUS) imaging techniques. Because these signals are less pronounced in tissue, harmonic-based CEUS imaging techniques yield improved microbubble-to-tissue contrast levels. In fact, power modulation (or pulse inversion) harmonic imaging is a relatively new technique with demonstrated improved image contrast over other ultrasound-based fundamental (ie, traditional gray scale) and harmonic imaging strategies.16 Moreover, power modulation harmonic imaging (PMHI) takes advantage of the unique nonlinear resonant behavior of UCAs for further improvements in microbubble detection sensitivity.17
The ability to visualize UCAs traversing tumor vasculature introduces a unique opportunity for quantifying tumor perfusion and flow kinetics. Because UCAs are sensitive to ultrasound exposure, insonation at high mechanical index (MI) levels is known to cause rapid microbubble destruction.18,19 Therefore, UCAs within an imaging field can be destroyed by a short sequence of high-MI (>0.8) pulses followed by visualization of UCA replenishment within that tissue region using low-MI (<0.2) ultrasound imaging. This destruction-replenishment technique allows analysis of the resultant time-intensity curves derived from a sequence of ultrasound images after microbubble destruction.20,21
An artificial neural network (ANN) is a nonlinear modeling technique inspired by the information-processing and learning behavior of biological nervous systems. In the past, neural networks have been used in conjunction with medical image analysis (findings) to categorize input patterns into 2 or more classes, such as benignity or malignancy, of breast22,23 and prostate24 tumors. By using histologic findings (eg, microvessel density [MVD]) as an objective measure (reference standard) of the breast cancer response or no response to bevacizumab therapy, ultrasound-based tumor metrics may be formulated in a neural network context to reveal patterns suggestive of therapeutic efficacy.
In this study, we investigate the feasibility of using CEUS for characterizing blood flow in a breast cancer xenograft model. Combined with ANN data modeling and trend recognition capability, a method for determining the breast cancer response to bevacizumab is introduced and analyzed.
Materials and Methods
Cell Culture
The 2LMP metastatic subclone of the human breast cancer cell line MDA-MB-231 was obtained from Donald Buchsbaum, PhD (University of Alabama), and maintained in Dulbecco’s modified Eagle’s medium (Mediatech Inc, Manassas, VA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT). Cells were cultured in 75-cm2 flasks. At approximately 80% confluency, cells were harvested by trypsinization, counted with a hemocytometer, and diluted to a final concentration of 10 × 106 cells/mL.
Animal Preparation
Animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Alabama. Twenty-five 4-week-old nude athymic mice (The Jackson Laboratory, Bar Harbor, ME) were implanted with 1 × 106 cancer cells in bilateral inguinal mammary fat pads. Approximately 3 weeks after cell implantation (ie, day 0) a group of animals (n = 15) received a 0.1-mg intraperitoneal injection of bevacizumab (Genentech, South San Francisco, CA). The remaining animals functioned as experimental controls (n = 10), receiving an equivalent intraperitoneal injection of saline only.
Ultrasound Imaging
Contrast-enhanced ultrasound images were acquired in all animals before bevacizumab administration and at 1, 2, 3, and 6 days thereafter. Approximately 3 to 5 minutes after a 30-µL bolus tail vein injection of a perflutren microsphere UCA (Definity; Lantheus Medical Imaging, North Billerica, MA) diluted to 100 µL with saline, scanning was performed by an experienced sonographer using an iU22 scanner (Philips Healthcare, Bothell, WA) equipped with an L9-3 transducer. Tumors were imaged in the transverse plane of the largest tumor dimensions. A gray scale ultrasound image was acquired for tumor size monitoring. Subsequently, a low-power (MI = 0.07) PMHI technique was used to show tumor perfusion after UCA destruction from the image plane by a multiframe (n = 5) high-power “flash” sequence (MI = 0.7). Uncompressed digital images (Digital Imaging Communications in Medicine format) were acquired for at least 20 seconds, allowing sufficient time for tumor reperfusion.25 All imaging was performed under isoflurane anesthesia (1%–2%) on a temperature-controlled heating pad (37°C).
Immunohistologic Analysis
After ultrasound imaging on day 6, animals were humanely euthanized, and tumors were surgically excised. Using established protocols, sectioned tumors underwent staining with hematoxylineosin (H&E) and CD31 antibody26 for quantification of tumor necrosis and MVD, respectively. Histologic specimens were reviewed by a board-certified anatomic pathologist blinded to the experimental groups. Hematoxylin-eosin–stained sections were examined for cellular necrosis and reported as the percentage of the entire tumor cross section (original magnification ×40). Each CD31 section was examined (original magnification ×40) to identify 5 separate areas containing the greatest number of microvessels. Individual vessels from these 5 areas were counted (original magnification ×200), averaged, and recorded as MVD.27
Data Processing
Custom programs were developed using the programming software package MATLAB (The Mathworks, Natick, MA) that allowed processing of ultrasound image data. User placement of a 0.75-cm-diameter circular region of interest (ROI) necessitated subjective interpretation of tumor location. A relatively large ROI was chosen to capture blood flow in adjacent tissue and to allow for tumor growth throughout the study period. After ROI placement, individual time-intensity curves (from each spatial location within the ROI) were generated from a 20-second sequence of CEUS images. The first frame of data after UCA destruction was used to normalize all sequential images. Subsequently, an averaged time-intensity curve was generated and used to derive the following descriptive tumor perfusion metrics, namely, area under the curve (AUC), peak intensity (IPK), and time to peak intensity (TPK). Tumor sizes (circumferential area) were measured from gray scale ultrasound images (acquired before PMHI) using a semiautomatic segmentation algorithm28 implemented in MATLAB.
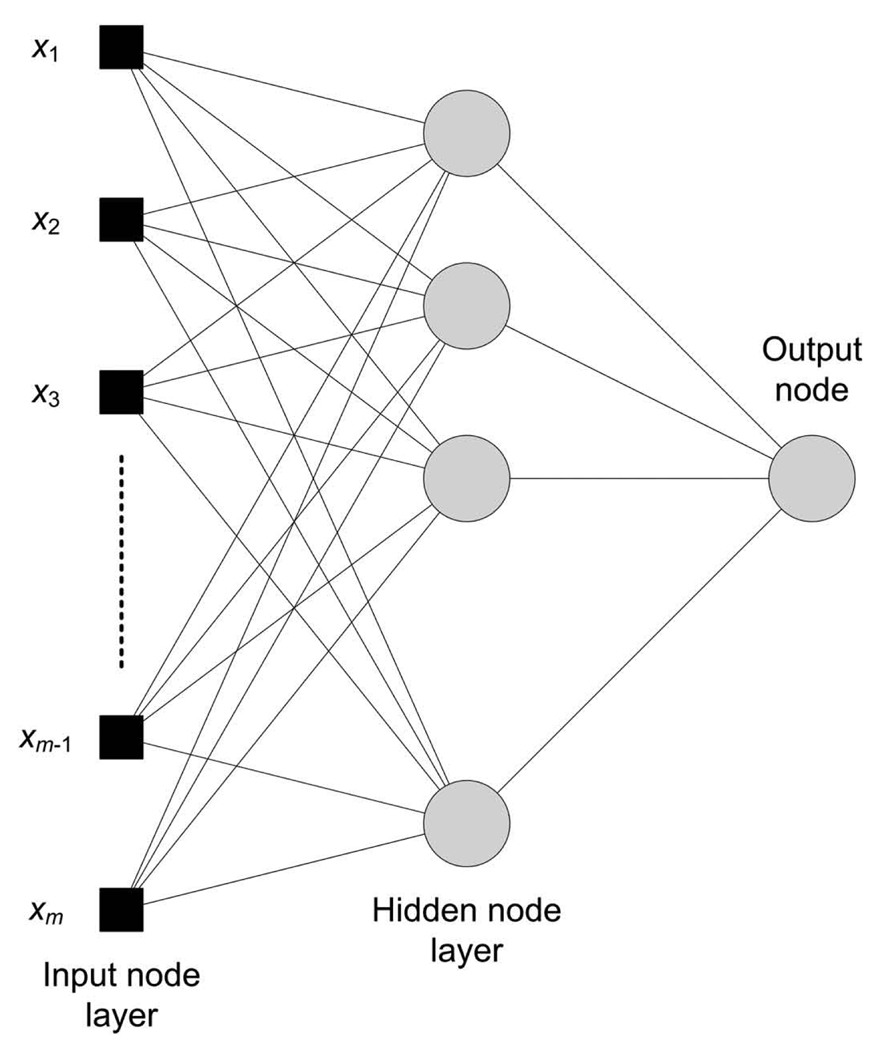
Contrast-enhanced ultrasound-derived tumor perfusion metrics and size measurements were modeled using a multilayer feedforward neural network (MLFN) implemented using the software package NeuralTools 5.5 (Palisade Corp, Ithaca, NY). Immunohistologic measures of MVD were classified as the dependent categorical variable whereby an average MVD threshold was used to classify the response (or tumor exposure) to bevacizumab therapy. Using different neural network configurations (2–25 nodes in a hidden layer), network training was conducted on randomized data sets (60% of cases) to give the most accurate prediction. The remaining data (40% of cases) were used for model testing and predicting known output values (ie, tumor response to therapy or lack thereof). A schematic diagram of the MLFN architecture is illustrated in Figure 1. Variable analysis provided insight into the impact of these metrics. Model accuracy was determined by the number of incorrect predictions and reported as the percentage of total cases.
Figure 1.
Architecture of the MLFN for predicting the breast cancer response to bevacizumab therapy.
Statistical Analysis
For each animal, the tumor exhibiting the greatest intratumoral blood flow on day 6 was selected for evaluation because bilateral CEUS-based tumor metrics have been shown to be statistically correlated.21 All experimental data were summarized as mean ± SD and reported as a percent change from baseline. Spearman rank correlation analyzed data relationships. A 2-sample t test evaluated statistical differences between control and therapy animal group results. Findings with P < .05 were considered statistically significant. All data was analyzed using either Excel (Microsoft Corporation, Redmond, WA) or an independent statistical software add-in (StatTools 5.5; Palisade Corp).
Results
The use of microbubble destruction-replenishment techniques allows real-time visualization of tumor perfusion and blood flow patterns. A representation of tumor perfusion immediately after UCA destruction in an orthotopic 2LMP xenograft is detailed in Figure 2. After UCA destruction from the imaging plane, microbubbles slowly reperfuse the tumor and surrounding vasculature. Analysis of tumor time-intensity curve details (eg, AUC, IPK, and TPK) derived from a multiday study introduces a potential strategy for monitoring changes in tumoral vasculature and efferent blood flow in response to antiangiogenic therapies.
Figure 2.
Representative ultrasound images (top) illustrating progression of breast cancer perfusion at times 0.1 (a), 2 (b), 10 (c), and 20 (d) seconds after UCA destruction from the image field. The bottom plot shows the average time-intensity curve derived from an ROI (0.75 cm diameter) centered and encompassing the tumor entirety.
Before treatment, there were no statistically significant differences in tumor sizes between the two animal groups (P = .74). The same was true at days 1 (P = .21), 2 (P = .15), and 3 (P = .30) from baseline. By day 6 of this study, however, control tumors were found to be statistically larger than those treated with bevacizumab (P = .029). The observed progressive change in tumor sizes (relative to baseline measurements) is summarized in Figure 3. These results indicate that bevacizumab therapy inhibited breast cancer growth on average by nearly 60% over the course of the study period.
Figure 3.
Changes in normalized tumor size for control and bevacizumab- treated animal groups.
Contrast-enhanced ultrasound tumor perfusion metrics derived from both control and therapy group animals are plotted in Figure 4. At baseline, there were no statistically significant differences between any control and therapy group metrics (P > .51). Inspection of Figure 4 reveals that bevacizumab-treated tumors exhibited an abrupt (but transient) increase in both the AUC and IPK metrics compared with the decreasing trend of the control group. Specifically, control and therapy group-based AUC and IPK metrics were significantly different at both days 1 (P < .015) and 2 (P < .011), whereas no statistically significant differences remained by days 3 (P > .41) and 6 (P > .63) of this study. Conversely, decreasing trends in the TPK metric were less distinct, and no significant differences between the two animal groups were observed (P > .053).
Figure 4.
Microbubble-enhanced ultrasound tumor perfusion metrics, namely, AUC (a), IPK (b), and TPK (c), mapped as a function of time. Microbubble perfusion was derived from averaged time-intensity curves for the corresponding day.
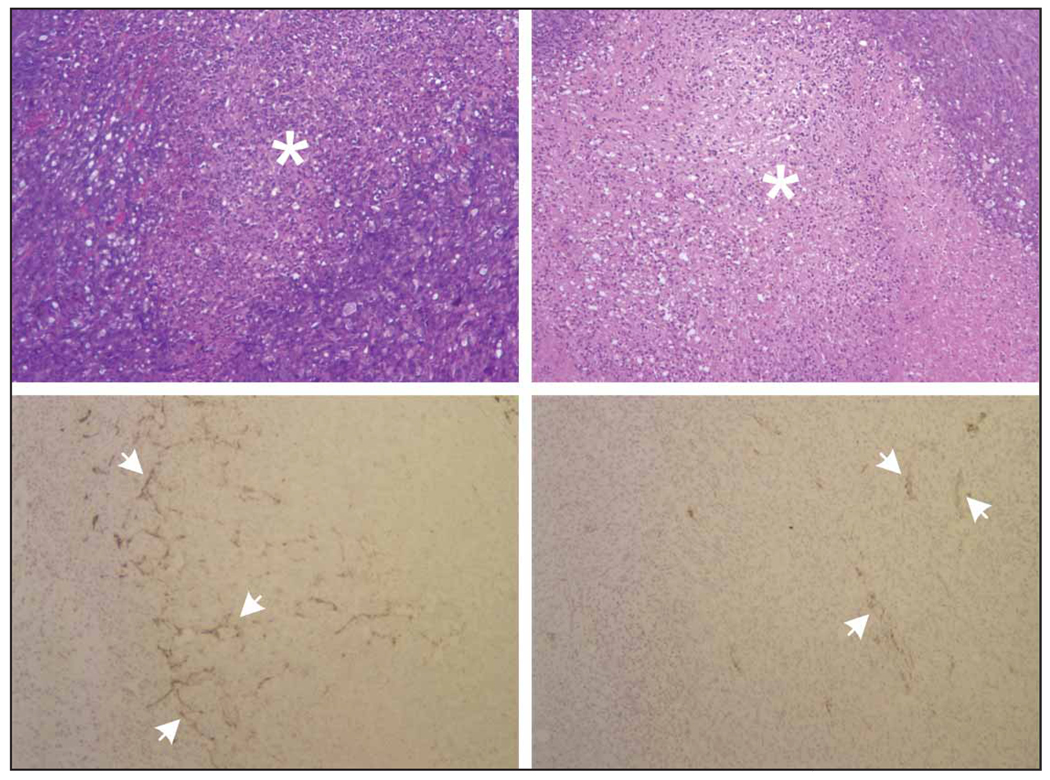
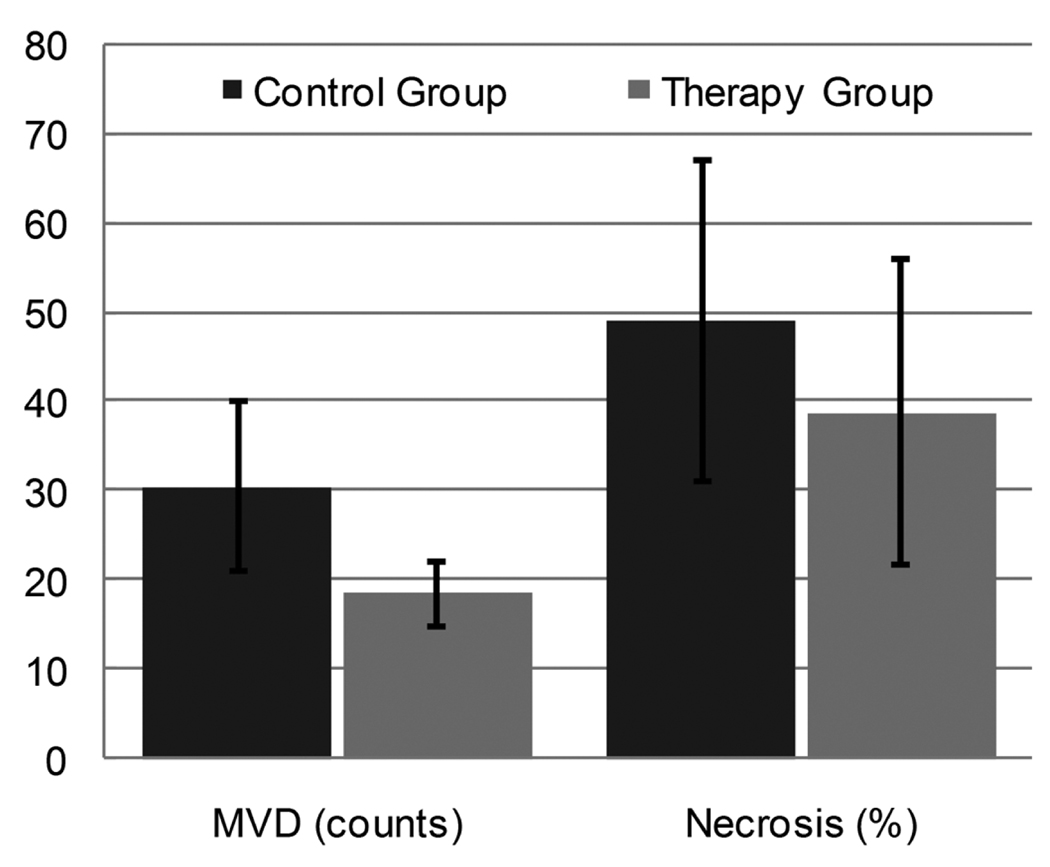
Figure 5 illustrates H&E and CD31 immunohistochemical results from day 6. Representative H&E sections reveal discernible focal necrosis in both control and bevacizumab-treated tumors. Necrotic areas in control tumors are attributed to high proliferative activity and increased levels of hypoxia due to elevated intratumoral pressures and reduced blood flow. Conversely, necrosis in bevacizumab-treated tumors may be attributed to microvessel pruning (compare Figures 4B and 5A), reduced intratumoral blood flow, and similar increased hypoxic cellular conditions. A summary of immunohistologic results for both tumor groups is presented in Figure 6. As the results show, MVD counts were significantly higher in the control group versus the bevacizumab-treated tumor group (P = .004). Analysis of tumor necrosis reveals considerable levels for both tumor groups but no statistically significant differences (P = .17).
Figure 5.
Effect of bevacizumab on histologic characteristics of breast cancer xenografts. Top, Representative H&E-stained sections for control (left) and bevacizumab-treated (right) tumors. Focal areas of tumor necrosis are seen on both groups, as indicated by asterisks. Bottom, Representative CD31 immunohistologic sections for control (left) and bevacizumab-treated (right) tumors. Arrows indicate tumor microvessels.
Figure 6.
Summary of immunohistologic results from control and therapy group tumors. Results are depicted for both MVD counts and percent intratumoral necrosis.
An analysis of ultrasound and histologic data from day 6 of this study was conducted to identify any correlation between end point variables. Using the control tumor data, no significant correlations between MVD and either AUC (r = 0.24; P = .51) or IPK (r = 0.26; P = .47) metrics were discovered. However, trends toward significance in the correlation of MVD and either AUC (r = −0.48; P = .069) or IPK (r = −0.48; P = .071) metrics were found in therapy tumor data. These results suggest an inverse relationship between ultrasound-based tumor perfusion metrics and MVD. Although no significant correlation existed between the control tumor area and MVD (r = 0.47; P = .17), a trend toward a significant correlation was found between the bevacizumab-treated tumor area and MVD (r = 0.48; P = .072). This relationship indicates that the size of a treated tumor is proportional to the density of intratumoral microvessels.
Inspection of the ultrasound results presented in Figures 3 and 4 reveals discernible differences between control and therapy-derived tumor metrics. Although differences may be transient in nature, such information may prove valuable in assessing the breast cancer response (or lack thereof) to antiangiogenic therapy. To that end, we evaluated the use of ANNs applied to ultrasound-based tumor metrics for determining the breast cancer response to bevacizumab therapy. Given histologic information from this study, it was subjectively determined that an average MVD count of 25 vessels was the clearest delineation between the control and therapy tumor groups. Using this threshold as the criterion for a tumor response (ie, MVD <25) or no response (ie, MVD ≥25) to bevacizumab therapy, ultrasound-based metrics were modeled using a MLFN. Note that control data were modeled as a therapy nonresponder despite not being administered bevacizumab. To standardize animal preparation, this approach was chosen in lieu of selecting a second breast cancer cell line with low-level VEGF receptor expression.
Changes in ultrasound-based tumor perfusion (ie, AUC, IPK, and TPK) and size data throughout the 6-day study were optimally modeled using an MLFN with 10 nodes in the hidden layer. Although training produced 0% incorrect predictions, neural network testing resulted in a false prediction rate of 20% in the testing sample. Noting that the most significant changes in experimental data occurred within 3 days of bevacizumab dosing, the MLFN was retrained using this reduced data set. Consequently, the optimal MLFN configuration contained 8 nodes in the hidden layer. Prediction errors were 0% for the training (n = 15) and 10% for the testing (n = 10) cases. A variable-impact analysis discovered that the trained neural network is more sensitive to changes in the AUC and IPK metrics compared with the TPK. Interestingly, initial changes in tumor size after therapy had the least impact on neural network configuration. This suggests that measurements of physical tumor size may be necessary but not sufficient to assess the breast cancer response to bevacizumab therapy.
Discussion
Antiangiogenic drugs such as bevacizumab effectively disrupt tumor development by blocking the VEGF signaling pathway. Although the exact mechanisms are still being unraveled, VEGF inhibition leads to vascular normalization and a reduction of intratumoral pressure, which can restore tumor blood flow to various degrees. Because UCAs are excellent intravascular tracers, their application for assessing changes in tumor vasculature and blood flow appear well suited. In this study, the use of CEUS for determining the tumor response to bevacizumab therapy was investigated in a breast cancer murine model.
Experimental results revealed an abrupt and significant increase in the CEUS-derived AUC and IPK tumor perfusion metrics after a single bevacizumab dose compared with controls. An analysis of these same metrics from day 6 of the study revealed that this difference between control and therapy tumor data had abated (P > .63). Collectively, these observations suggest that the antivascular effect of a single dose of bevacizumab in a breast cancer murine model is transient. Conversely, no significant changes in the TPK metric were observed (P > .053) throughout the study, suggesting that the TPK metric alone may be insufficient to describe transient changes in tumor vascularity.
A comparable study by Dickson et al8 using a neuroblastoma murine model evaluated the tumor response to a single dose of bevacizumab. Using CEUS and a time-intensity curve-derived IPK metric, this group noted similar trends in tumor perfusion in response to antiangiogenic therapy. Specifically, treated tumors exhibited significant increases in an IPK metric compared with control tumors on days 1 (P = .01) and 3 (P = .03) after bevacizumab dosing. However, no significant differences in tumor perfusion were found between bevacizumab-treated and control tumors on day 7 (P = .92). The transient nature of the change in tumor perfusion after bevacizumab dosing is highlighted by these corroborating studies.
Inspection of tumor growth data (see Figure 3) revealed that by day 6 of the study, bevacizumab-treated tumors were significantly smaller than controls (P = .029). Interestingly, several research articles document a progressive shrinking of tumor size in response to bevacizumab therapy,29,30 whereas others describe an increase in tumor volume.8,21,27 This disparity in the antitumor effect of bevacizumab treatment can be attributed to several experimental variables such as the strain of the murine model, the specific breast cancer cell line, mouse and tumor ages, and therapy dosing.
Histologic analysis of tumor sections revealed discernible focal necrosis in both control and bevacizumab-treated tumors. In bevacizumab-treated tumors, there was a significantly lower MVD count compared with controls (P = .004). In accord with these findings, it was shown in a neuroblastoma xenograft murine model that bevacizumab-induced transient remodeling of tumor vasculature is associated with drastic changes in measurements of MVD.8 It was shown that after a single bevacizumab dose, a progressive decrease in MVD occurred that equated to a more than 70% reduction within 7 days. Measured experimentally, this change in MVD coincided with a rapid decrease in both tumor vessel permeability and tumor interstitial fluid pressure, thereby increasing tumor perfusion. These results reflect an earlier study by Inai et al30 that also reported a more than 70% decrease in tumor MVD after 7 days using the VEGF signaling inhibitor AG013736. Although our histologic analysis was limited by the use of a single section to quantify MVD in each tumor, results presented by both Dickson et al8 and Inai et al30 corroborate and add further insight to our observed increase in tumor perfusion as measured using the AUC and IPK metrics.
Transient trends in tumor perfusion data and physical size were modeled using a neural network data classification scheme. Permutations of input data revealed that tumor perfusion data within 3 days of bevacizumab dosing in a murine model were sufficient to minimize the prediction error in the testing group to 10%. Although the breast cancer response to bevacizumab therapy was predicated on a subjective threshold measure of MVD and a very limited number of experimental cases, preliminary results are encouraging. To be deemed clinically feasible, more research in both animal models and humans is warranted. Notwithstanding, these results suggest that neural network modeling applied to noninvasive ultrasound-based tumor information may be a useful strategy for predicting the breast cancer response to bevacizumab therapy.
In conclusion, treatment of breast cancer–bearing mice with a single dose of bevacizumab is transient and appears to last only a few days. This tumor response was characterized by restricted tumor growth and a significant change in tumor blood flow. Moreover, these antiangiogenic effects were determined noninvasively using ultrasound-based techniques and data classification. Therefore, CEUS may be a useful tool for monitoring the restoration of blood flow to breast cancer and determining the response to bevacizumab therapy.
Acknowledgments
We thank Karri Folks and Sharon Samuel for assistance in animal experimentation and the Comprehensive Cancer Center’s Tissue Procurement Facility for histologic services. This research was supported by grant UL1RR025777 from the National Institutes of Health National Center for Research Resources and the Comprehensive Cancer Center at the University of Alabama.
Abbreviations
- ANN
artificial neural network
- AUC
area under the curve
- CEUS
contrast-enhanced ultrasound
- H&E
hematoxylin-eosin
- IPK
peak intensity
- MI
mechanical index
- MLFN
multilayer feedforward neural network
- MVD
microvessel density
- PMHI
power modulation harmonic imaging
- ROI
region of interest
- TPK
time to peak intensity
- UCA
ultrasound contrast agent
- VEGF
vascular endothelial growth factor
References
- 1.Relf M, LeJeune S, Scott PAE, et al. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor β-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- 2.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? Cancer Res. 1986;46:467–473. [PubMed] [Google Scholar]
- 4.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 5.Jain RK. Determinants of tumor blow flow: a review. Cancer Res. 1988;48:2641–2658. [PubMed] [Google Scholar]
- 6.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 7.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 8.Dickson PV, Hamner JB, Sims TL, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007;13:3942–3945. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]
- 9.Bergsland E, Dickler MN. Maximizing the potential of bevacizumab in cancer treatment. Oncologist. 2004;9:36–42. doi: 10.1634/theoncologist.9-suppl_1-36. [DOI] [PubMed] [Google Scholar]
- 10.Chen HX. Expanding the clinical development of bevacizumab. Oncologist. 2004;9:27–35. doi: 10.1634/theoncologist.9-suppl_1-27. [DOI] [PubMed] [Google Scholar]
- 11.Rugo HS. Bevacizumab in the treatment of breast cancer: rationale and current data. Oncologist. 2004;9:43–49. doi: 10.1634/theoncologist.9-suppl_1-43. [DOI] [PubMed] [Google Scholar]
- 12.Cameron D, Bell R. Future use of bevacizumab and other anti-angiogenic agents in breast cancer. Eur J Cancer. 2008;6:40–50. [Google Scholar]
- 13.Shih T, Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. 2006;28:1779–1802. doi: 10.1016/j.clinthera.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor: targeted therapy. Clin Cancer Res. 2008;14:6371–6375. doi: 10.1158/1078-0432.CCR-07-5287. [DOI] [PubMed] [Google Scholar]
- 15.de Jong N, Frinkling P, ten Cate F, van der Wouw P. Characteristics of contrast agents and 2D imaging. IEEE Ultrasonics Symp. 1996;1:1449–1458. [Google Scholar]
- 16.Kim AY, Choi BI, Kim TK, Kim KW, Lee JY, Han JK. Comparison of contrast-enhanced fundamental imaging, second-harmonic imaging, and pulse-inversion harmonic imaging. Invest Radiol. 2001;36:582–588. doi: 10.1097/00004424-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Eckersley RJ, Chin CT, Burns PN. Optimising phase and amplitude modulation schemes for imaging microbubble contrast agents at low acoustic power. Ultrasound Med Biol. 2005;31:213–219. doi: 10.1016/j.ultrasmedbio.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Shi WT, Forsberg F, Vaidyanathan P, Tornes A, Ostensen J, Goldberg BB. The influence of acoustic transmit parameters on the destruction of contrast microbubbles in vitro. Phys Med Biol. 2006;51:4031–4045. doi: 10.1088/0031-9155/51/16/010. [DOI] [PubMed] [Google Scholar]
- 19.Yeh CK, Su SY. Effects of acoustic insonation parameters on ultrasound contrast agent destruction. Ultrasound Med Biol. 2008;34:1281–1291. doi: 10.1016/j.ultrasmedbio.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97:473–483. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 21.Hoyt K, Warram JM, Umphrey H, et al. Assessing breast cancer response to bevacizumab using contrast-enhanced ultrasound: initial results using a murine model. IEEE Ultrasonics Symp. In press. [Google Scholar]
- 22.Floyd CE, Lo JY, Yun AJ, Sullivan DC, Kornguth PJ. Prediction of breast cancer malignancy using an artificial neural network. Cancer. 1994;74:2944–2948. doi: 10.1002/1097-0142(19941201)74:11<2944::aid-cncr2820741109>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Joo S, Yang TS, Moon WK, Kim HC. Computer-aided diagnosis of solid breast nodules: use of an artificial neural network based on multiple sonographic features. IEEE Trans Med Imaging. 2004;23:1292–1300. doi: 10.1109/TMI.2004.834617. [DOI] [PubMed] [Google Scholar]
- 24.Djavan B, Remzi M, Zlotta A, Zeitz C, Snow P, Marberger M. Novel artificial neural network for early detection of prostate cancer. J Clin Oncol. 2002;20:921–929. doi: 10.1200/JCO.2002.20.4.921. [DOI] [PubMed] [Google Scholar]
- 25.Chomas JE, Pollard RE, Sadlowski AR, et al. Contrast-enhanced US of microcirculation of superficially implanted tumors in rats. Radiology. 2003;229:439–446. doi: 10.1148/radiol.2292020536. [DOI] [PubMed] [Google Scholar]
- 26.Wang D, Stockard CR, Harkins L, et al. Immuno-histochemistry for the evaluation of angiogenesis in tumor xenografts. Biotech Histochem. 2008;83:179–189. doi: 10.1080/10520290802451085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 28.Ladak HM, Mao F, Wang Y, et al. Prostate boundary segmentation from 2D ultrasound images. Med Phys. 2000;27:1777–1788. doi: 10.1118/1.1286722. [DOI] [PubMed] [Google Scholar]
- 29.Selvakumaran M, Yao KS, Feldman MD, O’Dwyer PJ. Antitumor effect of the angiogenesis inhibitor bevacizumab is dependent on susceptibility of tumors to hypoxiainduced apoptosis. Biochem Pharmacol. 2008;75:627–638. doi: 10.1016/j.bcp.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inai T, Mancuso M, Hashizume H, et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]