Abstract
Bony craniofacial deficits resulting from injury, disease, or birth defects remain a considerable clinical challenge. In this study, microsphere-based scaffold fabrication methods were use to study the respective effects of scaffold pore size, open pore volume, and total void volume fraction on osseous tissue infiltration and bone regeneration in a critical size rat cranial defect. To compare the healing effects of these parameters, three different scaffolds types were fabricated: solid 100 μm spheres, solid 500 μm spheres, and hollow 500 μm spheres. These constructs were implanted into surgically created rat calvarial defects. By 90-days post op, results of micro computed tomography (CT) analysis showed that all scaffolds generated similar amounts of new bone which was significantly greater than untreated controls. Interestingly, the spatial distribution of new bone within the defect area varied by scaffold group. MicroCT and histological analysis demonstrated healing restricted to the dural side in the hollow 500 μm group, whereas the solid 500 μm group demonstrated healing along the dural side and within the center of the defect. Solid 100 μm groups demonstrated healing along the dural layer, periosteal layer, and within the center of the defect. These results suggest that pore size and closed void volume may both play important roles in scaffold degradation patterns and associated bone healing.
Keywords: bone tissue engineering, porosity, microsphere, scaffold, bone regeneration
INTRODUCTION
Craniofacial surgery to repair damaged, diseased, or congenitally missing bone is an ongoing clinical challenge. Traditional replacement materials, such as the autograft and allograft, suffer from several disadvantages including limited availability and cost, respectively. Developing synthetic biomaterials with reliable performance, low cost, and wide availability would help ameliorate the difficulty in reconstructing these defects. Fabrication of appropriate biomaterials for tissue regeneration requires knowledge of both the biomaterial and the normal repair mechanism of the tissue in question. In cranial bone, repair and regeneration is dependent upon the adjacent periosteum and dura mater. Recent findings have suggested that osteoprogenitor cell migrations into the defect site are solely derived from dural and/or periosteal layers whereas mature osteoblasts in adjacent bone contribute only minimally to the healing process.1 Others have also reported on the significant role of dura in cranial bone regeneration suggesting that this layer makes its contribution by donating osteoprogenitor cells and secreting critical growth factors such as FGFs and TGF-β.2–4 Membranes that completely block these tissue layers from the bony wound bed result in significantly less new bone formation.5 Therefore, creating biomaterials that direct the migration and enhance the proliferation/differentiation of these cell types is important. Scaffold design parameters such as degradation rate, pore volume, and pore size can be altered to control these cellular behaviors.
It is known that scaffold pore size affects both cell migration and diffusion of factors within the local scaffold environment. Furthermore, recent bone repair studies suggest that scaffold pore size may in fact influence the type of osteogenesis which occurs. Several ectopic bone studies using hydroxyapatite report that smaller pore sizes (90–120 μm) result in chondrogenesis before osteogenesis whereas larger pore sizes (350 μm) result in direct osteogenesis with enhanced vascularization. Additionally, the authors note that less porous scaffolds with lower interconnectivity retard the proliferation/differentiation of osteoblastic cells and allow for chondrogenesis to precede osteogenesis.6 Therefore, porosity, interconnectivity, and average pore size may be key parameters which can be altered to influence the type of ossification which occurs at tissue-specific sites of repair.
Utilizing poly-lactic-co-glycolic acid (PLAGA) microsphere-based scaffolds to heal cranial defects may be particularly advantageous given what is known about these scaffolds. Specifically, PLAGA microsphere-based scaffolds support cell attachment,7 are capable of releasing various bioactive factors,8 and demonstrate osteoconductivity in bony defect models.9 Microsphere-based scaffolds possess completely interconnected pore architecture and pore volume in the range of trabelcular bone (30–40%).10 Additionally, microsphere diameter is directly proportional to pore size. Smaller microspheres yield scaffolds with smaller pore sizes. Therefore, this scaffold fabrication technique ensures adequate pore volume for osseous tissue synthesis and regeneration, and also allows flexibility in choosing an optimal overall pore size that may be preferred in specific applications, such as those required for cranial bone reconstruction.
As with all scaffolding techniques which utilize PLAGA polymers, degradation rate can be tailored by using various ratios of the co-polymers, lactic, and glycolic acid. The spatial pattern of degradation can also be tailored using the microsphere-based scaffolding technique in ways that other fabrication systems cannot. This sphere-based technique allows for inclusion of component microspheres with altered degradation properties and or internal geometry. In this way, initial pore geometry can be fixed among varying scaffold groups, but scaffolds may be engineered to display distinct patterns of degradation based on the degradation rate of respective microspheres or the inclusion of additional void (hollow core) space within individual microspheres. Specifically, hollow core spheres with the same average pore size and interconnectivity as solid spheres, could have faster overall degradation which may be more permissive to tissue ingrowth, key migration behaviors, and perhaps neovascularization. The aim of the current study is to evaluate the effects of microsphere size distribution and inclusion of hollow core microspheres on the in vivo healing potential of PLAGA microsphere-based scaffolds in an adult rat critical size cranial defect model.
MATERIALS AND METHODS
Scaffold fabrication
Hollow and solid microsphere-based scaffolds were fabricated using biodegradable 85:15 PLAGA from Alkermes Mediasorb (Wilmington, OH). Individual hollow spheres were fabricated using a double emulsion method and solid spheres were fabricated using a single emulsion and sieved to preselected diameter size ranges.11 To confirm the average diameter distribution within each sieve size, a collection of microspheres from each group was spread across the stage of a stereo light microscope. Several fields of view were imaged at 100×. Image J software analysis was used to measure the diameter of five spheres per image. To fabricate 3D scaffolds, spheres of the appropriate size (50–300 μm and 500–710 μm) and void volume (hollow or solid) were poured into individual circular copper molds measuring 8 mm (diameter) by 1 mm (thickness). This geometrical shape was chosen so that the scaffold shape would be appropriate for implantation at the site of the cranial defect. All microsphere-based scaffolds were sintered at 80°C for 3 h in the copper molds. Following sintering, scaffolds were removed and stored in a desiccator for future use. Additionally, caliper measurements were used to determine scaffold thickness following sintering where the average thickness was 0.78 ± 0.03 mm.
Mechanical testing
For mechanical testing, a different copper mold was used to create scaffolds with a 2:1 aspect ratio (length to diameter). For each scaffold type, six samples were tested using an Instron 4511 machine with a crosshead speed of 1 mm/min.
Cranial defect model
Adult male retired breeders (400–550 g) were randomly assigned to four different experimental groups: control, solid 100 μm, solid 500 μm, hollow core 500 μm. Animals were anesthetized using 4.5 mg/100 g Nembutal (intraperitoneal injection). Following anesthetization, the head was shaved, sterilized with betadine and 70% ethanol, and mounted onto a stereotactic restraint. One half mL of 1% lidocaine was injected intradermally along the midline. Using a Zeiss surgical microscope, the skin was incised along the midline. The subcutaneous fascia was divided until the periostial layer was revealed. Using a scalpel, an H-flap incision was made in the periosteum to reflect the flaps laterally. An 8 mm diameter circular defect was excavated using drill. Continuous saline irrigation was used to remove residual bone chips and maintain moisture of the tissue. Care was taken to avoid damage to the underlying dura mater tissue. Polymer implants of choice were placed into the defect. The periosteum was closed using 6-0 nylon running suture and the skin was closed using 4-0 monocryl suture. Following closure, both antibiotics and analgesics were administered for 1 week following surgery. NIH guidelines for the care and use of laboratory animal12 have been observed and all surgeries were performed according to a protocol approved by the Institutional Animal Care Committee at the University of Virginia.
X-ray imaging analysis
Following in vivo end timepoints, the animals were euthanized and the defects were excised and placed in 10% formalin. Two animals were euthanized at day 30 (n = 2) and six animals were euthanized at day 90 (n = 6). Samples of the same implant type (control, solid 100 μm, solid 500 μm, or hollow 500 μm) and same end timepoint (day 30 or 90) were placed chronologically across Kodak X-ray film. Samples were imaged for 6 s at 12.5 kVp using a (Hewlett Packard Faxitron Series X-ray System, 43805N). The films were subsequently developed and placed into an Alpha Innotech imaging system (Fluor Chem IS-8800) to convert the X-ray films into digital files from which digital images could be obtained.
Micro ct imaging analysis (scaffold, In vivo, Ex vivo)
Before implantation, individual microsphere-based scaffolds were imaged using the quantitative micro-computed tomography specimen imaging scanner (Scanco, Bassersdorf, Switzerland). Four scaffolds from each group (solid 100 μm, solid 500 μm, hollow 500 μm) were imaged utilizing a high resolution 45 kVp scan. Following reconstruction of the 2D slices, representative images were chosen to confirm the void volume of each scaffold type. Subsequently, an appropriate threshold matching the original grayscale images was chosen, contour lines were drawn around the scaffold, and 3D images were evaluated. Porosity and pore size were estimated using the quantitative 3D evaluation program included with the micro computed tomography (CT) software package. The results of pore volume and pore size were confirmed using mercury porosimetry.
Bone formation in animals was followed over a 90 day time course. MicroCT was used to assess new bone formation in the defect at 0, 30, and 90 days. Two animals were euthanized at day 30 (yielding n = 8 in vivo scans) and six animals were euthanized at day 90 (yielding n = 6 in vivo scans). Animals were anesthetized by intraperitoneal injection of 4.5 mg/100 g Nembutal. Subsequently, animals were imaged for 14 min utilizing a low resolution 45 kVp scan. At end timepoints, animals were sacrificed, and ex vivo scans of each specimen were obtained utilizing a high resolution 45 kVp scan. Following reconstruction of the 2D slices, an appropriate threshold matching the original gray-scale images was chosen. Contour lines were drawn around the defect area to appropriately select a circular defect void volume (VOI) of 8 mm × 1 mm, taking care to exclude neighboring native bone. 3D images were created from 2D slices and the bone volume within the selected circular defect was calculated using the 3D evaluation program. Bone void volume, threshold (160), and scan parameters (support = 2, width = 1.2) were kept constant throughout the entire study.
Bone histology
Following ex vivo X-ray imaging and microCT scanning, each sample was decalcified using Richard Scientific Decalcifying Solution (Kalamazoo, MI) for 2 weeks at room temperature on a rotating rocker. Here, n = 2 for day 30 samples and n = 6 for day 90 samples. Following decalcification, samples were dehydrated overnight. Samples were then cut along the coronal plane at the midline of the defect and embedded in paraffin. Seven micron sections were mounted onto individual slides, and stained with hematoxylin and eosin. To determine measurements of tissue area per defect area, montage images of histological sections were imported into Image J. Images were converted to grayscale files, thresholded, and tissue areas were measured as the number of black pixels per total defect area. Remaining polymer is dissolved during paraffin embedding and therefore shows up as white in grayscale images.
Statistical significance
A single way ANOVA test was performed to measure statistical significance when comparing experimental groups. Significant differences were denoted as p < 0.05.
RESULTS
Scaffold characterization
The overall scaffold architecture, sphere void volume, and average sphere diameter of each implant type (solid 100 μm, solid 500 μm, hollow core 500 μm) was validated before implantation using microCT imaging. Sphere void volume was confirmed two different ways. First, a selection of microspheres from each group was imaged at 100× on a light microscope. Solid spheres remained clear throughout whereas hollow core spheres demonstrated a sphere within a sphere. The outer sphere of polymer picked up an intense reflection from the light source creating a bright ring not present in the solid spheres (Fig. 1). Before implantation, each microsphere-based scaffold was imaged using both light microscopy and microCT (Figs. 2 and 3) to confirm scaffold size and sphere type.
Figure 1.
Light microscopy images of individual microspheres comparing solid spheres with hollow core spheres. A: Solid 500 μm average diameter spheres. B: Hollow core 500 μm average diameter spheres.
Figure 2.

The geometrical dimensions of a microsphere-based scaffold can be tailored to fit within a critical sized rat cranial defect, shown on the left. A: Sintered scaffold using 100 μm average diameter spheres. B: Sintered scaffold using 500 μm average diameter spheres.
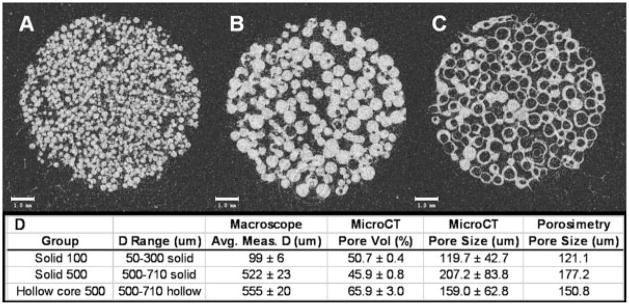
Figure 3.
MicroCT 2D slice reconstructions of cranial defect scaffolds before implantation. Scaffolds were sintered using three different sphere types. A: Solid 100 μm average diameter spheres. B: Solid 500 μm average diameter spheres. C: Hollow core 500 μm average diameter spheres. D: Sieve ranges for each scaffold group, average sphere diameter within each group measured from macroscope images, % pore volume calculated by microCT, and average pore size calculated by both microCT and mercury porosimetry analysis.
To examine individual microsphere size distribution within the selected sieve ranges (50–300 μm and 500–710 μm), light microscopy was used to image a collection of individual spheres within each size range (Fig. 1). With five measurements per image and 20 images per group, a total 120 measurements were taken. Average sphere diameters for solid 100 μm, solid 500 μm, hollow 500 μm, were 99 ± 6, 522 ± 23, 555 ± 20, respectively. In all cases, average measurements were very close to the lower sieve threshold (Fig. 3).
Pore size and pore volume were calculated for each scaffold type using the microCT 3D evaluation software (Fig. 3). For the solid 100 μm and solid 500 μm groups, the measured % pore volumes [(50.7 ± 0.4)% and (45.9 ± 0.8)%, respectively] were in close agreement with previously published estimates of this scaffold design.10,11 However, the hollow 500 μm group yielded a pore volume much higher than expected, (65.9 ± 3.0)%. Because the microCT software cannot discriminate between pore volume and hollow space within individual spheres, the pore volume reported in the hollow group was much higher than the actual value because hollow areas within spheres were included in this measurement. Pore size estimates using both microCT software and mercury porosimetry analysis were in close agreement. MicroCT evaluation reported pore sizes of 119.7 ± 42.7 μm, 207.2 ± 83.8 μm, 159.0 ± 62.8 μm for solid 100 μm, solid 500 μm, hollow 500 μm, respectively. Mercury porosimetry reported pore sizes of 121.1, 177.2, and 150.8 μm for solid 100, solid 500, hollow 500 μm, respectively.
To assess differences in mechanical properties of individual scaffold designs, constructs were molded with a 2:1 aspect ratio and tested on an Instron 4511 machine. Values for modulus, compressive strength, and maximum load were obtained for each implant type, using a sample size of n = 6 (Fig. 4). The average modulus for each group was found to be 57.01 ± 26.31 MPa, 68.89 ± 22.54 MPa, 30.43 ± 21.15 MPa, for solid 100 μm, solid 500 μm, hollow 500 μm, respectively. The hollow 500 μm scaffolds were found to have significantly lower modulus as compared with solid 500 μm. All measured moduli exceeded previously reported values of elastic modulus for rat braincase, 3–10 Mpa,13 noted with a dashed bar [Fig. 4(C)]. No significant differences in compressive strength were found between scaffold types, where the overall average was 1.18 MPa. The average maximum load for each group was found to be 0.05 ± 0.02 N, 0.02 ± 0.01 N, 0.07 ± 0.02 N for solid 100 μm, solid 500 μm, hollow 500 μm, respectively. Significant differences in maximum load existed for both comparisons: hollow 500 μm versus solid 500 μm and solid 100 μm versus solid 500 μm.
Figure 4.
Scaffolds fabricated using the various spheres types were sintered with a 2:1 aspect ratio and tested using an Instron 4511 machine. A: Max load. B: Compressive Strength. C: Modulus. For each scaffold type (solid 100 μm, solid 500 μm, hollow core 500 μm) an n = 6 was measured. *Significant from solid 500 μm. The horizontal line in (C) represents the in vivo measured value of rat braincase. Here, p < 0.05.
In vivo healing analysis
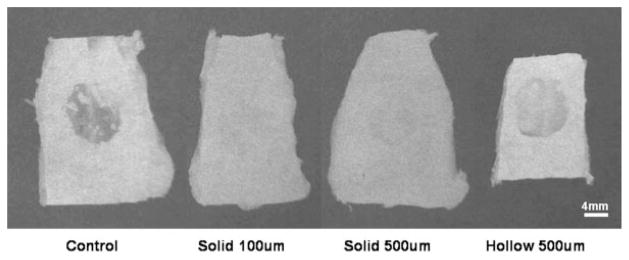
Comparison of in vivo healing potentials between implants made with hollow versus solid spheres was evaluated by qualitative X-ray analysis (Fig. 5), quantitative microCT imaging (Figs. 6–8), and histological sectioning and staining (Fig. 9). In vivo healing was followed for a total of 90 days. Following either 30 or 90 days healing, defects were removed from the animal and fixed in 10% buffered formalin. Both high resolution ex vivo microCT scans and X-ray images were obtained for all samples. Histological sectioning was performed only on day 90 samples. X-ray images showed particularly good bone coverage over the defect in the solid 100 μm and solid 500 μm day 90 samples. X-ray images of untreated controls showed the least bone healing as compared with the experimental groups. Quantitative microCT scans showed significantly greater bone volume in all groups over controls at day 90. At day 30, bone volume trends showed greater healing in the scaffold implanted groups compared with unloaded controls. Solid 100 μm showed greatest healing at day 90 although this trend was not significant. Qualitative images from both the high resolution microCT scans and the histological sectioning further supported the healing patterns measured in the quantitative results.
Figure 5.
The excised defect and surrounding native bone from the cranial defect site. X-ray imaging analysis was performed on each ex vivo sample. Representative images from each 90 day experimental group are shown above.
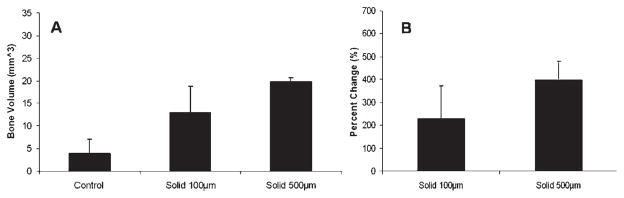
Figure 6.
Ex vivo evaluation of bone healing using microCT imaging analysis at 30 days comparing solid 100 μm vs. 500 μm average sized spheres. A: Bone volume as calculated by microCT evaluation. B: Percent change in bone volume compared to Day 30 control.
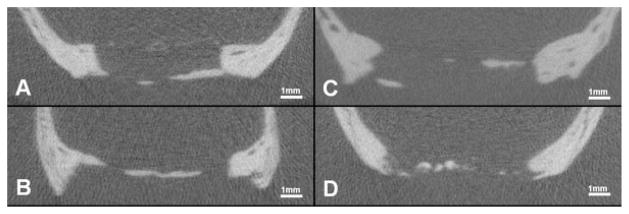
Figure 8.
Cross sections thru the center of the defect using 3D microCT evaluation. All samples are in vivo scans of solid 100 μm scaffolds following 90 days healing. A: Healing predominantly along periosteal side. B: Healing predominantly along dural side. C: Healing along both periosteal and dural sides. D: Healing located centrally within defect.
Figure 9.
Ex vivo 90 day samples were fixed in 10% buffered formalin, decalcified in Richard’s Decal Solution, embedded in paraffin, sectioned and stained for hematoxylin and eosin. Black squares (
 ) indicate edges of the scaffold to demonstrate differences in thickness. A: Percent of the defect area covered by tissue following 90 days in vivo. This measurement assumes a correlation between the amount of polymer degraded to allow for tissue integration. *Significant compared with hollow 500 μm, where p < 0.05. **Significant compared with hollow 500 μm, where p < 0.1. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
) indicate edges of the scaffold to demonstrate differences in thickness. A: Percent of the defect area covered by tissue following 90 days in vivo. This measurement assumes a correlation between the amount of polymer degraded to allow for tissue integration. *Significant compared with hollow 500 μm, where p < 0.05. **Significant compared with hollow 500 μm, where p < 0.1. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Defect thicknesses, including new tissue ingrowth and remaining polymer, were measured from histological sections. Several length measurements were taken within the defect area for several different sections for each rat at day 90. Average defect thicknesses for 500 μm hollow, 500 μm solid, and 100 μm solid groups were 1.31 ± 0.06, 1.04 ± 0.04, 0.94 ± 0.07, respectively. In all cases, the defect area increased in thickness from the original measurement, 0.78 μm. Increases in overall defect thickness were inversely proportional to the amount of new bone formed [Fig. 9(A)]. The 100 μm solid groups had significantly greater percentage of tissue ingrowth compared with 500 μm hollow group.
Both the 2D microCT images and histology sections revealed different spatial healing patterns within each group. Five hundred micrometer hollow groups showed minimal healing at dural edges. Five hundred micrometer solid groups showed healing predominantly along the bottom of the defect at the dural edge and centrally within the defect. One hundred micrometer solid groups showed healing along the dural side, the periosteal side, and within the center of the defect.
DISCUSSION
Many studies have investigated scaffold design parameters to optimize the osteoinductivity, osteoconductivity, biodegradability, and mechanical integrity of these implants at sites of bony repair.14 However, there are fundamental differences in bone formation,15 mechanical requirements,16,17 and local progenitor populations,18,19 related to the exact site of the bony defect. Therefore, scaffold design parameters should be tailored in a site-specific fashion to address distinct healing behaviors. The focus of this study was to investigate the effects of microsphere-based scaffold design parameters for site-specific cranial repair.
Material choice is an important parameter to consider for scaffold design. Material choice can influence mechanical properties, degradation rate, and host compatibility. For this study, 85:15 poly-lactic-co-glycolic acid, PLGA, was chosen for its slower degradation rate and lower acidic profile as compared to more rapidly degrading formulations. Additionally, we have previously shown that progenitor populations isolated from the underlying dural tissue can attach, proliferate, and differentiate towards an osteoblastic phenotype in long-term in vitro culture (28 days),20 suggesting that this biomaterial implanted in vivo adjacent to dural tissue will permit ingrowth and subsequent bone regeneration. Experimental groups in the current study demonstrated appreciable amounts of healing along the dural edge supporting previous in vitro results demonstrating PLAGA’s biocompatibility with dural cells.
Functionally, long bones, unlike cranial bones, must be able to support body weight with measured values for elastic modulus of rat femur reported at 12.1 Gpa.21 Therefore, it is critical to find material properties and architectures which can achieve large loading capacities to meet the mechanical needs of weight bearing long bones. However, cranial bones do not experience the same daily loading as long bones and thus the ability to sustain repeated high maximal loads may not be as stringent for cranial scaffolds. But, structural integrity remains important for tissue organization. Measured values for elastic modulus of the rat braincase (including brain, sutures, and skull) are reported between 3 and 10 Mpa.16 Therefore, in our study, the elastic modulus of all three scaffold designs exceeded this range up to six times the highest reported value. As expected, the hollow core scaffolds exhibited the lowest modulus, but would still maintain structural integrity for this site of repair.
Experimentally measured values of rat femur bone thickness, range from 3.63 to 4.13 mm.17 Average rat skull thickness ranges from 0.16 mm at neonate day 13 to 0.69 mm at neonate day 90.22 In this way, cranial scaffolds may avoid the mass diffusion limitations from which long bone scaffolds often suffer and may allow for tissue integration throughout the entire scaffold. The microsphere-based scaffolds used in this study had an initial average thickness of 0.78 ± 0.03 mm. Mass diffusion limitations are reported to become an issue with tissue thicknesses greater than 0.8 mm.23 Therefore, the scaffolds in this study should have minimal diffusion limitations, increasing the possibility of total tissue infiltration throughout the defect. However, it is important to note that translation to humans will amplify requirements of nutrient mass transport. Large mineralizing pools of new bone were found at the center of the defect following 90-days healing suggesting that tissue and nutrient infiltration to the thickest parts of the scaffold were possible.
Unlike ulna or femur long bone models, cranial bones develop predominantly by intramembranous ossification. In contrast, long bones develop via endochondral ossification. During this process in which cartilage formation precedes bone formation, blood vessels invade the hypertrophic cartilage region thus establishing an environment suitable for subsequent osteoblastic differentiation. This process requires great organization and collaboration between endothelium, cartilage, and osteoblasts. In contrast, cranial bones arise directly from mesenchymal stem cell differentiation following capillary ingrowth within a mesenchymal region.14 Therefore, an ideal cranial scaffold design would support migration, proliferation, and direct osteoblastic differentiation of local mesenchymal cells. Recent reports suggest that pore size may be an important influence in this regard.24
Determining optimal parameters of pore size and pore volume remains an active area of research. In addition to influencing the type of tissue formed, pore systems are an essential feature for transient biodegradable scaffolds allowing native cell and tissue infiltration. In this study, different microsphere size ranges were considered (50–300 μm and 500–710 μm) yielding different average pore sizes (0.12 mm for solid 100 μm to 0.21 mm for solid 500 μm). The closed-packed system of the microsphere-based scaffolds ensured 100% connectivity and pore volume was measured at 48%. Hollow core scaffolds show higher pore volumes because the microCT evaluation program is able to quantify additional pore volume located within the component microspheres. Quantitative microCT evaluation of bone volume within the defect, showed significant healing over the untreated control in all cases by 90 days post implantation. Interestingly, solid 500 μm implants did not exhibit any significant additional healing after day 30. However, the solid 100 μm implants increased new bone volume by 16 mm3 from day 30–90. This result may suggest smaller beads create pore systems more permissive of continued host tissue ingrowth. Although not significant, the difference in mean bone volume between groups may suggest a trend that smaller pore sizes influence greater amounts of bone healing within the defect area. Important limitations contributing to the healing outcomes observed in our studies include possible damage to the underlying dura, variations in periosteal closure, poor implant/host integration, infection local to the wound bed, poor revascularization of repair site, and/or lower nutrient/waste exchange that anticipated.
In general, healing trends between X-ray and microCT images were in agreement. However, the X-ray images suggested greater overall healing in all samples as compared with qualitative microCT analysis. This discrepancy can best be explained by the limit of resolution of the two systems used. The Faxitron X-ray system has a focal spot of 500 μm whereas the microCT system has a maximal resolution of 9 μm. The X-ray system resolution cannot distinguish individual bony islands closer than 500 μm which could potentially result in the appearance of greater bone coverage across the defect area compared with the microCT system’s higher resolution images.
Using 2D microCT reconstructed images, the spatial distribution and patterning of new bone formation was visually characterized. In the case of the solid 100 μm scaffolds, healing was observed equally along both edges of the defect and at the center edge of the defect. Additionally, healing was observed along the dural surface, the periosteal surface, and within the center of the defect. In contrast, both the solid and hollow 500 μm scaffolds had healing at both edges and minimally along the center edge but only along the dural surface. These observations suggest that pore size may directly influence the locations of healing within cranial defects.
Our original hypothesis stated that microsphere morphology will alter associated patterns of pore volume changes. Specifically, we anticipated hollow microspheres would generate additional pore volume for osseous tissue ingrowth as suggested by Huang et al.,25 a study which found slightly faster degradation rates using porous constructs compared with solid constructs. Although degradation rates were not measured directly in this study, previous investigations show at least 50% of this polymer will degrade over 80 weeks in vitro.26 In contrast to our original hypothesis, radiological analysis of new bone healing in animals receiving hollow core scaffold implants were not statistically different than other scaffold groups. Moreover, histological analysis suggests that remodeling of scaffold pore volume in this group may delay osseous tissue ingrowth possibly caused by swelling behaviors observed in histological sections. Specifically, end point thicknesses measured for each experiment group (1.05 ± 0.04 mm, 1.47 ± 0.05 mm, 1.91 ± 0.08 mm for solid 100 μm, solid 500 μm and hollow 500 μm respectively) suggested a correlation between increased scaffold thickness and reduced bone ingrowth. Furthermore, percent of the defect area covered by tissue following 90 days in vivo showed a similar trend of increasing tissue area with decreased scaffold thickness. This measurement suggests a correlation between the amount of polymer degraded and the amount of space allowed for tissue integration. Indeed, several other studies have also established such a link between increased swelling of PLAGA and slower degradation.27,28
CONCLUSIONS
Cranial bone is a unique tissue which has distinctive microenvironmental requirements during the repair process. Scaffold properties such as mechanical parameters, compatibility with local cell populations, and anatomical requirements of size and shape should be tailored to the specific site of repair. In this study, scaffolds were fabricated with an appropriate elastic modulus and overall thickness to approximate the defect site. A polymer choice of 85:15 PLAGA was previously shown to support proliferation and differentiation of local progenitor populations. Here, the cell-biomaterial compatibility may have been masked by the slow degradation rate, thereby potentially impeding maximal new bone growth. Interestingly, spatial locations of new bone growth within the defect area differed between pore size groups, suggesting scaffolds with smaller pore sizes are more permissible to mineralization throughout the entire defect region. Patterns of degradation of hollow core spheres did not significantly improve bony ingrowth as originally anticipated. In contrast, hollow sphere degradation patterns may have altered pore morphology such that bony ingrowth was delayed. However, our data also suggest that using the hollow core sphere technique may permit significant reductions in total implant volume after degradation begins, while maintaining a roughly equivalent functional outcome. Additional studies are required to determine whether bone healing by hollow core scaffolds may be improved, such as the tailoring of microcapsule wall thickness29 or changing co-polymer ratio and associated degradation rate of component microcapsules.
Figure 7.
Ex vivo evaluation of bone healing using microCT imaging analysis at 90 days comparing solid 100 μm, solid 500 μm, and hollow 500 μm average sized spheres. A: Bone volume as calculated by microCT evaluation. B: Percent change in bone volume compared to Day 30 control (data not shown). Shown are day 90 groups, n = 4. *Significant from control day 90. Here, p < 0.05.
Acknowledgments
Authors would like to acknowledge Rasesh Kapadia and Andres Laib from Scanco Medical for graciously lending their technical assistance.
Contract grant sponsor: National Institute of Health; contract grant number: K01AR052352-01A1
Contract grant sponsor: National Institute of Arthritis and Musculoskeletal and Skin Diseases; contract grant number: AR-052352-01A1
Contract grant sponsor: Whitaker Foundation faculty start up funds
Contract grant sponsor: National Institute of Dental & Craniofacial Research; contract grant number: DE-010369-08
Contract grant sponsor: Biotechnology Training Program; contract grant number: T32 GM-008715-03
References
- 1.Wang J, Glimcher MJ. Characterization of matrix-induced osteogenesis in rat calvarial bone defects. II. Origins of bone-forming cells. Calcif Tissue Int. 1999;65:486–493. doi: 10.1007/s002239900737. [DOI] [PubMed] [Google Scholar]
- 2.Hobar PC, Masson JA, Wilson R, Zerwekh J. The important of the dura in craniofacial surgery. Plast Reconstr Surg. 1998;98:217–225. doi: 10.1097/00006534-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Ogle RC, Tholpady SS, McGlynn KA, Ogle RA. Regulation of cranial suture morphogenesis. Cells Tissue Organs. 2004;176:54–66. doi: 10.1159/000075027. [DOI] [PubMed] [Google Scholar]
- 4.Warren SM, Greenwald JA, Nacamuli RP, Fong KD, Song HJ, Fang TD, Mathy JA, Longaker MT. Regional dura mater differentially regulates osteoblast gene expression. J Craniofacial Surg. 2003;14:363–370. doi: 10.1097/00001665-200305000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Gosain AK, Santoro TD, Song LS, Capel CC, Sudhakar PV, Madoub HS. Osteogenesis in calvarial defects: Contribution of the dura, the pericranium, and the surrounding bone in adult versus infant animals. Plast Reconstr Surg. 2003;112:515–527. doi: 10.1097/01.PRS.0000070728.56716.51. [DOI] [PubMed] [Google Scholar]
- 6.Kuboki Y, Jin QM, Takita H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J Bone Joint Surg Am. 2001;83A (2 Suppl 1):S105–S115. [PubMed] [Google Scholar]
- 7.El-Amin SF, Botchwey E, Tuli R, Kofron MD, Mesfin A, Sethuraman S, Tuan RS, Laurencin CT. Human osteoblast cells: Isolation, characterization, and growth on polymers for musculoskeletal tissue engineering. J Biomed Mater Res Part A. 2006;76:439–449. doi: 10.1002/jbm.a.30411. [DOI] [PubMed] [Google Scholar]
- 8.Kofron MD, Laurencin CT. Development of a calcium phosphate co-precipitate/poly(lactide-co-glycolide) DNA delivery system: Release kinetics and cellular transfection studies. Biomaterials. 2004;25:2637–2643. doi: 10.1016/j.biomaterials.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 9.Borden M, Attawia M, Khan Y, El-Amin SF, Laurencin CT. Tissue-engineered bone formation in vivo using a novel sintered polymeric microsphere matrix. J Bone and Joint Surg Br B. 2004;86:1200–1208. doi: 10.1302/0301-620x.86b8.14267. [DOI] [PubMed] [Google Scholar]
- 10.Borden M, Attawia M, Khan Y, Laurencin CT. Tissue engineered microsphere-based matrices for bone repair: Design, evaluation, and optimization. Biomaterials. 2001;23:551–559. doi: 10.1016/s0142-9612(01)00137-5. [DOI] [PubMed] [Google Scholar]
- 11.Botchwey EA, Pollack SR, Levine EM, Laurencin CT. Bone tissue engineering in a rotating bioreactor using a microcarrier matrix system. J Biomed Mater Res. 2001;55:242–253. doi: 10.1002/1097-4636(200105)55:2<242::aid-jbm1011>3.0.co;2-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NIH Publication #85–23 Rev. 1985. [Google Scholar]
- 13.Gefen A, Gefen N, Zhu Q, Raqhupathi R, Margulies SS. Age-dependent changes in material properties of the brain and braincase of the rat. J Neurotrauma. 2003;20:1163–1177. doi: 10.1089/089771503770802853. [DOI] [PubMed] [Google Scholar]
- 14.Laurencin CT, Khan Y, Kofron M, El-Amin S, Botchwey E, Yu X, Cooper JA. Tissue engineering of bone and ligament—A 15-year perspective. Clin Ortho Relat Res. 2006;447:221–236. doi: 10.1097/01.blo.0000194677.02506.45. [DOI] [PubMed] [Google Scholar]
- 15.Collin-Osdoby P. Role of vascular endothelial cells in bone biology. J Cell Biochem. 1994;55:304–309. doi: 10.1002/jcb.240550306. [DOI] [PubMed] [Google Scholar]
- 16.Gefen A, Gefen N, Zhu Q, Raqhupathi R, Margulies SS. Age-dependent changes in material properties of the brain and braincase of the rat. J Neurotrauma. 2003;20:1163–1177. doi: 10.1089/089771503770802853. [DOI] [PubMed] [Google Scholar]
- 17.Van der Merwe SW, van den Bogaerde JB, Goosen C, Maree FF, Milner RJ, Schnitzler CM. Hepatic osteodystrophy in rats results mainly from portasystemic shunting. GUT. 2003;52:580–585. doi: 10.1136/gut.52.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38:758–768. doi: 10.1016/j.bone.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 19.Everts V, Korper W, Jansen DC, Steinfort J, Lammerse I, Heera S, Docherty AJP, Beertsen W. Functional heterogeneity of osteoclasts: Matrix metalloproteinases participate in osteoclastic resorption of calvarial bone but not in resorption of long bone. FASEB J. 1999;13:1219–1230. doi: 10.1096/fasebj.13.10.1219. [DOI] [PubMed] [Google Scholar]
- 20.Petrie CE, Tholpady SS, Ogle RC, Botchwey EA. Proliferative capacity and osteogenic potential of novel dura mater stem cells on poly-lactic-co-glycolic acid (PLGA) J Biomed Mater Res. 2008;85:61–71. doi: 10.1002/jbm.a.31367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jorgensen PH, Bak B, Andreassen TT. Mechanical properties and biochemical composition of rat cortical femur and tibia after long-term treatment with biosynthetic human growth hormone. Bone. 1991;12:353–359. doi: 10.1016/8756-3282(91)90022-b. [DOI] [PubMed] [Google Scholar]
- 22.Levchakov A, Linder-Gantz E, Raghupathi R, Margulies SS, Gefen A. Computational studies of strain exposures in neonate and mature rat brains during closed head impact. J Neurotrauma. 2006;23:1570–1580. doi: 10.1089/neu.2006.23.1570. [DOI] [PubMed] [Google Scholar]
- 23.Ishaug SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997;36:17–28. doi: 10.1002/(sici)1097-4636(199707)36:1<17::aid-jbm3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 24.Jin QM, Takita H, Kohgo T, Atsumi K, Itoh H, Kuboki Y. Effects of geometry of hydroxyapatite as a cell substratum in BMP-induced ectopic bone formation. J Biomed Mater Res. 2000;51:491–499. [PubMed] [Google Scholar]
- 25.Huang YY, Qi M, Liu HZ, Zhao H, Yang DZ. Degradation of porous poly(D,L-lactic-co-glycolic acid) films based on water diffusion. J Biomed Mater Res A. 2007;15(80):909–915. doi: 10.1002/jbm.a.30980. [DOI] [PubMed] [Google Scholar]
- 26.Li S. Hydrolytic degradation characteristics of aliphatic poly-esters derived from lactic and glycolic acids. J Biomed Mater Res. 1999;48:342–353. doi: 10.1002/(sici)1097-4636(1999)48:3<342::aid-jbm20>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 27.Witt C, Kissel T. Morphological characterization of microspheres, films and implants prepared from poly(lactide-co-glycolide) and ABA triblock copolymers: Is the erosion controlled by degradation, swelling or diffusion? Eur J Pharm Biopharm. 2001;51:171–181. doi: 10.1016/s0939-6411(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 28.Yoon JJ, Park TG. Degradation behaviors of biodegradable macroporous scaffolds prepared by gas foaming of effervescent salts. J Biomed Mater Res. 2001;55:401–408. doi: 10.1002/1097-4636(20010605)55:3<401::aid-jbm1029>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 29.Jeyanthi R, Thanoo BC, Metha RC, DeLuca PP. Effect of solvent removal technique on the matrix characteristics of poly-lactide/glycolide microspheres for peptide delivery. J Controlled Release. 1996;38:235–244. [Google Scholar]