Abstract
The interaction of Bordetella bronchiseptica, toxigenic Pasteurella multocida serotype D, and the mycotoxin fumonisin B1 (FB1) was studied. On day 0 of the experiment, 28 artificially reared 3-day-old piglets were divided into 4 groups (n = 7 each): a control group (A), a group fed FB1 toxin (B), a group infected with the 2 pathogens (C), and a group infected with the 2 pathogens and fed FB1 toxin (D). The B. bronchiseptica infection [with 106 colony-forming units (CFU)/mL] was performed on day 4 and the P. multocida infection (with 108 CFU/mL) on day 16. From day 16 a Fusarium verticillioides fungal culture (dietary FB1 toxin content 10 mg/kg) was mixed into the feed of groups B and D. In groups C and D, clinical signs including mild serous nasal discharge, sneezing, panting, and hoarseness appeared from day 4, and then from day 16 some piglets had coughing and dyspnea as well. Computed tomography (CT) performed on day 16 demonstrated lung lesions attributable to colonization by B. bronchiseptica in the infected groups. By day 25 the number of piglets exhibiting lesions had increased, and the lesions appeared as well-circumscribed, focal changes characterized by a strong density increase in the affected areas of the lungs. The gross pathological findings confirmed the results obtained by CT. These results indicate that, when combined with dual infection by B. bronchiseptica and P. multocida, dietary exposure of pigs to FB1 toxin raises the risk of pneumonia and increases the extent and severity of the pathological changes.
Résumé
L’interaction entre Bordetella bronchiseptica, Pasteurella multocida toxinogène de sérotype D, et la mycotoxine fumonisine B1 (FB1) a été étudiée. Au jour 0 de l’expérience, 28 porcelets âgés de 3 j et élevés dans des conditions artificielles ont été séparés en 4 groupes de 7 porcelets : un groupe témoin (A), un groupe nourri avec la toxine FB1 (B), un groupe infecté avec les 2 agents pathogènes (C), et un groupe infecté avec les 2 agents pathogènes et nourri avec la toxine FB1 (D). L’infection avec B. bronchiseptica [106 unités formatrices de colonies (UFC)/mL] a été effectuée au jour 4 et l’infection avec P. multocida (108 UFC/mL) au jour 16. À partir du jour 16 une culture fongique de Fusarium verticillioides (contenu alimentaire en toxine FB1 de 10 mg/kg) a été mélangée dans l’aliment des groupes B et D. Dans les groupes C et D, des signes cliniques incluant un léger écoulement nasal séreux, des éternuements, du halètement et une raucité sont apparus à partir du jour 4, et par la suite à partir du jour 16 certains porcelets présentaient de la toux ainsi que de la dyspnée. Une tomodensitométrie (CT) effectuée au jour 16 a montré, dans les groupes infectés, des lésions pulmonaires attribuables à la colonisation par B. bronchiseptica. Au jour 25, le nombre de porcelets démontrant des lésions avait augmenté, et les lésions apparaissaient comme des zones focales de changements bien circonscrites, caractérisées par une forte augmentation de la densité dans les régions affectées du poumon. Les trouvailles pathologiques ont confirmé les résultats obtenus par CT. Ces résultats indiquent que, lorsque combinée à une infection mixte par B. bronchiseptica et P. multocida, l’exposition alimentaire des porcs à la toxine FB1 augmente le risque de pneumonie et l’étendue et la sévérité des changements pathologiques.
(Traduit par Docteur Serge Messier)
Introduction
Porcine respiratory disease complex is a major health problem in modern pig production (1). Diseases occurring in the simultaneous presence of multiple pathogens coupled with environmental predisposing factors are common in this industry and have enormous importance for profitability. The primary pathogens can be viruses or bacteria; the secondary pathogens are mostly bacteria (1).
Bordetella bronchiseptica is frequently isolated from respiratory conditions produced by multiple etiologic factors. Its dermonecrotic toxin has a fundamental role in producing respiratory disease in swine (2). Research findings suggest that the concurrent presence of B. bronchiseptica and other respiratory pathogens results in more severe disease than infection with B. bronchiseptica alone (3–5). It is known that B. bronchiseptica and toxigenic Pasteurella multocida work together to produce the progressive form of porcine atrophic rhinitis (6), and B. bronchiseptica has been demonstrated to produce pneumonia in young piglets (7).
One of the pathogens most frequently isolated from the lungs is P. multocida (8). Capsular serotype A is believed to be dominant among the pulmonary isolates of P. multocida (9,10); however, some investigators have found no difference in the frequency of the 2 serotypes (A and D) isolated from the lungs (11). Studies on diseases caused by P. multocida in combination with other respiratory pathogens have demonstrated that mixed infections produce a more severe disease course (12–15).
Among the predisposing factors of environmental origin, mycotoxins occurring in feeds and exerting a harmful effect on the health of animals may play an important role. Fumonisins (FB1, FB2, FB3, and FB4), produced by Fusarium verticillioides (16), may be present worldwide, mainly in maize in considerable amounts (17,18). Short-term exposure of pigs to FB1 toxin causes pulmonary edema; long-term exposure results in pulmonary fibrosis (19). Clinical signs develop only after exposure to high doses (more than 100 to 300 mg/kg of feed or 15 mg/kg body weight) of the toxin (20,21).
In this experiment the interactions of B. bronchiseptica, toxigenic P. multocida, and FB1 toxin were studied to determine whether the toxin can influence the type or severity of pulmonary diseases of bacterial origin.
Materials and methods
Experimental animals and housing
The piglets included in the experiment originated from a herd of high health status in which the incidence of respiratory diseases was negligible. The sows were free from toxigenic P. multocida, and the prevalence of B. bronchiseptica infection was very low. On the 3rd day of life, 28 female piglets were selected and transported to the experimental animal facility.
On the day of their arrival (day 0 of the experiment) the piglets were placed in battery cages in 2 rooms. On day 16 the 14 piglets in each room were divided into 2 groups; thus there were 2 groups of 7 piglets each in both rooms. Group A, serving as controls, and group B, piglets to be fed FB1 toxin, were housed in 1 room. Piglets to be experimentally infected with B. bronchiseptica and P. multocida (group C) as well as those to be infected with B. bronchiseptica and P. multocida and fed FB1 toxin (group D) were kept in the 2nd room.
Air temperature was adjusted to 27°C. The cages and the rooms were cleaned twice a day, and the piglet-rearing equipment was cleaned every 2nd day. Animal tenders entering the rooms wore protective clothing and disinfected their hands and feet with an aqueous solution of Virkon S (Antec, Novo Mesto, Croatia) when entering the rooms.
Feeding of the animals
Up to day 16 the piglets were fed a milk replacer diet consisting of skim milk powder, vegetable fats, and whey powder that contained 23% crude protein, 23% ether extract, and 1.6% lysine (Salvana Ferkel Ammen Milch; Salvana Tiernahrung, Sparrieshoop, Germany) from a Mambo automatic feeder (Sloten, Deventer, the Netherlands).
From day 7 a dry coarse meal containing 16 MJ/kg of metabolizable energy, 18.5% crude protein, 9% ether extract, and 1.65% lysine (Salvana Pre-meal; Salvana Tiernahrung) was also given to the piglets ad libitum, and then from day 16 to the end of the experiment only the dry coarse meal was available to them.
Drinking water was provided from nipple drinkers, and initially this was complemented with water offered from plastic drinking bowls.
Experimental infection
Groups C and D were infected with B. bronchiseptica [strain KM22, 106 colony-forming units (CFU)/mL] on day 4 and with toxigenic P. multocida serotype D (strain LFB-3, 108 CFU/mL) on day 16. The bacterial suspensions were prepared as described previously (22). A volume of 0.5 mL was inoculated through an endotracheal tube in all cases.
Mycotoxin treatment
From day 16 until the end of the experiment (day 39) groups B and D were fed a diet into which an F. verticillioides fungal culture (23) containing 3691 mg/kg of FB1 toxin was added in an amount to give a dietary FB1 concentration of 10 mg/kg of feed. This diet and those fed to groups A and C were checked for mycotoxin content (17) and found not to contain detectable amounts of other mycotoxins (T-2, zearalenone, DON, and OTA).
Studies
Clinical signs were recorded daily during the experiment. The piglets were weighed on days 4, 16, 25, and 39.
Sphingolipid profile test
On 2 occasions (on days 25 and 39) the free sphinganine to sphingosine ratio, the most sensitive biomarker of fumonisin toxicosis (24), was determined in the blood by a method described previously (25).
Computed tomography (CT)
On days 4, 16, 25, and 39 CT was used to detect lesions in the lung. Combinations of the following active ingredients, administered intramuscularly, were used for premedication: azaperone (Stresnil; Janssen Pharmaceutica, Beerse, Belgium), 4 mg/kg of body weight (BW); ketamine (CP-Ketamin 10%; CP-Pharma, Burgdorf, Germany), 10 mg/kg BW; xylazine (CP-Xylazine 2%; CP Pharma), 1 mg/kg BW; and atropine (Atropinum sulphuricum 0.1%; EGIS, Budapest, Hungary), 0.04 mg/kg BW.
After premedication a balloon-type endotracheal tube was introduced into the trachea, and then anesthesia was induced through inhalation of isoflurane (Forane; Abbott Laboratories, Abbott Park, Illinois, USA) in a mixture with 2% (v/v) oxygen. The animal was placed supine on a special supporting structure. Artificial breath-holding was applied during the thoracic scan.
Scans of the entire volume of the lungs were made with a Somatom Emotion 6 multislice CT scanner (Siemens, Erlangen, Germany) with a tube voltage of 130 kV, a dose of 100 mAs, and a field of view of 200 mm. From the collected data cross-sectional images of slices 2 and 5 mm thick were reconstructed, with full overlapping. The images were analyzed with the use of Medical Image Processing software (version 1.0, Ferenc Závoda, Kaposvár, Hungary).
Gross and histopathological examination
Postmortem examinations were performed on day 39. Macroscopic examination of the lungs was performed as described for routine slaughter check (26). Results were expressed as the percentage of lung area affected. For histopathological examination, samples were taken from lung areas showing pathological changes. Tissue samples were fixed in 4% formalin solution, embedded in paraffin, and sectioned; the sections were stained with hematoxylin and eosin.
Statistical evaluation
Differences between the groups were studied by 1-way analysis of variance (ANOVA) and Tukey’s post hoc test with use of the SAS 9.1 program (SAS System for Windows, release 9.1; SAS Institute, Cary, North Carolina, USA). The level of statistical significance was set at P ≤ 0.05.
Authorization
The experimental infection and CT examinations applied in this study were authorized by the Food Chain Safety and Animal Health Directorate of the Somogy County Agricultural Office (permission 438/2/SOM/2005).
Results
Clinical signs and weight
Groups A and B did not show clinical signs during the experiment. In groups C and D, clinical signs including mild serous nasal discharge, sneezing, panting, and hoarseness appeared from day 4 after B. bronchiseptica infection, and then from day 16 (infection with P. multocida and start of FB1 toxin consumption) some piglets had coughing and dyspnea as well. The number of pigs with clinical signs was the same as the number with lung lesions: 4 out of 7 in group C and 6 out of 7 in group D.
Three piglets died during the experiment: 1 piglet on day 17 in group C and 2 piglets on days 24 and 34, respectively in group D. The 3 piglets had severe dyspnea and the characteristic signs of hypoxia.
No significant differences were found between the groups in the weight gain of the piglets (Table I). However, the growth rate of those in group D was somewhat inferior to that of the animals in the other 3 groups (P > 0.05). The piglets in the mycotoxinfed groups (B and D) showed a pronounced heterogeneity of body weight on day 39, as indicated by the rather high standard deviations.
Table I.
Body weights in the piglet treatment groups (n = 7 each)
| Day of experiment | Piglet group; mean weight ± standarded deviation (kg)
|
P-value | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| 4a | 2.14 ± 0.35 | 2.11 ± 0.33 | 1.95 ± 0.20 | 2.10 ± 0.15 | 0.561 |
| 16b | 3.08 ± 0.46 | 2.94 ± 0.50 | 2.79 ± 0.33 | 2.77 ± 0.27 | 0.442 |
| 25 | 5.13 ± 0.71 | 4.80 ± 0.92 | 4.64 ± 0.62 | 4.41 ± 0.89 | 0.443 |
| 39 | 9.08 ± 1.09 | 8.95 ± 1.56 | 8.94 ± 1.07 | 8.57 ± 2.67 | 0.959 |
A — control group; B — group fed fumonisin B1 (FB1); C — group infected with Bordetella bronchiseptica and Pasteurella multocida serotype D; D — group infected with B. bronchiseptica and P. multocida serotype D and fed FB1.
Day of infection with B. bronchiseptica.
Day of infection with P. multocida and start of feeding with FB1.
Blood sphingolipid profile
On day 39 the free sphinganine to sphingosine ratio in the blood was elevated (P < 0.05) in groups B and D (0.65 and 0.47, respectively) as compared with the groups not fed FB1 toxin (0.22 and 0.27, respectively), indicating the effect of the toxin at the cellular level.
Evaluation of the CT scans
No lung lesions were seen in groups A and B on any of the test dates. Table II presents the numbers of piglets in groups C and D with lesions on the various test dates.
Table II.
Numbers of piglets with pathological lung lesions detected by computed tomography and by gross examination at necropsya
| Day of experiment
|
|||||
|---|---|---|---|---|---|
| Piglet group | 4 | 16 | 25 | 39 | Necropsy |
| A | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 |
| B | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 |
| C | 0/7 | 3/7 | 3/6 (4/7)b | 3/6 (4/7)b | 3/6 (4/7)b |
| D | 0/7 | 5/7 | 5/6 (6/7)b | 4/5 (6/7)b | 4/5 (6/7)b |
Three piglets died during the experiment: 1 piglet on day 17 in group C and 2 piglets on days 24 and 34, respectively in group D. The 3 piglets had severe dyspnea and the characteristic signs of hypoxia.
Number of piglets with lung lesions/total number of piglets at the start of the experiment.
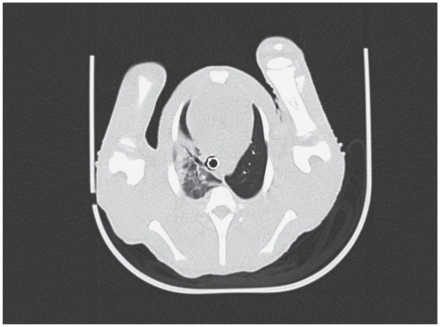
On day 16 lung lesions (Figure 1) were seen in 3 piglets in group C and 5 piglets in group D. The lesions were characterized by a mild or moderate density increase [about −600/−300 Hounsfield units (HU)], as compared with the normal density in the pneumatized parenchymal areas of the lung (about −700/−800 HU). This density increase was the result of an inflammatory process (exudate formation and cell proliferation).
Figure 1.
Lung lesions at the level of the 4th thoracic vertebrae on day 16 as shown by computed tomography (CT) of the lung of a piglet in group D infected with Bordetella bronchiseptica and Pasteurella multocida sero-type D and fed fumonisin B1 (FB1).
By day 25 the number of piglets showing well-circumscribed, focal lung lesions had increased. As a result of chronic inflammation, necrotic processes, and connective tissue formation, the affected lung areas showed foci of very high density (0 to 150 HU) surrounded by a zone of low or medium density (Figure 2).
Figure 2.
Demonstration of the progressively focal character of the lung lesions in serial CT scans of a piglet in group C on days 16 (A), 25 (B), and 39 (C) at the level of the 6th thoracic vertebra.
Gross and histopathological findings
In groups A and B none of the piglets had changes in the lung. In contrast, the lungs of 3 of the 6 surviving animals in group C and 4 of the 5 surviving animals in group D showed pathological lesions (Table II). The average percentage of affected lung area was 8.9% ± 16.0% (standard deviation) in group C and 16.9% ± 22.3% in group D (P = 0.087).
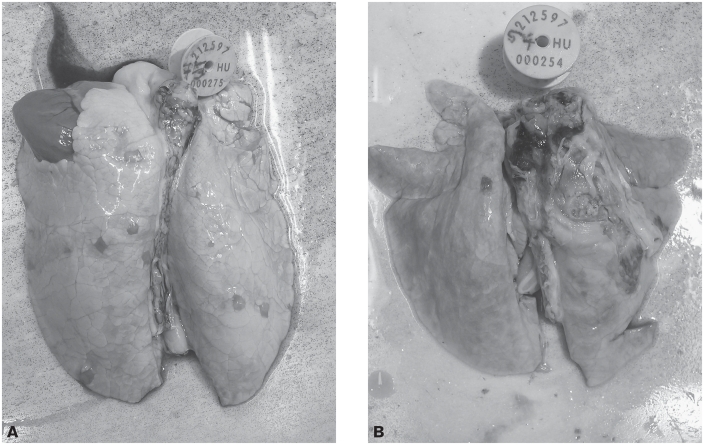
The lesions were located mainly in the anterior and intermediate lobes and in the cranial third of the posterior lobe. Involvement ranged from a few lobules (Figure 3A) to the entire lobe (Figure 3B). The lesions occurred mainly in the form seen in chronic catarrhal pneumonia, with necrotic foci demarcated by a fibrous capsule. Areas showing acute catarrhal changes were also observed around the chronic lesions. In 6 piglets a chronic adhesive pleuritis had also developed. The piglets that died during the experiment typically had acute or subacute serous–hemorrhagic catarrhal pneumonia.
Figure 3.
Diffuse inflammation extending to a lung lobule in the pulmonary parenchyma (A) and chronic inflammation of the pulmonary parenchyma extending to most of the left anterior, intermediate, and posterior lobes, accompanied by adhesive pleuritis (B), in piglets in group D.
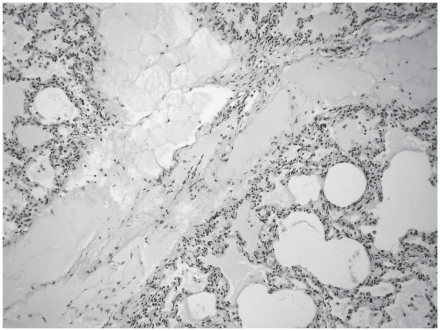
The histopathological changes observed in group C included an acute serous–hemorrhagic catarrhal pneumonia in the piglet that died on day 17 (with infiltration of lymphocytes, histiocytes, and neutrophil granulocytes in the lumen of the alveoli), which was seen also in the piglets undergoing necropsy at the end of the experiment. In addition, alveolar emphysema, focal atelectasis, catarrhal infiltration, fibrotic encapsulation, necrosis, and subacute pleuritis occurred (Figure 4). In group D the changes seen in group C were accompanied by mild or moderate alveolar and interstitial edema in some animals (Figure 5).
Figure 4.
Inflammatory and necrotic foci surrounded by a fibrous capsule in the pulmonary parenchyma of a piglet in group C. Hematoxylin and eosin (H&E); original magnification ×20.
Figure 5.
Alveolar and interstitial edema in the lung of a piglet in group D. H&E; original magnification ×100.
Discussion
The negative impact of respiratory pathogens (including B. bronchiseptica and P. multocida) on the weight gain of piglets has been demonstrated by several studies (3,7,27). Tóth et al (28) found that FB1 toxin did not affect feed intake and body weight gain even at a dose of 40 mg/kg of feed, despite the fact that such levels of FB1 cause rather severe but not clinically manifest pulmonary edema. On the other hand, Halloy et al (29) detected a significant depression in the body weight gain of toxin-fed piglets when the effects exerted by the combination of FB1 toxin and P. multocida were studied in experimentally infected piglets. The role played by FB1 toxin in dual infection with B. bronchiseptica and P. multocida and the interactions of the toxin and these pathogens had not yet been studied. In the present experiment there was no significant difference found in the average body weight of the groups, although the infected and FB1-treated group D piglets had the lowest growth rate among the groups.
The results of this study support the earlier finding that in young piglets B. bronchiseptica infection can cause lung lesions (7,30). Infection with P. multocida and the dietary intake of FB1 toxin starting 12 d after B. bronchiseptica infection increased the incidence of clinical signs, indicating an interaction between the bacterial infections and the mycotoxin.
The progressive nature of the pneumonia was confirmed with serial CT examination. By day 25, 71% of the piglets in the infected groups (57% of those in group C and 86% of those in group D) had pathological lung lesions that were increasing in size and becoming progressively focal. By the end of the experiment (day 39) the focal pneumonic nature had become more pronounced, whereas its severity was similar to that found on day 25.
At necropsy the incidence of lung lesions was the highest and their extent the most pronounced in the piglets of group D. This finding is in accord with the observation of Halloy et al (29) that P. multocida produces more severe and more extensive pneumonic lesions in piglets also exposed to fumonisin B1 toxin.
From the results of this experiment it can be concluded that, when coupled with dual infection by B. bronchiseptica and P. multocida, dietary exposure to the mycotoxin FB1 above the advised level of 5 mg/kg of feed (31) raises the risk of pneumonia and increases the extent and severity of the pathological changes produced.
Computed tomography is potentially suitable for the early detection of pneumonia and for monitoring its course and could thus provide useful information for the study of other respiratory conditions. We are currently designing a method to quantify the extent of lung lesions detected on CT scans that may improve the applicability of this technique.
Acknowledgement
The research was supported by the OTKA Foundation (project No. K 81690).
References
- 1.Brockmeier SL, Halbur PG, Thacker EL. Porcine respiratory disease complex (PRDC) In: Brogden KA, Guthmiller JM, editors. Polymicrobial Disease. Washington, DC: ASM Press; 2002. pp. 231–258. [PubMed] [Google Scholar]
- 2.Brockmeier SL, Register KB, Magyar T, Lax AJ, Pullinger GD, Kunkle RA. Role of the dermonecrotic toxin of Bordetella bronchiseptica in the pathogenesis of respiratory disease in swine. Infect Immun. 2002;70:481–490. doi: 10.1128/IAI.70.2.481-490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brockmeier SL, Palmer MV, Bolin SR. Effects of intranasal inoculation of porcine reproductive and respiratory syndrome virus, Bordetella bronchiseptica, or a combination of both organisms in pigs. Am J Vet Res. 2000;61:892–899. doi: 10.2460/ajvr.2000.61.892. [DOI] [PubMed] [Google Scholar]
- 4.Brockmeier SL. Prior infection with Bordetella bronchiseptica increases nasal colonization by Haemophilus parasuis in swine. Vet Microbiol. 2004;99:75–78. doi: 10.1016/j.vetmic.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Brockmeier SL, Loving CL, Nicholson TL, Palmer MV. Coinfection of pigs with porcine respiratory coronavirus and Bordetella bronchiseptica. Vet Microbiol. 2008;128:36–47. doi: 10.1016/j.vetmic.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chanter N, Magyar T, Rutter JM. Interactions between Bordetella bronchiseptica and toxigenic Pasteurella multocida in atrophic rhinitis of pigs. Res Vet Sci. 1989;47:48–53. [PubMed] [Google Scholar]
- 7.Underdahl NR, Socha TE, Doster AR. Long-term effect of Bordetella bronchiseptica infection in neonatal pigs. Am J Vet Res. 1982;43:622–625. [PubMed] [Google Scholar]
- 8.Palzer A, Ritzmann M, Wolf G, Heinritzi K. Associations between pathogens in healthy pigs and pigs with pneumonia. Vet Rec. 2008;162:267–271. doi: 10.1136/vr.162.9.267. [DOI] [PubMed] [Google Scholar]
- 9.Davies RL, MacCorquodale R, Baillie S, Caffrey B. Characterization and comparison of Pasteurella multocida strains associated with porcine pneumonia and atrophic rhinitis. J Med Microbiol. 2003;52:59–67. doi: 10.1099/jmm.0.05019-0. [DOI] [PubMed] [Google Scholar]
- 10.Ross RF. Pasteurella multocida and its role in porcine pneumonia. Anim Health Res Rev. 2006;7:13–29. doi: 10.1017/S1466252307001211. [DOI] [PubMed] [Google Scholar]
- 11.Djordjevic SP, Eamens GJ, Ha H, Walker MJ, Chin JC. Demonstration that Australian Pasteurella multocida isolates from sporadic outbreaks of porcine pneumonia are non-toxigenic (toxA–) and display heterogeneous DNA restriction endonuclease profiles compared with toxigenic isolates from herds with progressive atrophic rhinitis. J Med Microbiol. 1998;47:679–688. doi: 10.1099/00222615-47-8-679. [DOI] [PubMed] [Google Scholar]
- 12.Amass SF, Clark LK, van Alstine WG, et al. Interaction of Mycoplasma hyopneumoniae and Pasteurella multocida infections in swine. J Am Vet Med Assoc. 1994;204:102–107. [PubMed] [Google Scholar]
- 13.Chung WB, Bäckström LR, Collins MT. Experimental model of swine pneumonic pasteurellosis using crude Actinobacillus pleuropneumoniae cytotoxin and Pasteurella multocida given endobronchially. Can J Vet Res. 1994;58:25–30. [PMC free article] [PubMed] [Google Scholar]
- 14.Fuentes MC, Pijoan C. Pneumonia in pigs induced by intranasal challenge exposure with pseudorabies virus and Pasteurella multocida. Am J Vet Res. 1987;48:1446–1448. [PubMed] [Google Scholar]
- 15.Halloy DJ, Kirschvink NA, Mainil J, Gustin PG. Synergistic action of E. coli endotoxin and Pasteurella multocida type A for the induction of bronchopneumonia in pigs. Vet J. 2005;169:417–426. doi: 10.1016/j.tvjl.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Gelderblom WCA, Jaskiewicz K, Marasas WFO, et al. Fumonisins — novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl Environ Microbiol. 1988;54:1806–1811. doi: 10.1128/aem.54.7.1806-1811.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fazekas B, Bajmócy E, Glávits R, Fenyvesi A, Tanyi J. Fumonisin B1 contamination of maize and experimental acute fumonisin toxicosis in pigs. Zentralbl Veterinarmed B. 1998;45:171–181. doi: 10.1111/j.1439-0450.1998.tb00780.x. [DOI] [PubMed] [Google Scholar]
- 18.Dutton MF. The African fusarium/maize disease. Mycotoxin Res. 2009;25:29–39. doi: 10.1007/s12550-008-0005-8. [DOI] [PubMed] [Google Scholar]
- 19.Zomborszky-Kovács M, Kovács F, Horn P, Vetési F, Repa I, Tornyos G. Investigations into the time- and dose-dependent effect of fumonisin B1 in order to determine tolerable limit values. Livestock Prod Sci. 2002;76:251–256. [Google Scholar]
- 20.Harrison LR, Colvin BM, Greene JT, Newman LE, Cole JR. Pulmonary edema and hydrothorax in swine produced by fumonisin-B1, a toxic metabolite of Fusarium moniliforme. J Vet Diagn Invest. 1990;2:217–221. doi: 10.1177/104063879000200312. [DOI] [PubMed] [Google Scholar]
- 21.Haschek WM, Motelin G, Ness DK, et al. Characterization of fumonisin toxicity in orally and intravenously dosed swine. Mycopathologia. 1992;117:83–96. doi: 10.1007/BF00497283. [DOI] [PubMed] [Google Scholar]
- 22.Magyar T, King VL, Kovács F. Evaluation of vaccines for atrophic rhinitis — a comparison of three challenge models. Vaccine. 2002;20:1797–1802. doi: 10.1016/s0264-410x(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 23.Fodor J, Kametler L, Kovács M. Practical aspects of fumonisin production under laboratory conditions. Mycotoxin Res. 2006;22:211–216. doi: 10.1007/BF02946744. [DOI] [PubMed] [Google Scholar]
- 24.Riley RT, An NH, Showker JL, et al. Alteration of tissue and serum sphinganine to sphingosine ratio: an early biomarker of exposure to fumonisin-containing feeds in pigs. Toxicol Appl Pharmacol. 1993;118:105–112. doi: 10.1006/taap.1993.1015. [DOI] [PubMed] [Google Scholar]
- 25.Kametler L, Fodor J, Kovács M, Horn P. Biomarker in the detection of fumonisin toxicosis in pigs. Acta Agraria Kaposvariensis. 2006;10:285–291. [Google Scholar]
- 26.Straw BE, Tuovinen VK, Bigras-Poulin M. Estimation of the cost of pneumonia in swine herds. J Am Vet Med Assoc. 1989;195:1702–1706. [PubMed] [Google Scholar]
- 27.Cowart RP, Lipsey RJ, Hedrick HB. Measurement of conchal atrophy and pneumonic lesions and their association with growth rate in commingled feeder pigs. J Am Vet Med Assoc. 1990;196:1262–1264. [PubMed] [Google Scholar]
- 28.Tóth Á, Zomborszky-Kovács M, Tornyos G, Szalai N, Kübler K. Effect of low doses of the mycotoxin fumonisin B1 on the body mass gain, feed intake and feed conversion rate of pigs. Agriculture. 2000;6:149–151. [Google Scholar]
- 29.Halloy DJ, Gustin PG, Bouhet S, Oswald IP. Oral exposure to culture material extract containing fumonisins predisposes swine to the development of pneumonitis caused by Pasteurella multocida. Toxicology. 2005;213:34–44. doi: 10.1016/j.tox.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Brassinne M, Dewaele A, Gouffaux M. Intranasal infection with Bordetella bronchiseptica in gnotobiotic piglets. Res Vet Sci. 1976;20:162–166. [PubMed] [Google Scholar]
- 31.Commission recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding (2006/576/EC) Off J Eur Union. 2006 Aug 23;49(L229):7–9. [Google Scholar]